Intracranial Flow Velocity Quantification Using Non-Contrast Four-Dimensional Flow MRI: A Prospective Comparative Study with Transcranial Doppler Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Magnetic Resonance Imaging
2.3. Transcranial Ultrasound Examination
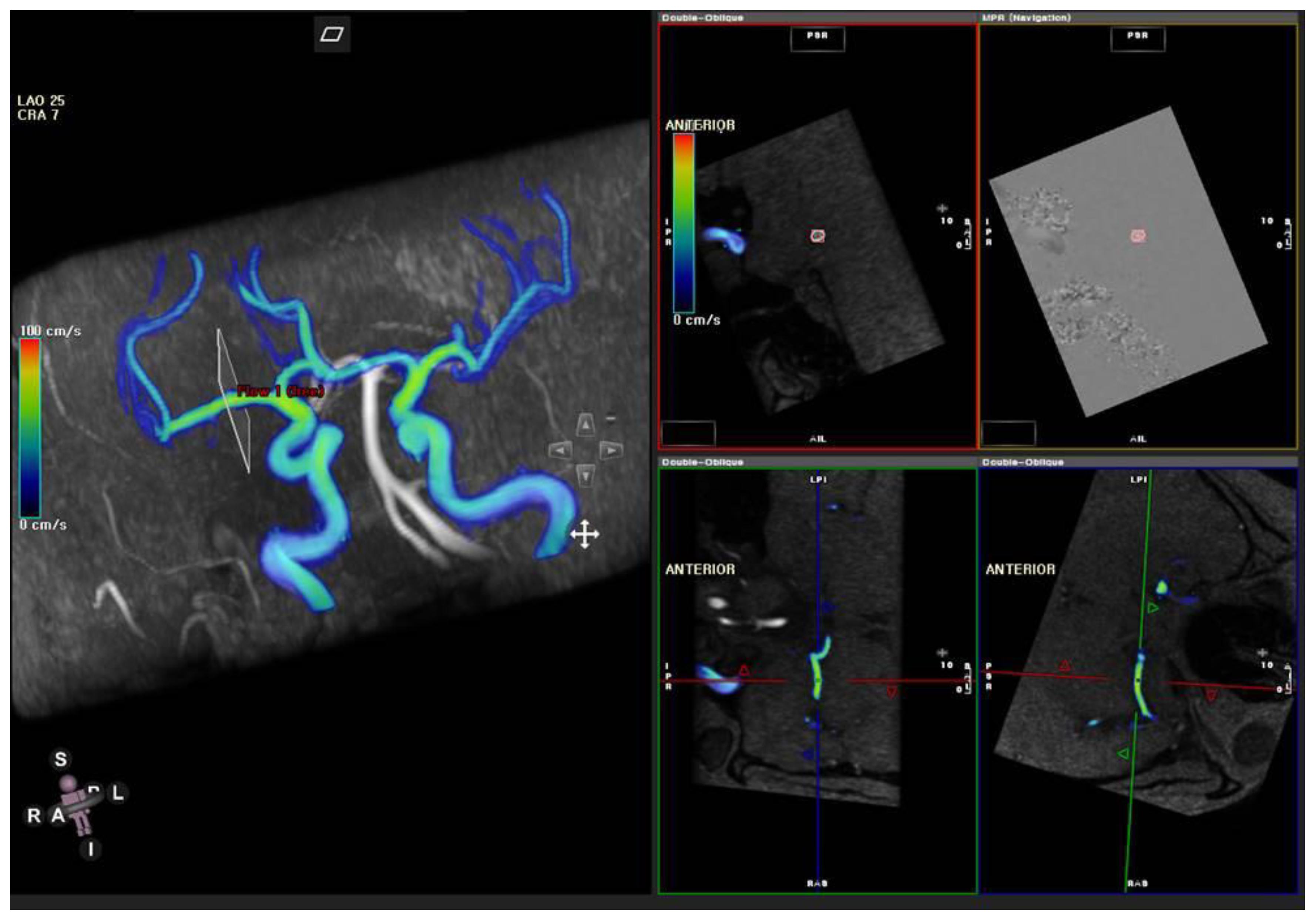
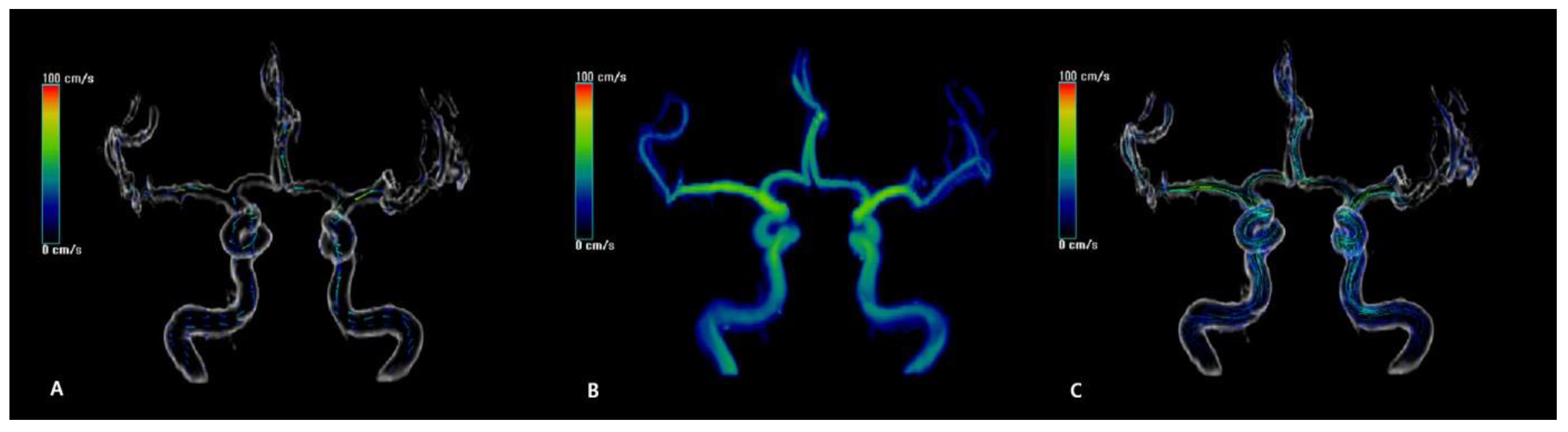
2.4. MR Flow Velocity Analysis
2.5. Statistical Analysis
3. Results
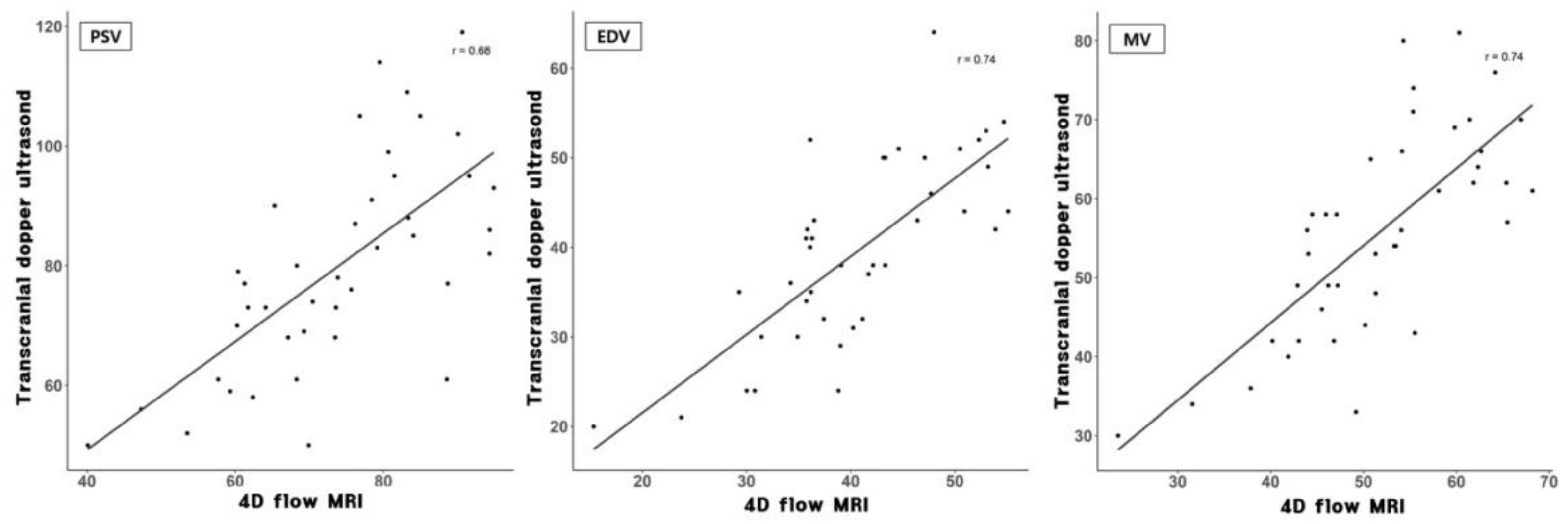
3.1. Flow Velocity Comparison between TCD and 4D Flow MRI
3.2. Differences of Bilateral MCA Flow Velocities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandrov, A.V.; Bladin, C.F.; Norris, J.W. Intracranial blood flow velocities in acute ischemic stroke. Stroke 1994, 25, 1378–1383. [Google Scholar] [CrossRef] [Green Version]
- Newell, D.W.; Winn, H.R. Transcranial Doppler in cerebral vasospasm. Neurosurg. Clin. N. Am. 1990, 1, 319–328. [Google Scholar] [CrossRef]
- Lee, M.; Zaharchuk, G.; Guzman, R.; Achrol, A.; Bell-Stephens, T.; Steinberg, G.K. Quantitative hemodynamic studies in moyamoya disease: A review. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef]
- Rivera-Rivera, L.A.; Turski, P.; Johnson, K.M.; Hoffman, C.; Berman, S.E.; Kilgas, P.; Rowley, H.A.; Carlsson, C.M.; Johnson, S.C.; Wieben, O. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Jung, C.; Kim, J.H.; Choi, B.S.; Kim, E. Quantitative Magnetic Resonance Angiography in Internal Carotid Artery Occlusion with Primary Collateral Pathway. J. Stroke 2015, 17, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Conte, M.; Scarafile, R.; Riegler, L.; Cocchia, R.; Pezzullo, E.; Cavallaro, M.; Carbone, A.; Natale, F.; Russo, M.G.; et al. Transcranial Doppler Ultrasound: Physical Principles and Principal Applications in Neurocritical Care Unit. J. Cardiovasc. Echogr. 