Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Estimation of LOX Levels in Serum Samples
2.3. Detection of rs1800449 Polymorphism
2.4. Sequencing
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for ovarian cancer: US preventive services task force recommendation statement. JAMA 2018, 319, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Anderson, M.L.; Heo, M.; Hosgood, H.D., III; Kamensky, V.; Bea, J.W.; Hou, L.; Lane, D.S.; Wactawski-Wende, J.; Manson, J.E.; et al. Adult stature and risk of cancer at different anatomic sites in a cohort of postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1353–1363. [Google Scholar] [CrossRef]
- Nieto, J.J.; Rolfe, K.J.; MacLean, A.B.; Hardiman, P. Ovarian cancer and infertility: A genetic link? Lancet 1999, 354, 649. [Google Scholar] [CrossRef]
- Hereditary Cancer Syndromes and Risk Assessment: ACOG committee opinion, number 793. Obstet. Gynecol. 2019, 134, e143–e149. [CrossRef] [PubMed]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Ovarian Cancer Risk Factors. Available online: https://www.cancer.org/cancer/ovarian-cancer/causes-risks-prevention/risk-factors.html (accessed on 17 October 2021).
- Amendola, P.G.; Reuten, R.; Erler, J.T. Interplay between LOX enzymes and integrins in the tumor microenvironment. Cancers 2019, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Bu, M.; Li, L.; Zhang, Y.; Xu, Y.; An, S.; Hou, F.; Jie, X. Lysyl oxidase genetic variants affect gene expression in cervical cancer. DNA Cell Biol. 2014, 33, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Mi, R.; Koupenova, M.; Eliades, A.; Patterson, S.; Toselli, P.; Thon, J.; Italiano, J.E., Jr.; Trackman, P.C.; Papadantonakis, N.; et al. Lysyl oxidase is associated with increased thrombosis and platelet reactivity. Blood 2016, 127, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Foreman, K.M.; Soriano, M.I.; Rossen, N.S.; Shehade, H.; Fregoso, D.R.; Eggold, J.T.; Krishnan, V.; Dorigo, O.; Krieg, A.J.; et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res. 2019, 79, 2271–2284. [Google Scholar] [CrossRef]
- Nishioka, T.; Eustace, A.; West, C. Lysyl oxidase: From basic science to future cancer treatment. Cell Struct. Funct. 2012, 37, 75–80. [Google Scholar] [CrossRef]
- Sterzyńska, K.; Klejewski, A.; Wojtowicz, K.; Świerczewska, M.; Nowacka, M.; Kaźmierczak, D.; Andrzejewska, M.; Rusek, D.; Brązert, M.; Brązert, J.; et al. Mutual expression of ALDH1A1, LOX and collagens in ovarian cancer cell lines as combined CSCs- and ECM-related models of drug resistance development. Int. J. Mol. Sci. 2019, 20, 54. [Google Scholar] [CrossRef]
- De Donato, M.; Petrillo, M.; Martinelli, E.; Filippetti, F.; Zannoni, G.F.; Scambia, G.; Gallo, D. Uncovering the role of nuclear Lysyl oxidase (LOX) in advanced high grade serous ovarian cancer. Gynecol. Oncol. 2017, 146, 170–178. [Google Scholar] [CrossRef]
- Grau-Bové, X.; Ruiz-Trillo, I.; Rodriguez-Pascual, F. Origin and evolution of lysyl oxidases. Sci. Rep. 2015, 5, 10568. [Google Scholar] [CrossRef]
