Abstract
This systematic review and meta-analysis aimed to investigate the ultrasonographic variation of the diameter of the inferior vena cava (IVC), internal jugular vein (IJV), subclavian vein (SCV), and femoral vein (FV) to predict fluid responsiveness in critically ill patients. Relevant articles were obtained by searching PubMed, EMBASE, and Cochrane databases (articles up to 21 October 2021). The number of true positives, false positives, false negatives, and true negatives for the index test to predict fluid responsiveness was collected. We used a hierarchical summary receiver operating characteristics model and bivariate model for meta-analysis. Finally, 30 studies comprising 1719 patients were included in this review. The ultrasonographic variation of the IVC showed a pooled sensitivity and specificity of 0.75 and 0.83, respectively. The area under the receiver operating characteristics curve was 0.86. In the subgroup analysis, there was no difference between patients on mechanical ventilation and those breathing spontaneously. In terms of the IJV, SCV, and FV, meta-analysis was not conducted due to the limited number of studies. The ultrasonographic measurement of the variation in diameter of the IVC has a favorable diagnostic accuracy for predicting fluid responsiveness in critically ill patients. However, there was insufficient evidence in terms of the IJV, SCV, and FV.
1. Introduction
Achieving a satisfactory response to fluid replacement in critically ill patients has remained a challenging issue [1,2,3,4]. An insufficient fluid volume can lead to low cardiac output (CO), which may result in reduced tissue perfusion [1,2]. However, excessive fluid infusion might also be detrimental. As depicted by the Frank–Starling curve, an increase in preload does not correspond to an equal increase in stroke volume (SV) when it reaches the maximum slope and plateau [5]. Excessive fluid volume is a significant risk factor for acute lung injury, bowel edema, and compartment syndrome [6,7]. Therefore, it is crucial to determine whether the patient needs additional fluid or not. However, it is not easy to precisely predict fluid responsiveness before fluid administration because the etiology of shock associated with diverse aspects of fluid balance is difficult to ascertain. This is despite the fact that many clinical manifestations of shock such as low blood pressure, tachycardia, altered mental state, cool clammy skin, or low urine output have been described [8]. To evaluate fluid responsiveness, information about the increase in SV after fluid challenge is useful. Invasive methods used in the past include the Swan–Ganz catheter, which directly measures capillary wedge pressure, and has been the gold standard for CO or SV measurement [9]. However, it is a substantially invasive and difficult procedure, especially in patients with cardiovascular instability [4,9]. Therefore, to overcome the shortcomings of the Swan–Ganz measurement method, a pulse wave analysis method that attempts to measure CO or SV has been proposed [10]. Other minimally invasive and non-invasive methods such as arterial pulse wave analysis also have several limitations in terms of artifact validation, arterial compliance, alteration in vasomotor tone, or non-pulsatile blood flow [10]. Moreover, the above-mentioned techniques are unable to predict fluid responsiveness before the fluid challenge.
Recently, several researchers have applied a point-of-care ultrasound for critically ill patients [11]. Ultrasonography is non-invasive, and its cost is relatively low. It can measure SV effectively [11]. In addition, ultrasonography can detect the variation of IVC diameter (ΔIVC), which reflects the cardiac preload [11,12]. By measuring the cardiac preload, it is possible to predict volume status and fluid responsiveness. The measurement of the IVC diameter is easily performed via a subxiphoid view even by non-highly trained operators, whereas measuring the SV via an echocardiogram requires an experienced intensivist or cardiologist. In addition, the internal jugular vein (IJV), subclavian vein (SCV), and femoral vein (FV) are easier to visualize because they are more superficial than the IVC. The diameter of the IVC varies with inspiration and expiration [12]. In patients who breathe spontaneously, intrathoracic pressure decreases during inspiration; this results in accelerated venous return. During expiration, intrathoracic pressure increases, and venous return decreases [12]. Consequently, the IVC diameter decreases during inspiration and increases during expiration. When mechanical ventilation is employed, this phenomenon reverses. However, the ΔIVC is not always visible in patients with obesity, intraabdominal fluid collection, or bowel gas. Thus, other large veins might be used as alternatives in such patients.
To date, several meta-analyses have demonstrated that ΔIVC showed favorable outcomes [12,13]. However, these evaluated data up to 2017, and many more studies have been published since then. Moreover, there has been no systematic review and meta-analysis regarding other large veins such as the IJV, SCV, or FV. To update the evidence on ΔIVC and explore its alternatives, we conducted a systematic review and meta-analysis for respiratory variation in the diameters of the IVC, IJV, SCV, and FV.
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy (PRISMA-DTA) statement published study search and selection criteria [14]. The preset protocol of this study was registered on PROSPERO (CRD42020206037, https://www.crd.york.ac.uk/prospero/, last accessed date: 21 October 2021). Relevant articles were obtained by searching PubMed, EMBASE, and Cochrane databases through 21 October 2021. These databases were searched using the following keywords: “((subclavian vein) OR (inferior vena cava) OR (internal jugular vein) OR (femoral vein)) AND ((fluid responsiveness) OR volume) AND (diameter OR collapsibility OR measurement) AND (ultrasonography OR ultrasound OR sonography OR sonographic OR (point of care))”. We also manually searched the reference lists of relevant articles. The titles and abstracts of all searched articles were screened for exclusion. Review articles and previous meta-analyses were also screened to obtain additional eligible studies. The search results were then reviewed and articles were included if the study investigated the diagnostic accuracy of the IVC, SCV, IJV, and FV to predict fluid responsiveness.
The inclusion criteria for diagnostic test accuracy (DTA) reviews were as follows: (1) the study population included patients who received fluid replacement due to sepsis, hypovolemia, or circulatory failure; (2) an ultrasonographic measurement of the respiratory variability of the IVC, SCV, IJV, and FV diameter was performed as an index test; (3) tests that enabled measurement of fluid responsiveness were performed for reference standard; (4) the primary outcome of the study was the diagnostic accuracy of ultrasonographic respiratory variability of the diameters of IVC, SCV, IJV, and FV to predict fluid responsiveness; (5) adequate information was provided to build a 2-by-2 contingency table consisting of true positive (TP), false positive (FP), false negative (FN), and true negative (TN) outcomes. Articles that involved another disease, those that did not provide 2-by-2 contingency table information, non-original articles, non-human studies, pediatric studies, or those published in a language other than English were excluded.
2.2. Data Extraction
Data from all eligible studies were extracted by two investigators. Extracted data from each of the eligible studies included: the first author’s name, year of publication, study location, study design and period, number of patients analyzed, measured vein, index test, threshold of index test, reference standard, device used for the reference standard, threshold of reference standard, and fluid responsiveness. The number of TP, FP, FN, and TN for the index test in predicting fluid responsiveness were collected. If the eligible study reported multiple thresholds and accuracy of the index test, we extracted the subset with optimal threshold or highest performance.
2.3. Quality Assessment
All studies were independently reviewed by two investigators. Disagreements concerning the study selection and data extraction were resolved by consensus. As recommended by the Cochrane Collaboration, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool was used to evaluate the risk of bias in DTA [15]. Disagreements in this regard were resolved by discussion with the third independent author. The QUADAS-2 assesses four domains for bias and applicability as follows: (1) patient selection; (2) index test; (3) reference standard; (4) flow and timing.