2016, 26, 28–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meckel, S.; Leitner, L.; Bonati, L.H.; Santini, F.; Schubert, T.; Stalder, A.F.; Lyrer, P.; Markl, M.; Wetzel, S.G. Intracranial artery velocity measurement using 4D PC MRI at 3 T: Comparison with transcranial ultrasound techniques and 2D PC MRI. Neuroradiology 2013, 55, 389–398. [Google Scholar] [CrossRef]
- Topcuoglu, M.A. Transcranial Doppler ultrasound in neurovascular diseases: Diagnostic and therapeutic aspects. J. Neurochem. 2012, 123, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spilt, A.; Box, F.M.; van der Geest, R.J.; Reiber, J.H.; Kunz, P.; Kamper, A.M.; Blauw, G.J.; van Buchem, M.A. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J. Magn. Reson. Imaging 2002, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Srichai, M.B.; Lim, R.P.; Wong, S.; Lee, V.S. Cardiovascular applications of phase-contrast MRI. Am. J. Roentgenol. 2009, 192, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Markl, M.; Frydrychowicz, A.; Kozerke, S.; Hope, M.; Wieben, O. 4D flow MRI. J. Magn. Reson. Imaging 2012, 36, 101536. [Google Scholar] [CrossRef]
- Ha, H.; Kim, G.B.; Kweon, J.; Lee, S.J.; Kim, Y.H.; Lee, D.H.; Yang, D.H.; Kim, N. Hemodynamic Measurement Using Four-Dimensional Phase-Contrast MRI: Quantification of Hemodynamic Parameters and Clinical Applications. Korean J. Radiol. 2016, 17, 445–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarine, A.; Garcon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. Radiographics 2019, 39, 632–648. [Google Scholar] [CrossRef] [Green Version]
- Gottwald, L.M.; Toger, J.; Markenroth Bloch, K.; Peper, E.S.; Coolen, B.F.; Strijkers, G.J.; van Ooij, P.; Nederveen, A.J. High Spatiotemporal Resolution 4D Flow MRI of Intracranial Aneurysms at 7T in 10 Minutes. Am. J. Neuroradiol. 2020, 41, 1201–1208. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhall, C.J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.A.; Schnell, S.; Carroll, T.; Vakil, P.; Hurley, M.C.; Wu, C.; Carr, J.; Bendok, B.R.; Batjer, H.; Markl, M. Intracranial 4D flow MRI: Toward individualized assessment of arteriovenous malformation hemodynamics and treatment-induced changes. Am. J. Neuroradiol. 2013, 34, 1922–1928. [Google Scholar] [CrossRef] [Green Version]
- Meckel, S.; Stalder, A.F.; Santini, F.; Radu, E.W.; Rufenacht, D.A.; Markl, M.; Wetzel, S.G. In vivo visualization and analysis of 3-D hemodynamics in cerebral aneurysms with flow-sensitized 4-D MR imaging at 3 T. Neuroradiology 2008, 50, 47384. [Google Scholar] [CrossRef] [PubMed]
- Medero, R.; Ruedinger, K.; Rutkowski, D.; Johnson, K.; Roldan-Alzate, A. In Vitro Assessment of Flow Variability in an Intracranial Aneurysm Model Using 4D Flow MRI and Tomographic PIV. Ann. Biomed. Eng. 2020, 48, 248493. [Google Scholar] [CrossRef]