- Kumari, S.; Tarun, K.P.; Tapaswini, P. Lysyl oxidase: Its diversity in health and diseases. Indian J. Clin. Biochem. 2017, 32, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, Y. The function and mechanisms of action of LOXL2 in cancer. Int. J. Mol. Med. 2015, 36, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Song, Y.; Pan, S.; Chu, M.; Wang, Z.W.; Zhu, X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 2020, 215, 107633. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Masuda, T.; Kuramitsu, S.; Tobo, T.; Sato, K.; Kidogami, S.; Nambara, S.; Ueda, M.; Tsuruda, Y.; Kuroda, Y.; et al. Potential association of LOXL1 with peritoneal dissemination in gastric cancer possibly via promotion of EMT. PLoS ONE 2020, 15, e0241140. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, H.; Yashiro, M.; Okuno, T.; Miki, Y.; Kitayama, K.; Masuda, G.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; et al. Significance of the lysyl oxidase members lysyl oxidase like 1, 3, and 4 in gastric cancer. Digestion 2018, 98, 238–248. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Ferreira, S.; Saraiva, N.; Rijo, P.; Fernandes, A.S. LOXL2 inhibitors and breast cancer progression. Antioxidants 2021, 10, 312. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Li, W. LOX/LOXL in pulmonary fibrosis: Potential therapeutic targets. J. Drug. Target. 2019, 27, 790–796. [Google Scholar] [CrossRef]
- Ji, F.; Wang, Y.; Qiu, L.; Li, S.; Zhu, J.; Liang, Z.; Wan, Y.; Di, W. Hypoxia inducible factor 1α-mediated LOX expression correlates with migration and invasion in epithelial ovarian cancer. Int. J. Oncol. 2013, 42, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Hudson, A.L.; Yuen, S.; Tran, N.; Howell, V.M.; Colvin, E.K. LOX and LOXL2 inhibition as a treatment for ovarian cancer. In Proceedings of the 107th Annual Meeting of the American Association for Cancer Research, New Orleans, LA, USA, 16–20 April 2016. [Google Scholar]
- Ye, M.; Zhou, J.; Gao, Y.; Pan, S.; Zhu, X. The prognostic value of the lysyl oxidase family in ovarian cancer. J. Clin. Lab. Anal. 2020, 34, e23538. [Google Scholar] [CrossRef]
- Rodriguez, H.M.; Vaysberg, M.; Mikels, A.; McCauley, S.; Velayo, A.C.; Garcia, C.; Smith, V. Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J. Biol. Chem. 2010, 285, 20964–20974. [Google Scholar] [CrossRef] [PubMed]
- Zaffryar-Eilot, S.; Marshall, D.; Voloshin, T.; Bar-Zion, A.; Spangler, R.; Kessler, O.; Ghermazien, H.; Brekhman, V.; Suss-Toby, E.; Adam, D.; et al. Lysyl oxidase-like-2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis 2013, 34, 2370–2379. [Google Scholar] [CrossRef][Green Version]
- Csiszar, K.; Mariani, T.J.; Gosin, J.S.; Deak, S.B.; Boyd, C.D. A restriction fragment length polymorphism results in a nonconservative amino acid substitution encoded within the first exon of the human lysyl oxidase gene. Genomics 1993, 16, 401–406. [Google Scholar] [CrossRef]
- Gene: LOX, Lysyl Oxidase (Minus Strand). Current Build 155, Released 9 April 2021. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1800449?vertical_tab=true (accessed on 17 November 2021).