2.4. Statistical Analysis
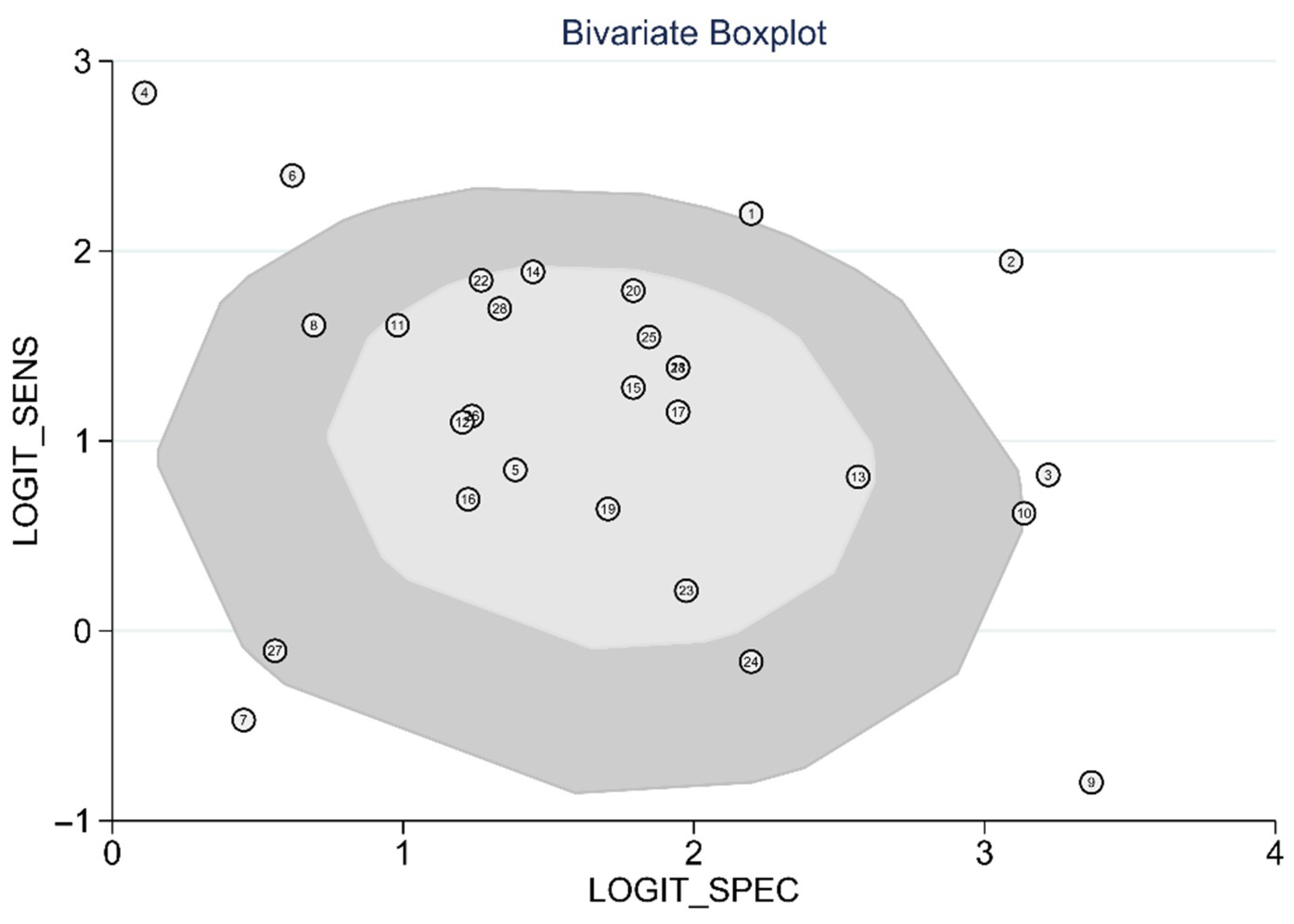
For statistical analysis using meta-analysis, we used the “metandi” and “midas” modules of Stata version 17.0 (Stata Corporation, College Station, TX, USA) and “mada” package of the R programming language, version 4.0.3 (R foundation, Vienna, Austria). QUADAS-2 assessment was performed using Review Manager Software 5.4 (The Cochrane Collaboration, Oxford, Copenhagen, Denmark). We constructed a 2-by-2 contingency table (TP, FP, FN, TN) by calculating or extracting from each primary study. For rigorous statistical analysis and heterogeneity across the studies, we used both the hierarchical summary receiver operating characteristics (HSROC) model [16] and the bivariate model [17]. A bivariate mixed-effects regression model for the synthesis of diagnostic test data and the derived logit estimates of sensitivity, specificity, and respective variances was used to construct a hierarchical summary ROC curve [17]. The HSROC model assumes that there is an underlying ROC curve in each study with parameters that characterize the accuracy and asymmetry of the curve [16]. An area under the ROC curve (AUROC) close to 1 and 0.5 indicated a strong test and poor test, respectively. Results with p-values < 0.05 were considered statistically significant. To investigate the heterogeneity, I2 was calculated from results as I2 = 100% × (Q − df)/Q, where Q is Cochran’s heterogeneity statistics and df is the degree of freedom [18]. I2 lies between 0% and 100%. A value of 0% indicates no observed heterogeneity and values greater than 50% are considered to indicate substantial heterogeneity. To detect the threshold effect, Spearman’s correlation coefficient between sensitivity and specificity was calculated after logit transformation. The HSROC shape (asymmetry) parameter was β (beta), where β = 0 corresponds to a symmetric ROC curve in which the diagnostic odds ratio does not vary along the curve [16]. Due to the trade-off between sensitivity and specificity, we used bivariate random-effects modeling of sensitivity and specificity as we expected that this pair of performance measures will be interdependent. We used the bivariate box plot that describes the degree of interdependence including the central location and identification of any outliers [19]. The inner oval represents the median distribution while the outer oval represents the 95% confidence bound. The skewness provides indirect evidence of some threshold variability [19]. A multiple univariable bivariate meta-regression was conducted to investigate the possible source of heterogeneity. Covariates were manipulated as mean-centered continuous or dichotomous (yes = 1. No = 0) fixed effects. Publication bias was first assessed visually using a scatter plot. We used the diagnostic log odds ratio (lnDOR), which should have a symmetrical funnel shape when publication bias is absent [20]. Formal testing for publication bias was conducted by the regression of lnDOR against the square root of the effective sample size, with p < 0.05 for the slope coefficient indicating significant asymmetry [20].
3. Results
3.1. Selection and Characteristics
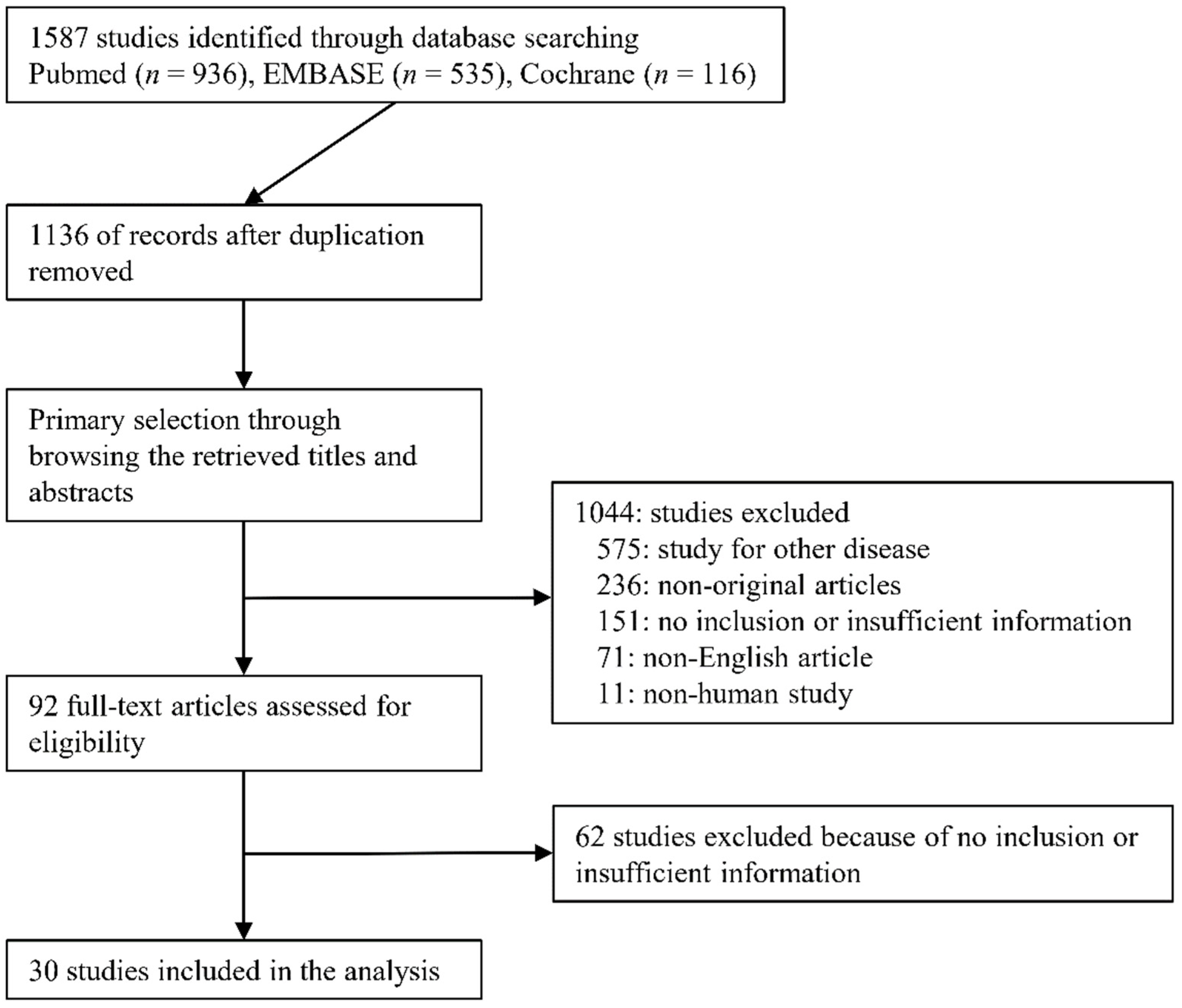
A total of 1587 studies were identified through searching databases. After removing duplicates, 1136 studies were retrieved. We excluded 1044 studies through a title and abstract review because they were non-original (n = 236), studied other diseases (n = 575), were non-human studies (n = 11), or were written in a non-English language (n = 71). We reviewed 92 full-text articles. After the full-text review, 62 articles were excluded due to insufficient data (n = 36), lack of 2-by-2 data (n = 25), and not being original (n = 1). Finally, 30 studies [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] comprising 1719 patients were included in this review (Figure 1); detailed information about the eligible studies is shown in Table 1. In cases of the IJV [29,36,42], FV (was not detected), and SCV [41], we were not able to conduct the meta-analysis due to an insufficient number of studies. Two studies [36,42] reported on both the IVC and IJV. He et al. [45] reported on three subsets according to tidal volume (TV) (6 mL/kg, 9 mL/kg, 12 mL/kg) and the subset of 9 mL/kg TV showed the highest AUROC. Thus, we extracted the subset of 9 mL/kg TV. Three studies [39,40,48] reported on subsets of patients with standardized breathing and spontaneous breathing. We extracted subsets of spontaneous breathing because other studies included only patients with spontaneous breathing. Corl et al. [44] reported results obtained by both experts and novices. We extracted the results of experts because other studies were conducted by experts. One study by Blavius [50] was a comparative study between artificial intelligence and human. We extracted the result of the training dataset by humans because the number of test datasets was much smaller than the test set (20 vs. 175). Caplan et al. [48] reported different results according to the measuring site (1, 2, 3, and 5 cm apart from the aortocaval junction). We extracted a subset of 3 cm from the aortocaval junction because it was similar to other eligible studies.
