Mycoplasma pneumoniae and Chlamydia pneumoniae Coinfection with Acute Respiratory Distress Syndrome: A Case Report
Abstract
1. Introduction
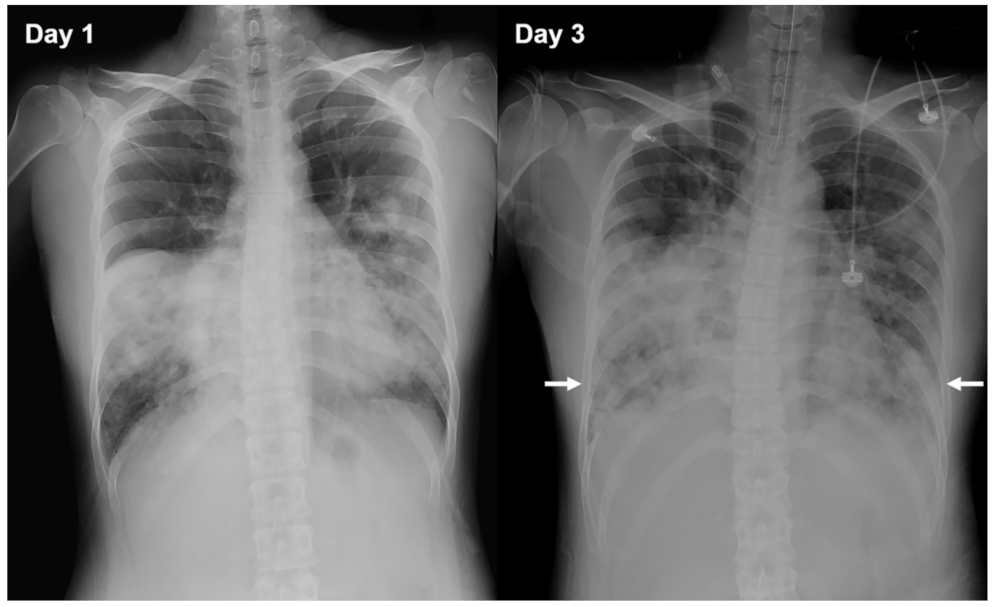
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.T.; Ewig, S.; Rodloff, A.C.; Müller, E.E. Acute respiratory distress syndrome and pneumonia: A comprehensive review of clinical data. Clin. Infect. Dis. 2006, 43, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Fukano, H.; Okimoto, N.; Hara, H.; Yoshida, K.; Niki, Y.; Matsushima, T. Clinical presentation of community-acquired Chlamydia pneumoniae pneumonia in adults. Chest 2002, 121, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Niki, Y.; Nakajima, M.; Fukano, H.; Matsushima, T. Prevalence of asymptomatic infection with Chlamydia pneumoniae in subjectively healthy adults. Chest 2001, 119, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliţ, L.E.; Simu, I.; Săsăran, M.O. The Association Between Mycoplasma pneumoniae and Chlamydia pneumoniae, a Life-Threatening Condition in Small Children-A Case Report and a Review of the Literature. Front. Pediatr. 2020, 8, 558941. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Fukuda, Y.; Wakabayashi, S.; Kono, M.; Ono, S.; Kato, A.; Kondo, E.; Nakamura, Y.; Tanaka, Y.; Teranishi, H.; et al. Low prevalence of Chlamydia pneumoniae infections during the Mycoplasma pneumoniae epidemic season: Results of nationwide surveillance in Japan. J. Infect. Chemother. 2020, 26, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Lochindarat, S.; Suwanjutha, S.; Prapphal, N.; Chantarojanasiri, T.; Bunnag, T.; Deerojanawong, J.; Kunakorn, M.; Srisan, P. Mycoplasma pneumoniae and Chlamydophila pneumoniae in children with community-acquired pneumonia in Thailand. Int. J. Tuberc. Lung Dis. 2007, 11, 814–819. [Google Scholar]
- Izumikawa, K.; Izumikawa, K.; Takazono, T.; Kosai, K.; Morinaga, Y.; Nakamura, S.; Kurihara, S.; Imamura, Y.; Miyazaki, T.; Tsukamoto, M.; et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: A review of the Japanese literature. J. Infect. Chemother. 2014, 20, 181–185. [Google Scholar] [CrossRef]
- Liu, K.T.; Yang, K.Y.; Lee, Y.C.; Perng, R.P. Risk factor analysis of acute respiratory distress syndrome among hospitalized patients with Chlamydophila pneumoniae pneumonia. J. Chin. Med. Assoc. 2007, 70, 318–323. [Google Scholar] [CrossRef]