- Ren, J.; Wu, X.; He, W.; Shao, J.; Cheng, B.; Huang, T. Lysyl oxidase 473 G>A polymorphism and breast cancer susceptibility in Chinese Han population. DNA Cell Biol. 2011, 30, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mongkolrob, R.; Tharabenjasin, P.; Bualuang, A.; Jarjanazi, H.; Pabalan, N. Influence of lysyl oxidase polymorphisms in cancer risk: An updated meta-analysis. Genet. Test. Mol. Biomark. 2021, 25, 411–418. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Zhu, Z. Lysyl oxidase rs1800449 polymorphism and cancer risk among Asians: Evidence from a meta-analysis and a case-control study of colorectal cancer. Mol. Genet. Genom. 2015, 290, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Y.; Wang, F.; Tian, Z.; Ma, B.; Li, Z.; Wang, B.; Zhao, W. Lysyl oxidase assists tumor-initiating cells to enhance angiogenesis in hepatocellular carcinoma. Int. J. Oncol. 2019, 2, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Liang, Z.; Mei, Z.; Wu, T.; Cui, A.; Liu, C.; Cui, L. Lysyl oxidase: A colorectal cancer biomarker of lung and hepatic metastasis. Thorac. Cancer 2018, 9, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, C.; Tong, D.; Hou, H. Lysyl oxidase G473A polymorphism is associated with increased risk of ovarian cancer. Genet. Test. Mol. Biomark. 2012, 16, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cong, J.-L.; Qu, L.-Y.; Jiang, L.; Wang, Y. Association between lysyl oxidase G473A polymorphism and ovarian cancer in the Han Chinese population. J. Int. Med. Res. 2012, 40, 917–923. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J. Lysyl oxidase G473A promotes migration, invasion and metastasis of ovarian cancer cells through regulating p38/Akt signaling pathways. Int. J. Clin. Exp. Pathol. 2017, 10, 3093–3100. [Google Scholar]
- Hussain, M.A.; Pati, S.; Swain, S.; Prusty, M.; Kadam, S.; Nayak, S. Pattern and trends of cancer in Odisha, India: A retrospective study. Asian Pac. J. Cancer Prev. 2012, 13, 6333–6336. [Google Scholar] [CrossRef][Green Version]
- Bhatla, N.; Meena, J.; Kumari, S.; Banerjee, D.; Singh, P.; Natarajan, J. Cervical cancer prevention efforts in India. Indian J. Gynecol. Oncol. 2021, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, M.R.; Wadgave, U.; Shimpi, P.V. Kuppuswamy’s socio-economic status scale: A revision of occupation and income Criteria for 2016. Indian J. Pediatr. 2017, 84, 3–6. [Google Scholar] [CrossRef]
- Bhatla, N.; Dar, L.; Patro, A.R.; Kumar, P.; Kriplani, A.; Gulati, A.; Iyer, V.K.; Mathur, S.R.; Sreenivas, V.; Shah, K.V.; et al. Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009, 33, 446–450. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, B.; He, Z.; Zhou, Y.; Han, C.; Shi, G.; Gao, R.; Wang, C.; Yang, L.; Song, H.; et al. Lysyl oxidase polymorphisms and susceptibility to osteosarcoma. PLoS ONE 2012, 7, e41610. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, G.; Shen, W.; Huang, Z.; He, H.; Cui, L. Lysyl oxidase-like 2 is highly expressed in colorectal cancer cells and promotes the development of colorectal cancer. Oncol. Rep. 2018, 40, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Bird, D.; Lang, G.; Cox, T.R.; Erler, J.T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 2013, 32, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Trackman, P.C.; Mäki, J.M.; Myllyharju, J.; Kirsch, K.H.; Sonenshein, G.E. The Ras signaling inhibitor LOX-PP interacts with Hsp70 and c-Raf to reduce Erk activation and transformed phenotype of breast cancer cells. Mol. Cell Biol. 2011, 31, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.K.; Lu, J. Cancer cells remodel themselves and vasculature to overcome the endothelial barrier. Cancer Lett. 2016, 380, 534–544. [Google Scholar] [CrossRef]
| Parameters | Control Subjects (n = 86) | Cases (Ovarian Cancer) (n = 83) |
|---|---|---|
| Age (in Years) | 59 ± 3.4 | 58 ± 4.1 |
| Socioeconomic Status n (%) | ||
| Low | 41 (48%) | 45 (54%) |
| Middle | 45 (52%) | 38 (46%) |
| Family history of cancer n (%) | ||
| Yes | 07 (8%) | 24 (29%) * |
| No | 79 (92%) | 59 (71%) |
| Menopausal status n (%) | ||
| Postmenopausal | 47 (55%) | 44 (53%) |
| Premenopausal | 39 (45%) | 39 (47%) |
| Parity n (%) | ||
| Multiparous | 53 (62%) | 67 (81%) * |
| Uniparous | 33 (38%) | 16 (19%) |
| History of PCOD n (%) | ||
| Yes | 02 (2%) | 31 (37%) * |
| No | 84 (98%) | 52 (63%) |
| History of intake of hormone replacement therapy n (%) | NA | |
| Yes | 22 (27%) | |
| No | 61 (73%) | |
| FIGO staging n (%) | NA | |
| Stage II | 15 (18%) | |
| Stage III | 22 (27%) | |
| Stage IV | 46 (55%) |
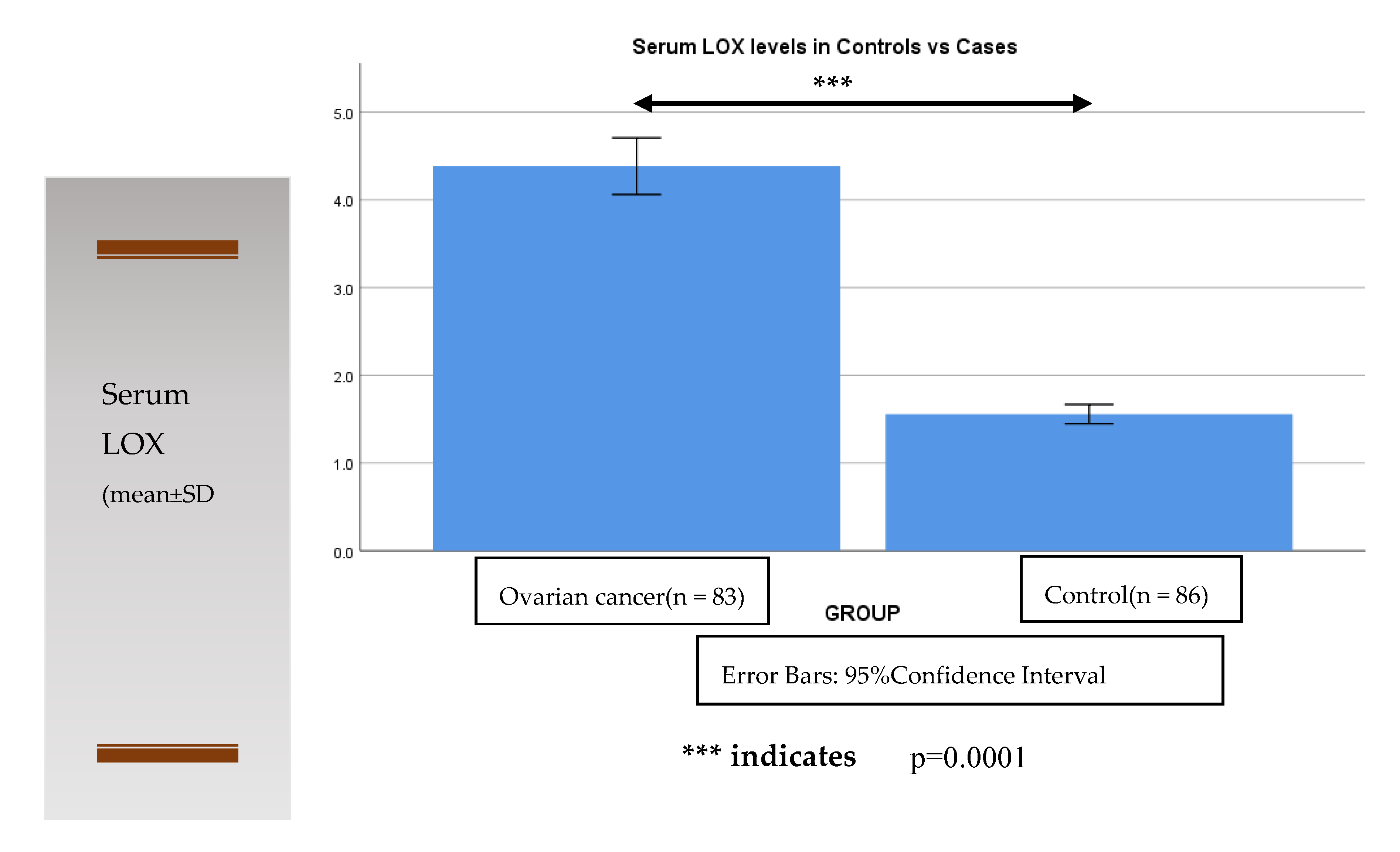
| Serum Lysyl Oxidase (ng/mL) | Control Subjects (n = 86) | Cases (Ovarian Cancer) (n = 83) FIGO Stage | |
| I, II | III, IV | ||
| 1.61 ± 0.47 | 3.28 ± 0.66 | * 5.01 ± 1.05 | |
| TNM grade | |||
| 1,2 | 3 | ||
| 1.61 ± 0.47 | 3.42 ± 0.78 | * 4.89 ± 1.16 | |
| FIGO: International Federation of Gynecology and Obstetrics staging TNM: Tumor, Lymph Node and Metastasis grade | |||
| Healthy Control (n = 86) | Cases Ovarian Cancer (n = 83) | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|---|
| Genotype | ||||
| GG | 61 (70.93%) | 49 (59.32%) | Referent | |
| GA | 20 (23.25%) | 21 (26.23%) | 1.305 (0.6319–2.702) | 0.4716 |
| AA | 5 (5.81%) | 13 (14.45%) | 3.208 (1.092–10.64) | 0.0332 |
| Allele | ||||
| G | 142 (82.6%) | 119 (71.69%) | Referent | |
| A | 30 (17.4%) | 47 (28.31%) | 1.866 (1.112–3.16) | 0.0178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Patro, A.R.K.; Mishra, B.; Jena, S.K.; Singh, S. Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population. Diagnostics 2022, 12, 53. https://doi.org/10.3390/diagnostics12010053
Kumari S, Patro ARK, Mishra B, Jena SK, Singh S. Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population. Diagnostics. 2022; 12(1):53. https://doi.org/10.3390/diagnostics12010053
Chicago/Turabian StyleKumari, Suchitra, A. Raj Kumar Patro, Baijayantimala Mishra, Saubhagya Kumar Jena, and Sweta Singh. 2022. "Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population" Diagnostics 12, no. 1: 53. https://doi.org/10.3390/diagnostics12010053
APA StyleKumari, S., Patro, A. R. K., Mishra, B., Jena, S. K., & Singh, S. (2022). Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population. Diagnostics, 12(1), 53. https://doi.org/10.3390/diagnostics12010053