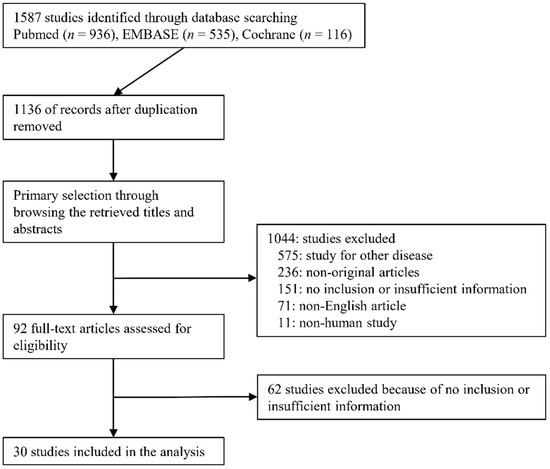
Figure 1.
Flow diagram for identification of eligible studies.

Table 1.
Main characteristics of the eligible studies.
3.2. Clinical Characteristics of Patients
All 30 eligible studies were summarized in Table 1. Twenty-eight studies [21,22,23,24,25,26,27,28,30,31,32,33,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50] comprised the measurement of the IVC. Only three studies [29,36,42] comprised that of the IJV and one study [41] comprised that of the SCV. In our searches, there was no relevant study that comprised measurements of the FV. Twenty-three studies used the M-mode of ultrasonography [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,37,39,41,42,43,45,47,50]. Seventeen studies used echocardiography as a reference standard [21,22,24,25,26,27,30,31,32,38,39,40,45,47,48,49]. Four studies used invasive devices that extracted the waveform in arteries [29,33,34,42]. Ten studies comprised patients with sepsis [21,22,26,27,29,33,36,37,39,48] and 18 comprised those on mechanical ventilation [21,22,23,24,27,29,31,32,33,34,37,38,41,42,43,45,46,47]. In the eligible studies, the ΔIVC was measured in three ways: first, the IVC collapsibility [25,26,28,30,35,36,39,40,44,48,49,50] denotes (maximal IVC diameter—minimal IVC diameter) / IVC diameter (maximum); second, the IVC distensibility [21,23,24,27,31,32,33,34,37,38,45,46,47] denotes (maximal IVC diameter—minimal IVC diameter)/minimal IVC diameter; third, the IVC variability [22,42,43] denotes (maximal IVC diameter—minimal IVC diameter)/(minimal IVC diameter + maximal IVC diameter)/2). ΔIVC was measured near the origin of the hepatic vein via the subxiphoid view in all eligible studies. The IJV diameter was measured at the level of the cricoid cartilage. The SCV diameter was measured at the level of the clavicle.
3.3. DTA Review
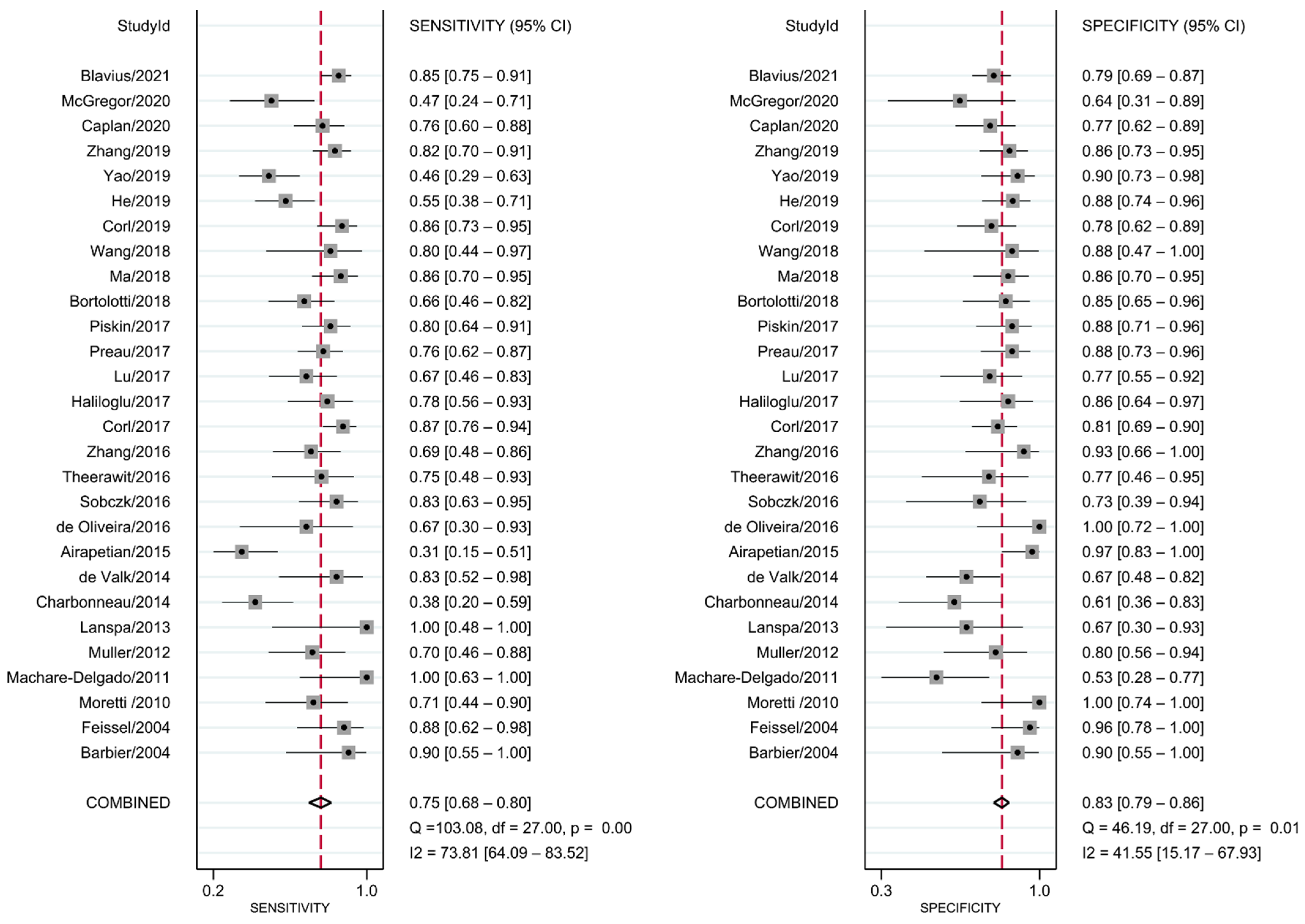
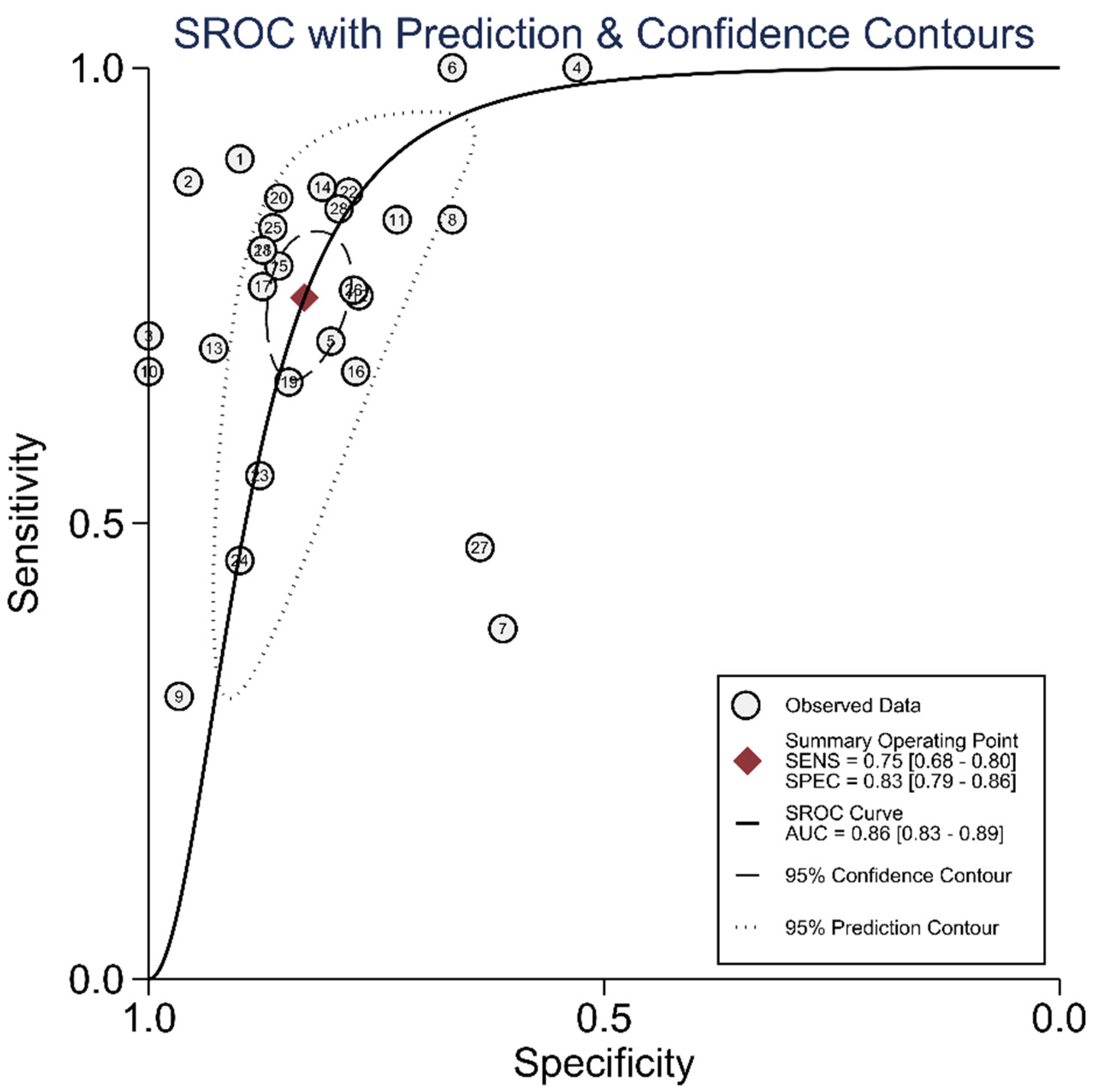
The diagnostic test accuracy of eligible studies was summarized in Table 2. The threshold of index test (ΔIVC) ranged from 11.1 to 49%. The pooled sensitivity of ΔIVC in 28 eligible studies [21,22,23,24,25,26,27,28,30,31,32,33,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50] was 0.75 (95% CI, 0.68–0.80, I2 = 73.8%) and the pooled specificity was 0.83 (95% CI, 0.79–0.86, I2 = 41.6%; Figure 2). The pooled positive likelihood ratio was 4.37 (95% CI, 3.58–5.33, I2 = 10.7%) and