- Reilly, J.P.; Christie, J.D.; Meyer, N.J. Fifty years of research in ARDS. Genomic contributions and opportunities. Am. J. Respir. Crit. Care Med. 2017, 196, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.P.; Christie, J.D. Linking genetics to ARDS pathogenesis: The role of the platelet. Chest 2015, 147, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Blumhagen, R.Z.; Hedin, B.R.; Malcolm, K.C.; Burnham, E.L.; Moss, M.; Abraham, E.; Huie, T.J.; Nick, J.A.; Fingerlin, T.E.; Alper, S. Alternative pre-mRNA splicing of Toll-like receptor signaling components in peripheral blood mononuclear cells from patients with ARDS. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L930–L939. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, N.; Koseki, N.; Kaiho, M.; Ariga, T.; Kikuta, H.; Togashi, T.; Oba, K.; Morita, K.; Nagano, N.; Nakanishi, M.; et al. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS ONE 2017, 12, e0173635. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Pereyre, S.; Goret, J.; Bébéar, C. Mycoplasma pneumoniae: Current knowledge on macrolide resistance and treatment. Front. Microbiol. 2016, 7, 974. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, M.; Ye, Z.; Tan, T.; Liu, X.; You, X.; Zeng, Y.; Wu, Y. Insights into the pathogenesis of Mycoplasma pneumoniae. Mol. Med. Rep. 2016, 14, 4030–4036. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Obase, Y.; Ouchi, K.; Kawasaki, K.; Kawai, Y.; Kobashi, Y.; Oka, M. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J. Med. Microbiol. 2007, 56, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Thurman, K.A.; Walter, N.D.; Schwartz, S.B.; Mitchell, S.L.; Dillon, M.T.; Baughman, A.L.; Deutscher, M.; Fulton, J.P.; Tongren, J.E.; Hicks, L.A.; et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin. Infect. Dis. 2009, 48, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Hvidsten, D.; Halvorsen, D.S.; Berdal, B.P.; Gutteberg, T.J. Chlamydophila pneumoniae diagnostics: Importance of methodology in relation to timing of sampling. Clin. Microbiol. Infect. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-K.; Lai, C.-H.; Tsai, C.; Chen, G.-L. Mycoplasma pneumoniae and Chlamydia pneumoniae Coinfection with Acute Respiratory Distress Syndrome: A Case Report. Diagnostics 2022, 12, 48. https://doi.org/10.3390/diagnostics12010048
Tsai M-K, Lai C-H, Tsai C, Chen G-L. Mycoplasma pneumoniae and Chlamydia pneumoniae Coinfection with Acute Respiratory Distress Syndrome: A Case Report. Diagnostics. 2022; 12(1):48. https://doi.org/10.3390/diagnostics12010048
Chicago/Turabian StyleTsai, Meng-Ko, Chao-Hung Lai, Chris Tsai, and Guan-Liang Chen. 2022. "Mycoplasma pneumoniae and Chlamydia pneumoniae Coinfection with Acute Respiratory Distress Syndrome: A Case Report" Diagnostics 12, no. 1: 48. https://doi.org/10.3390/diagnostics12010048
APA StyleTsai, M.-K., Lai, C.-H., Tsai, C., & Chen, G.-L. (2022). Mycoplasma pneumoniae and Chlamydia pneumoniae Coinfection with Acute Respiratory Distress Syndrome: A Case Report. Diagnostics, 12(1), 48. https://doi.org/10.3390/diagnostics12010048