the pooled negative likelihood ratio was 0.30 (95% CI, 0.24–0.39, I2 = 75.8%). The pooled diagnostic odds ratio was 14.3 (95% CI, 10.1–20.4, I2 = 100%). The summary ROC curve (SROC) with prediction and confidence contours is depicted in Figure 3. The AUROC was 0.86 (95% CI, 0.83–0.89, I2 = 93%). To evaluate the degree of interdependence, we used a bivariate boxplot that plotted the correlation of logit-transformed sensitivity and specificity (Figure 4). Seven studies were outliers, in that these were outside the 95% confidence interval area [21,22,23,24,26,27,30]. In the test for threshold effect, Spearman’s rank correlation rho was −0.20 (p = 0.30). HSROC asymmetry parameter, β, was −0.676 (p = 0.237). Therefore, we concluded that there was no threshold effect.

Table 2.
Diagnostic test accuracy of eligible studies.
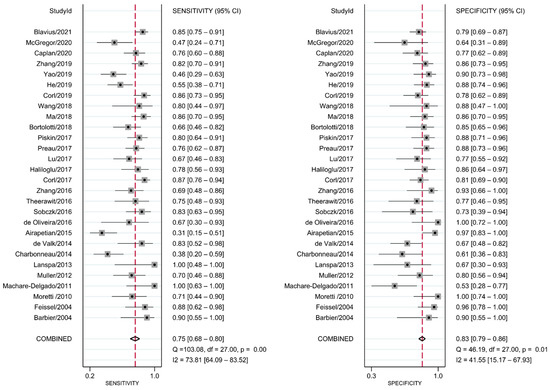
Figure 2.
Forest plot for pooled sensitivity and specificity of respiratory variability of IVC diameter.
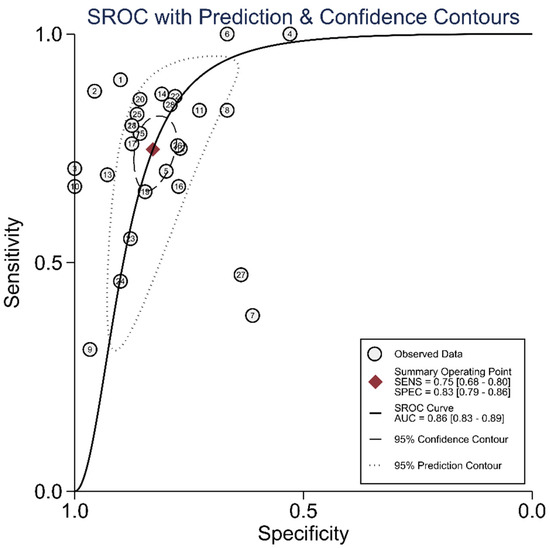
Figure 3.
Summary ROC curve of respiratory variability of IVC diameter.
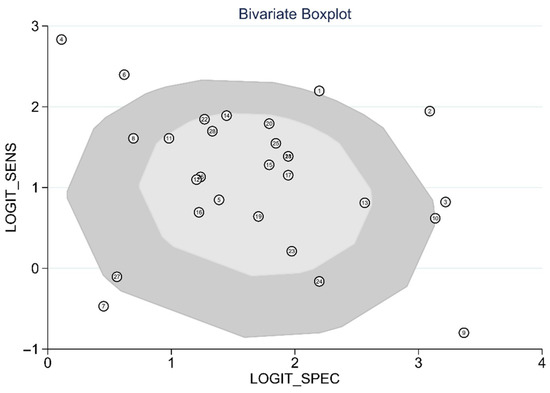
Figure 4.
Bivariate boxplot of respiratory variability of IVC diameter.
3.4. Meta-Regression, Subgroup Analysis, and Evaluation of Heterogeneity
The univariable meta-regression and subgroup analysis using possible confounders are summarized in Table 3. We conducted the subgroup analysis according to possible confounders as follows: ΔIVC, IVC collapsibility index, reference test, ICU admission, sepsis, fluid infusion, mechanical ventilation, and the heterogeneity on a bivariate boxplot. In the meta-regression test, there was no significance of any of the moderators. There was no statistical significance in meta-regression. As in the previous meta-analysis conducted by Si et al. [13], we divided two groups who underwent MV. One group underwent MV with TV ≥ 8 mL/kg or positive end expiratory pressure (PEEP) ≤ 5 cm H2O [21,22,23,27,31,32,34,38,42,45]. The other group underwent MV with TV < 8 mL/kg or PEEP > 5 cm H2O [24,33,37,43,46]. There was no statistical significance in the meta-regression test (p = 0.31). We also conducted a subgroup analysis according to inliers (within 95% CI) [25,28,31,32,33,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50] and outliers [21,22,23,24,26,27,30] on a bivariate boxplot, and the meta-regression showed no significant difference (p = 0.83).

Table 3.
Meta-regression and subgroup analysis of respiratory variability of IVC diameter.
3.5. Publication Bias
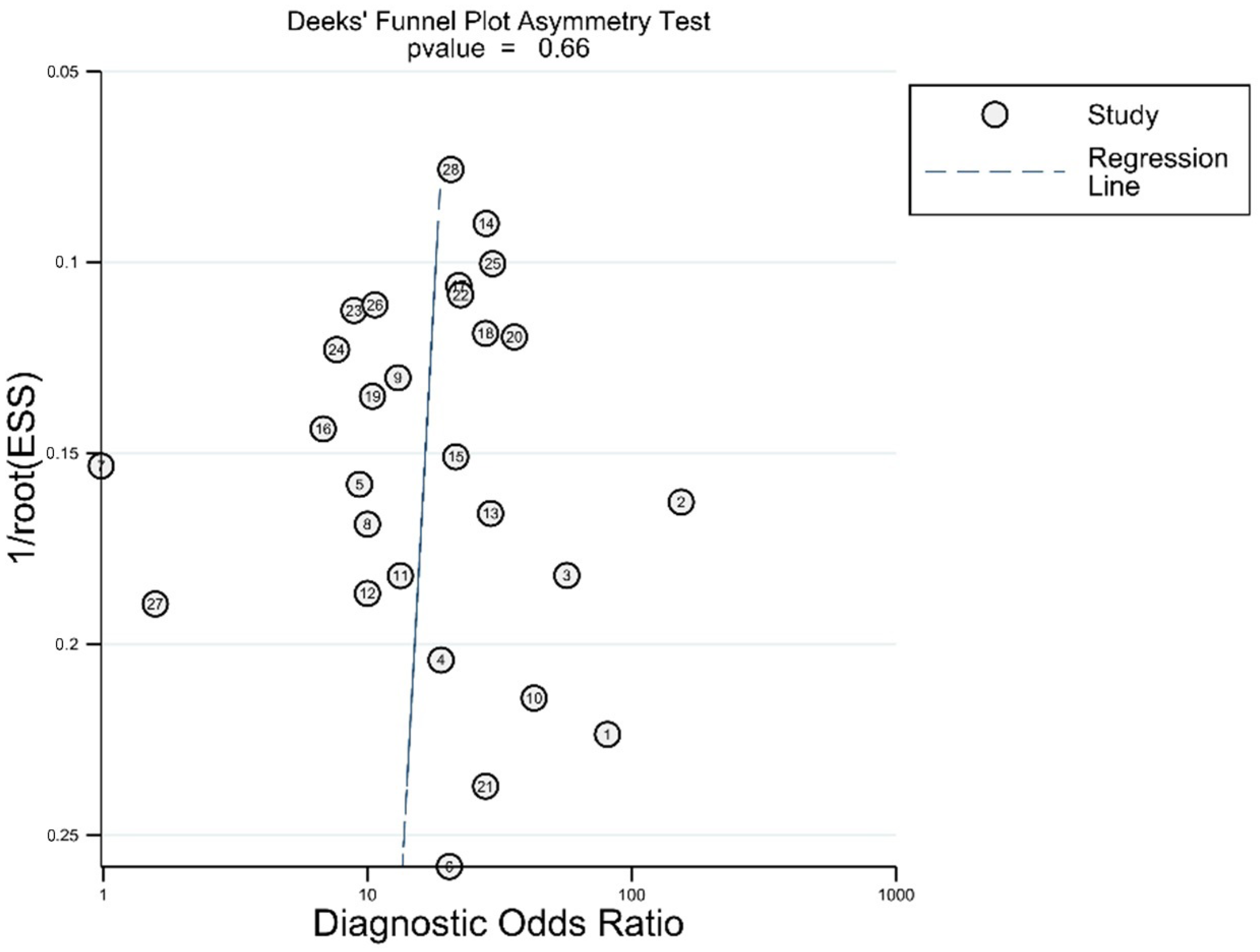
In Deek’s funnel plot using the diagnostic odds ratio, there was no asymmetry on visual inspection (Figure 5). There was also no statistically significant asymmetry (p = 0.66).
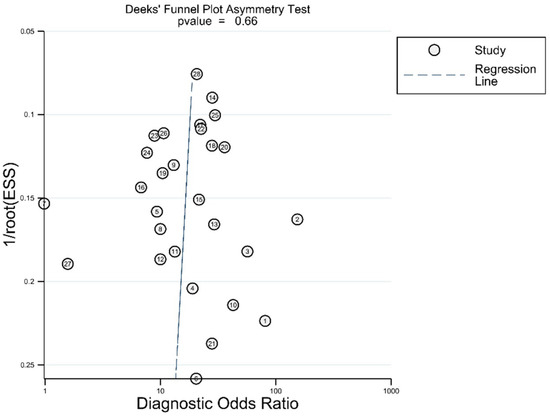
Figure 5.
Asymmetry test for publications bias.
3.6. Quality Assessment
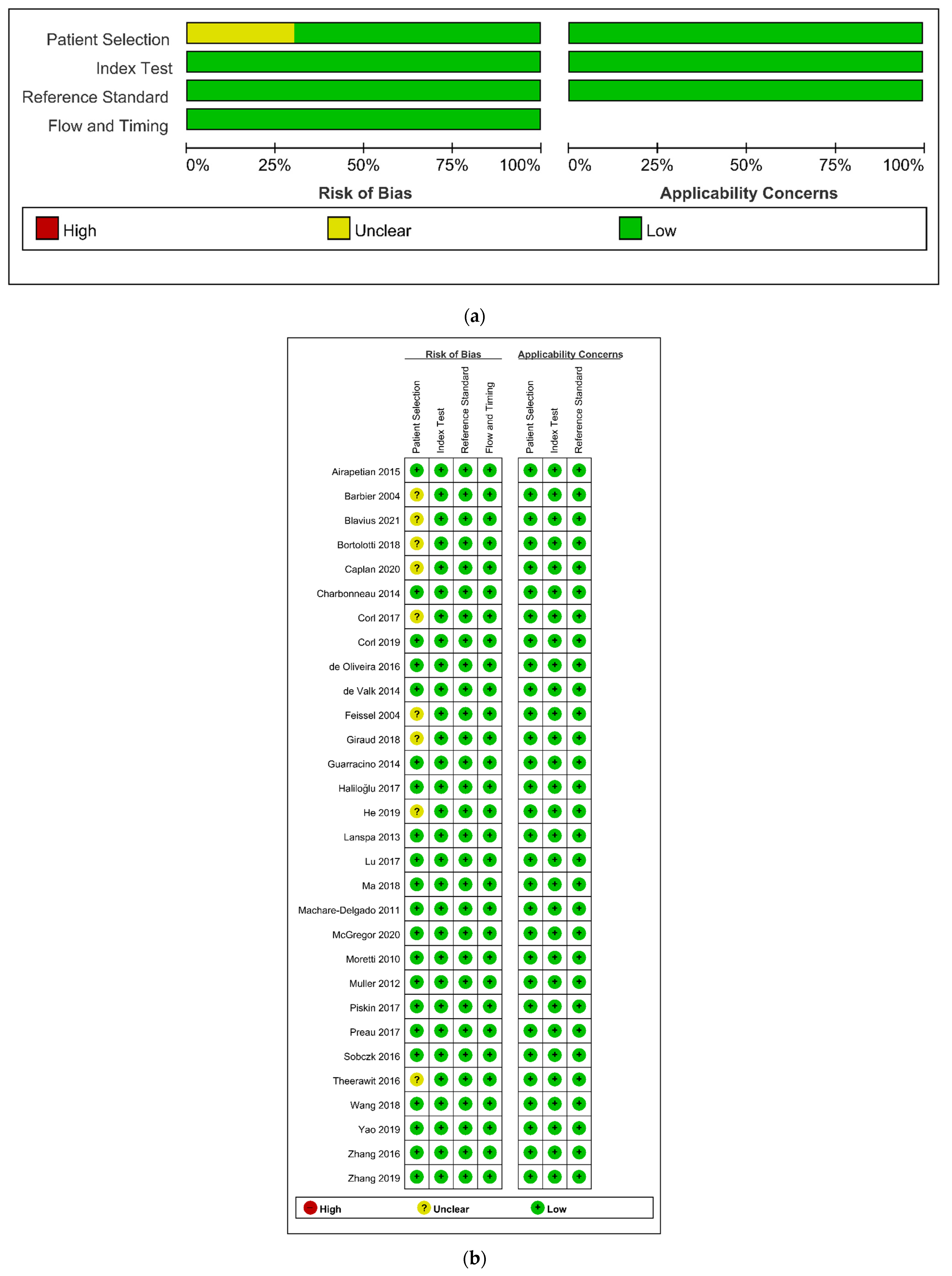
The details of the quality assessment are depicted in Figure 6. In terms of patient selection, the risk of bias was unclear in nine studies (30.0%) [21,22,33,35,40,41,45,48,50]. Consequently, these studies showed no consecutive patient selection or no description of it. In other domains of QUDAS-2 assessment, all studies showed a low risk of bias.
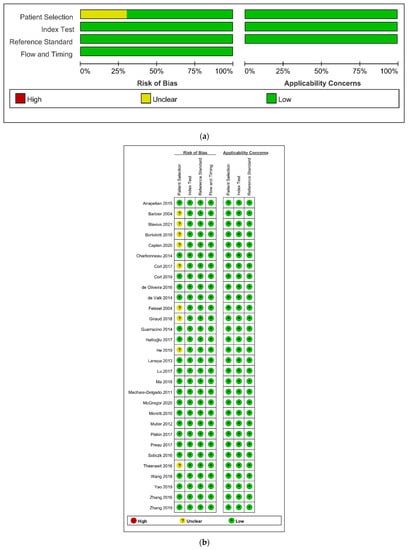
Figure 6.
Risk of bias and applicability concerns graph (a) and summary (b): review authors’ judgements about each domain presented as percentages across included studies.
4. Discussion
Our results suggest that the diagnostic accuracy of ultrasonographic ΔIVC for predicting fluid responsiveness is acceptable. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and AUROC of ΔIVC were 0.75, 0.83, 4.37, 0.30, 14.3, and 0.86, respectively. In the subgroup analysis, there was no difference between patients on MV and those breathing spontaneously. Despite the systematic review, we found only three studies on the IJV and one on the FV. We found no study on the SCV. There was insufficient evidence to support the diametric measurement of these large veins as an alternative to that of the IVC. More prospective studies are warranted, which should consider the threshold of the index test and the heterogeneity of the reference standard.
Recently, several previous systematic reviews and meta-analyses were conducted to investigate the diagnostic accuracy of ΔIVC. Orso et al. [12], in a meta-analysis including 20 studies with ΔIVC, reported that the pooled sensitivity, specificity, and AUROC were 0.71, 0.75, and 0.71, respectively. They included several studies of pediatric patients, whereas we excluded these studies. Si et al. [13], in a meta-analysis including 12 studies comprising only patients on MV, reported a sensitivity, specificity, and AUROC of 0.73, 0.82, and 0.85, respectively. In our subgroup analysis, studies comprising patients on MV showed a sensitivity, specificity, and AUROC of 0.74, 0.85, and 0.87, respectively, whereas studies comprising patients with spontaneous breathing showed similar results, with a sensitivity, specificity, and AUROC of 0.75, 0.81, and 0.85, respectively. Si et al. [13] concluded that ΔIVC was a poor predictor in patients with TV < 8 mL/kg or PEEP > 5 cm H2O through subgroup analysis (k = 6) (sensitivity, specificity, and AUROC of 0.66, 0.68, and 0.70, respectively). However, in our subgroup analysis (k = 5), ΔIVC in this setting showed better results, which were a sensitivity, specificity, and AUROC of 0.73, 0.77, and 0.82, respectively. In our analysis, similar to that of Si et al. [13], the performance of ΔIVC was higher in patients with TV ≥ 8 mL/kg or PEEP ≤ 5 cm H2O (sensitivity, specificity, and AUROC of 0.74, 0.88, and 0.90), but the meta-regression test did not show a significant difference (p = 0.31). Overall, compared with previous meta-analyses [12,13], we updated our interpretation with data from 11 studies that have been published since 2018. However, two studies [51,52] in the previous meta-analyses by Orso et al. [12] and one study [53] in the other meta-analysis by Si et al. [13] did not have 2-by-2 contingency data in our recalculation. Thus, we excluded these three studies. Only one previous meta-analysis investigated the IVC diameter, without a delineation of respiratory variation [54]. They analyzed two case–control and three before-and-after studies. They found a significantly lower diameter of the IVC in hypovolemic status and the mean difference was 6.3 mm (95% CI, 6.0–6.5). However, this effect size is apparently too small to use in clinical practice. Indeed, the inherent size of the IVC may vary in each patient. Similar static index tests, such as central venous pressure, showed no clinical significance in the previous study [55]. Since this study was published, there has been no meta-analysis investigating the IVC diameter alone. In common with ΔIVC, a more dynamic index would be appropriate for evaluating volume status.
Due to the limited number of studies that met our inclusion criteria, we did not conduct the meta-analysis for the IJV, SCV, and FV. We found only three studies that evaluated the IJV [29,36,42]. The specificity, sensitivity, and AUROC of these studies were sufficiently high for predicting fluid responsiveness. The AUROC of the IJV ranged from 0.825 to 0.915. The AUROC of the SCV was also sufficient, with a value of 0.970. Both the IJV and the SCV are located in proximity of the right atrium. Thus, these would be alternative vessels to investigate. However, the FV would be limited due to its distance from the right atrium. One eligible study in our meta-analysis reported a strong correlation between IVC-CI and IJV-CI (r = 0.976, n = 44) [36]. One study that was excluded because there was no fluid challenge, reporting a moderately strong correlation between IVC-CI and SCV-CI (r = 0.781, n = 34) [56]. In the case of the FV, only one study was excluded because it reported only a modest correlation between IVC-CI and FV-CI (r = 0.642, 57) [57]. In future reviews, the IJV and SCV need to be further investigated.
In the eligible studies of our meta-analysis, several conventional reference standards were used after fluid loading to determine the fluid responsiveness. The increase in CO or SV was considered as a response to fluid replacement. Therefore, the accurate measurement of CO or SV is crucial. To measure CO or SV, the most reliable method is the insertion of a Swan–Ganz catheter [4]. This involves an injection of ice-cold water into the right atrium through a pulmonary artery catheter and measurement of CO or SV using the temperature change [58]. It measures SvO2 to reflect accurate, real-time change in hemodynamics [59]. However, it is a difficult technique to perform in practice, especially if indicated often, and has limitations because it is invasive and even more difficult to perform in the presence of arrhythmias, pulmonary infarction, or catheter injury with vascular complications [60]. In our analysis, no study used a Swan–Ganz catheter as a reference standard. Another way to measure CO or SV is to extract the arterial waveform. Since the SV is estimated using the area under the dicrotic notch at the start of the rise of arterial pressure, the SV can be calculated for every heartbeat [59,61]. VigileoTM (Edwards life science, Irvine, CA, USA), MostCare™ (Vytech, Padova, Italy) using PRAM (Pressure Recording Analytical Method), and PiCCO® (Pulsion Medical Systems, Munich, Germany), which uses blood pressure waveforms, were proposed as less invasive methods [60,61]. These involve the insertion of a central venous catheter and a relatively small-sized device, approximately 4–5 Fr, into the artery, and allow the monitoring of continuous values even when the patient is unstable. The arterial waveform analysis method is less invasive than the Swan–Ganz method. However, re-calibration is required every 6–12 h in the case of vascular elasticity, aortic insufficiency, or inaccurate arterial pressure waveforms [60,61]. The method of measuring CO or SV using echocardiography involves measuring the velocity-time integral using the diameter of the left ventricular outlet and Doppler ultrasound [62]. Echocardiography is useful because it can also provide the differential diagnosis of cardiac dysfunction and hypovolemia by measuring chamber size and cardiac function. However, it is not able to detect continuous changes like the Swan–Ganz catheter and should be performed by an expert who has a high level of experience in general [63]. The bioimpedance method can measure the CO or SV only by direct contact [64]. The fluctuation of the volume of the body with pulsatile changes results in electrical impedance, and the variation of the systolic period is measured, allowing the value of the CO or SV to be monitored [65]. However, reliability is limited in some critically ill patients, and appropriate improvements are likely to be necessary in future studies. Evidence for the superiority of one method over another from the above techniques is limited [10]. We assumed that these reference standards have similar diagnostic accuracy.
Our analysis has several limitations. First, all eligible studies were observational. Second, several eligible studies have an unclear risk of bias in terms of patient selection. Third, the threshold of the index test varied and there was considerable heterogeneity. To overcome this issue, we investigated the correlation between sensitivity and specificity to detect the threshold effect. Fourth, the reference standard was heterogeneous. We also conducted a meta-regression test to evaluate the heterogeneity. Fifth, both patients on MV and those breathing spontaneously were included, although the physiology of the two is antonymous. We conducted a meta-regression, which showed no significance. Sixth, we did not find sufficient eligible studies involving the IJV and SCV. We found only three studies on the IJV. We did not conduct the meta-analysis due to statistical instability. We found no study that measured the respiratory variation of the FV diameter. Future studies are needed to investigate and correct the above deficiencies. Seventh, there would exist a “grey zone” to discriminate response to fluid resuscitation even though the ΔIVC is an easy-to-determine quantitative variable. Thus, integrating an additional qualitative sonographic evaluation may be more helpful in future study [66]. Finally, we included only published original articles and those written in English. This would be expected to introduce publication bias; however, this was not noted in our analysis.
5. Conclusions
Our systematic review and meta-analysis suggest that the ultrasonographic measurement of the respiratory variation in the diameter of the IVC has a favorable diagnostic accuracy for predicting fluid responsiveness in critically ill patients. However, we concluded that there is insufficient evidence in the case of the IJV, SCV, and FV diameters to have clinical application.
Author Contributions
Conceptualization, D.-W.K., S.C. and W.-S.K.; methodology, W.-S.K.; software, W.-S.K.; validation, D.-W.K., S.C. and W.-S.K.; formal analysis, W.-S.K..; investigation, D.-W.K., S.C. and W.-S.K.; resources, D.-W.K., S.C. and W.-S.K.; data curation, D.-W.K., S.C. and W.-S.K.; writing—original draft preparation, D.-W.K., S.C., W.-S.K. and J.K.; writing—review and editing, D.-W.K., S.C., W.-S.K. and J.K.; visualization, W.-S.K.; supervision, W.-S.K.; project administration, W.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT; Ministry of Trade, Industry and Energy; Ministry of Health and Welfare; and Ministry of Food and Drug Safety) (KMDF_PR_20200901_0095), Chonnam National University Hospital Biomedical Research Institute (CRI18013-1) and by grant NRF-2021R1I1A3047390 through the National Research Foundation of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ansari, B.M.; Zochios, V.; Falter, F.; Klein, A.A. Physiological controversies and methods used to determine fluid responsiveness: A qualitative systematic review. Anaesthesia 2016, 71, 94–105. [Google Scholar] [CrossRef]
- Bednarczyk, J.M.; Fridfinnson, J.A.; Kumar, A.; Blanchard, L.; Rabbani, R.; Bell, D.; Funk, D.; Turgeon, A.F.; Abou-Setta, A.M.; Zarychanski, R. Incorporating dynamic assessment of fluid responsiveness into goal-directed therapy: A systematic review and meta-analysis. Crit. Care Med. 2017, 45, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Harvey, S.; Harrison, D.A.; Singer, M.; Ashcroft, J.; Jones, C.M.; Elbourne, D.; Brampton, W.; Williams, D.; Young, D.; Rowan, K. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet 2005, 366, 472–477. [Google Scholar] [CrossRef]
- Jacob, R.; Dierberger, B.; Kissling, G. Functional significance of the Frank-Starling mechanism under physiological and pathophysiological conditions. Eur. Heart J. 1992, 13 (Suppl. E), 7–14. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Azim, A.; Zangbar, B.; Bauman, Z.; O’Keeffe, T.; Ibraheem, K.; Kulvatunyou, N.; Tang, A.; Latifi, R.; Rhee, P. Improving mortality in trauma laparotomy through the evolution of damage control resuscitation: Analysis of 1030 consecutive trauma laparotomies. J. Trauma Acute Care Surg. 2017, 82, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.W.; Khan, M.A.; Raja, A.S.; Cohen, M.J.; Como, J.J.; Cotton, B.A.; Dubose, J.J.; Fox, E.E.; Inaba, K.; Rodriguez, C.J.; et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2017, 82, 605–617. [Google Scholar] [CrossRef]
- Kalla, M.; Herring, N.J.S. Physiology of shock and volume resuscitation. Surgery 2013, 31, 545–551. [Google Scholar]
- Chatterjee, K.J.C. The Swan-Ganz catheters: Past, present, and future: A viewpoint. Circulation 2009, 119, 147–152. [Google Scholar] [CrossRef]
- Saugel, B.; Kouz, K.; Scheeren, T.W.L.; Greiwe, G.; Hoppe, P.; Romagnoli, S.; de Backer, D. Cardiac output estimation using pulse wave analysis-physiology, algorithms, and technologies: A narrative review. Br. J. Anaesth. 2021, 126, 67–76. [Google Scholar] [CrossRef]
- Pourmand, A.; Pyle, M.; Yamane, D.; Sumon, K.; Frasure, S.E. The utility of point-of-care ultrasound in the assessment of volume status in acute and critically ill patients. World J. Emerg. Med. 2019, 10, 232–238. [Google Scholar] [CrossRef]
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: A systematic review and meta-analysis. J. Intensive Care Med. 2020, 35, 354–363. [Google Scholar] [CrossRef]
- Si, X.; Xu, H.; Liu, Z.; Wu, J.; Cao, D.; Chen, J.; Chen, M.; Liu, Y.; Guan, X. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness in mechanically ventilated patients? A systematic review and meta-analysis. Anesth. Analg. 2018, 127, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Dwamena, B. A Midas Retouch Regarding Diagnostic Meta-Analysis. In Proceedings of the 2014 Stata Conference, Boston, MA, USA, 31 July–1 August 2014. [Google Scholar]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Barbier, C.; Loubières, Y.; Schmit, C.; Hayon, J.; Ricôme, J.L.; Jardin, F.; Vieillard-Baron, A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004, 30, 1740–1746. [Google Scholar] [CrossRef]
- Feissel, M.; Michard, F.; Faller, J.P.; Teboul, J.L. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004, 30, 1834–1837. [Google Scholar] [CrossRef]
- Moretti, R.; Pizzi, B. Inferior vena cava distensibility as a predictor of fluid responsiveness in patients with subarachnoid hemorrhage. Neurocrit. Care 2010, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Machare-Delgado, E.; Decaro, M.; Marik, P.E. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: A prospective cohort study. J. Intensive Care Med. 2011, 26, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Bobbia, X.; Toumi, M.; Louart, G.; Molinari, N.; Ragonnet, B.; Quintard, H.; Leone, M.; Zoric, L.; Lefrant, J.Y. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit. Care 2012, 16, R188. [Google Scholar] [CrossRef] [PubMed]
- Lanspa, M.J.; Grissom, C.K.; Hirshberg, E.L.; Jones, J.P.; Brown, S.M. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock 2013, 39, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, H.; Riu, B.; Faron, M.; Mari, A.; Kurrek, M.M.; Ruiz, J.; Geeraerts, T.; Fourcade, O.; Genestal, M.; Silva, S. Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Crit. Care 2014, 18, 473. [Google Scholar] [CrossRef]
- De Valk, S.; Olgers, T.J.; Holman, M.; Ismael, F.; Ligtenberg, J.J.; Ter Maaten, J.C. The caval index: An adequate non-invasive ultrasound parameter to predict fluid responsiveness in the emergency department? BMC Anesthesiol. 2014, 14, 114. [Google Scholar] [CrossRef]
- Guarracino, F.; Ferro, B.; Forfori, F.; Bertini, P.; Magliacano, L.; Pinsky, M.R. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit. Care 2014, 18, 647. [Google Scholar] [CrossRef]
- Airapetian, N.; Maizel, J.; Alyamani, O.; Mahjoub, Y.; Lorne, E.; Levrard, M.; Ammenouche, N.; Seydi, A.; Tinturier, F.; Lobjoie, E.; et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit. Care 2015, 19, 400. [Google Scholar] [CrossRef]
- De Oliveira, O.H.; Freitas, F.G.; Ladeira, R.T.; Fischer, C.H.; Bafi, A.T.; Azevedo, L.C.; Machado, F.R. Comparison between respiratory changes in the inferior vena cava diameter and pulse pressure variation to predict fluid responsiveness in postoperative patients. J. Crit. Care 2016, 34, 46–49. [Google Scholar] [CrossRef]
- Sobczyk, D.; Nycz, K.; Andruszkiewicz, P.; Wierzbicki, K.; Stapor, M. Ultrasonographic caval indices do not significantly contribute to predicting fluid responsiveness immediately after coronary artery bypass grafting when compared to passive leg raising. Cardiovasc. Ultrasound 2016, 14, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Theerawit, P.; Morasert, T.; Sutherasan, Y. Inferior vena cava diameter variation compared with pulse pressure variation as predictors of fluid responsiveness in patients with sepsis. J. Crit. Care 2016, 36, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, J.; Zhu, P.; Luan, H.; Wu, Y.; Zhao, Z. Ultrasonographic measurements of the inferior vena cava variation as a predictor of fluid responsiveness in patients undergoing anesthesia for surgery. J. Surg. Res. 2016, 204, 118–122. [Google Scholar] [CrossRef]
- Corl, K.A.; George, N.R.; Romanoff, J.; Levinson, A.T.; Chheng, D.B.; Merchant, R.C.; Levy, M.M.; Napoli, A.M. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J. Crit. Care 2017, 41, 130–137. [Google Scholar] [CrossRef]
- Haliloğlu, M.; Bilgili, B.; Kararmaz, A.; Cinel, İ. The value of internal jugular vein collapsibility index in sepsis. Ulusal travma ve acil cerrahi dergisi. Turk. J. Trauma Emerg. Surg. 2017, 23, 294–300. [Google Scholar] [CrossRef][Green Version]
- Lu, N.; Xi, X.; Jiang, L.; Yang, D.; Yin, K. Exploring the best predictors of fluid responsiveness in patients with septic shock. The Am. J. Emerg. Med. 2017, 35, 1258–1261. [Google Scholar] [CrossRef]
- Pişkin, Ö.; Öz, İ. Accuracy of pleth variability index compared with inferior vena cava diameter to predict fluid responsiveness in mechanically ventilated patients. Medicine 2017, 96, e8889. [Google Scholar] [CrossRef]
- Preau, S.; Bortolotti, P.; Colling, D.; Dewavrin, F.; Colas, V.; Voisin, B.; Onimus, T.; Drumez, E.; Durocher, A.; Redheuil, A.; et al. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit. Care Med. 2017, 45, e290–e297. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, P.; Colling, D.; Colas, V.; Voisin, B.; Dewavrin, F.; Poissy, J.; Girardie, P.; Kyheng, M.; Saulnier, F.; Favory, R.; et al. Respiratory changes of the inferior vena cava diameter predict fluid responsiveness in spontaneously breathing patients with cardiac arrhythmias. Ann. Intensive Care 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Giraud, R.; Abraham, P.S.; Brindel, P.; Siegenthaler, N.; Bendjelid, K. Respiratory changes in subclavian vein diameters predicts fluid responsiveness in intensive care patients: A pilot study. J. Clin. Monit. Comput. 2018, 32, 1049–1055. [Google Scholar] [CrossRef]
- Ma, G.G.; Hao, G.W.; Yang, X.M.; Zhu, D.M.; Liu, L.; Liu, H.; Tu, G.W.; Luo, Z. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann. Intensive Care 2018, 8, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Wu, H.; Wang, R.; Wang, Y.; Du, C. Assessment of fluid responsiveness by inferior vena cava diameter variation in post-pneumonectomy patients. Echocardiography 2018, 35, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Corl, K.A.; Azab, N.; Nayeemuddin, M.; Schick, A.; Lopardo, T.; Zeba, F.; Phillips, G.; Baird, G.; Merchant, R.C.; Levy, M.M.; et al. Performance of a 25% inferior vena cava collapsibility in detecting fluid responsiveness when assessed by novice versus expert physician sonologists. J. Intensive Care Med. 2020, 35, 1520–1528. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Thapa, S.; Li, C.; Luo, J.; Dai, W.; Liu, J. Evaluation of volume responsiveness by pulse pressure variability and inferior vena cava dispensability index at different tidal volumes by mechanical ventilation. Braz. J. Med. Biol. Res./Rev. Bras. Pesqui. Med. Biol. 2019, 52, e8827. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Liu, J.Y.; Sun, Y.B.; Zhao, Y.X.; Li, L.D. The value of the inferior vena cava area distensibility index and its diameter ratio for predicting fluid responsiveness in mechanically ventilated patients. Shock 2019, 52, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Chen, X.; Wang, X.; Liu, D. Respiratory variations of inferior vena cava fail to predict fluid responsiveness in mechanically ventilated patients with isolated left ventricular dysfunction. Ann. Intensive Care 2019, 9, 113. [Google Scholar] [CrossRef]
- Caplan, M.; Durand, A.; Bortolotti, P.; Colling, D.; Goutay, J.; Duburcq, T.; Drumez, E.; Rouze, A.; Nseir, S.; Howsam, M.; et al. Measurement site of inferior vena cava diameter affects the accuracy with which fluid responsiveness can be predicted in spontaneously breathing patients: A post hoc analysis of two prospective cohorts. Ann. Intensive Care 2020, 10, 168. [Google Scholar] [CrossRef]
- McGregor, D.; Sharma, S.; Gupta, S.; Ahmed, S.; Harris, T. Emergency department non-invasive cardiac output study (EDNICO): An accuracy study. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 8. [Google Scholar] [CrossRef]
- Blaivas, M.; Blaivas, L.; Philips, G.; Merchant, R.; Levy, M.; Abbasi, A.; Eickhoff, C.; Shapiro, N.; Corl, K. Development of a deep learning network to classify inferior vena cava collapse to predict fluid responsiveness. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2021, 40, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Vignon, P.; Repessé, X.; Bégot, E.; Léger, J.; Jacob, C.; Bouferrache, K.; Slama, M.; Prat, G.; Vieillard-Baron, A. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1022–1032. [Google Scholar] [CrossRef]
- Brun, C.; Zieleskiewicz, L.; Textoris, J.; Muller, L.; Bellefleur, J.P.; Antonini, F.; Tourret, M.; Ortega, D.; Vellin, A.; Lefrant, J.Y.; et al. Prediction of fluid responsiveness in severe preeclamptic patients with oliguria. Intensive Care Med. 2013, 39, 593–600. [Google Scholar] [CrossRef]
- Baker, A.K.; Partridge, R.J.; Litton, E.; Ho, K.M. Assessment of the plethysmographic variability index as a predictor of fluid responsiveness in critically ill patients: A pilot study. Anaesth. Intensive Care 2013, 41, 736–741. [Google Scholar] [CrossRef]
- Dipti, A.; Soucy, Z.; Surana, A.; Chandra, S. Role of inferior vena cava diameter in assessment of volume status: A meta-analysis. Am. J. Emerg. Med. 2012, 30, 1414–1419. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef]
- Kent, A.; Bahner, D.P.; Boulger, C.T.; Eiferman, D.S.; Adkins, E.J.; Evans, D.C.; Springer, A.N.; Balakrishnan, J.M.; Valiyaveedan, S.; Galwankar, S.C.; et al. Sonographic evaluation of intravascular volume status in the surgical intensive care unit: A prospective comparison of subclavian vein and inferior vena cava collapsibility index. J. Surg. Res. 2013, 184, 561–566. [Google Scholar] [CrossRef]
- Kent, A.; Patil, P.; Davila, V.; Bailey, J.K.; Jones, C.; Evans, D.C.; Boulger, C.T.; Adkins, E.; Balakrishnan, J.M.; Valiyaveedan, S.; et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann. Thorac. Med. 2015, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Teboul, J.L. Transpulmonary thermodilution: Advantages and limits. Crit. Care 2017, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Kobe, J.; Mishra, N.; Arya, V.K.; Al-Moustadi, W.; Nates, W.; Kumar, B. Cardiac output monitoring: Technology and choice. Ann. Card. Anaesth. 2019, 22, 6–17. [Google Scholar] [CrossRef]
- Marqué, S.; Cariou, A.; Chiche, J.D.; Squara, P. Comparison between Flotrac-Vigileo and Bioreactance, a totally noninvasive method for cardiac output monitoring. Crit. Care 2009, 13, R73. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; Morgan, M. The PiCCO monitor: A review. Anaesth. Intensive Care 2012, 40, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, J.; Pazos, A.; Florio, L.; Américo, C.; Lluberas, N.; Parma, G.; Lluberas, R. Cardiac output measured by transthoracic echocardiography and Swan-Ganz catheter. A comparative study in mechanically ventilated patients with high positive end-expiratory pressure. Rev. Bras. Ter. Intensiva 2019, 31, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Mercado, P.; Maizel, J.; Beyls, C.; Titeca-Beauport, D.; Joris, M.; Kontar, L.; Riviere, A.; Bonef, O.; Soupison, T.; Tribouilloy, C.; et al. Transthoracic echocardiography: An accurate and precise method for estimating cardiac output in the critically ill patient. Crit. Care 2017, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Kupersztych-Hagege, E.; Teboul, J.L.; Artigas, A.; Talbot, A.; Sabatier, C.; Richard, C.; Monnet, X. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br. J. Anaesth. 2013, 111, 961–966. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.H.; Chang, B.C.; Shim, J.K. Efficacy of goal-directed therapy using bioreactance cardiac output monitoring after valvular heart surgery. Yonsei Med. J. 2015, 56, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Trauzeddel, R.F.; Ertmer, M.; Nordine, M.; Groesdonk, H.V.; Michels, G.; Pfister, R.; Reuter, D.; Scheeren, T.W.L.; Berger, C.; Treskatsch, S. Perioperative echocardiography-guided hemodynamic therapy in high-risk patients: A practical expert approach of hemodynamically focused echocardiography. J. Clin. Monit. Comput. 2021, 35, 229–243. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).