- Bock, J.; Frydrychowicz, A.; Stalder, A.F.; Bley, T.A.; Burkhardt, H.; Hennig, J.; Markl, M. 4D phase contrast MRI at 3 T: Effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn. Reson. Med. 2010, 63, 330–338. [Google Scholar] [CrossRef]
- Futami, K.; Uno, T.; Misaki, K.; Tamai, S.; Nambu, I.; Uchiyama, N.; Nakada, M. Identification of Vortex Cores in Cerebral Aneurysms on 4D Flow MRI. Am. J. Neuroradiol. 2019, 40, 2111–2116. [Google Scholar] [CrossRef]
- Li, C.Q.; Hsiao, A.; Hattangadi-Gluth, J.; Handwerker, J.; Farid, N. Early Hemodynamic Response Assessment of Stereotactic Radiosurgery for a Cerebral Arteriovenous Malformation Using 4D Flow MRI. Am. J. Neuroradiol. 2018, 39, 67881. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Landgraf, B.; Johnson, K.M.; Kecskemeti, S.; Wu, Y.; Velikina, J.; Rowley, H.; Wieben, O.; Mistretta, C.; Turski, P. Velocity measurements in the middle cerebral arteries of healthy volunteers using 3D radial phase-contrast HYPRFlow: Comparison with transcranial Doppler sonography and 2D phase-contrast MR imaging. Am. J. Neuroradiol. 2011, 32, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Lagerstrand, K.M.; Vikhoff-Baaz, B.; Starck, G.; Forssell-Aronsson, E. Contrast agent influences MRI phase-contrast flow measurements in small vessels. Magn. Reson. Med. 2010, 64, 42–46. [Google Scholar] [CrossRef]
- Oktar, S.O.; Yucel, C.; Karaosmanoglu, D.; Akkan, K.; Ozdemir, H.; Tokgoz, N.; Tali, T. Blood-flow volume quantification in internal carotid and vertebral arteries: Comparison of 3 different ultrasound techniques with phase-contrast MR imaging. Am. J. Neuroradiol. 2006, 27, 363–369. [Google Scholar] [PubMed]
- Seitz, J.; Strotzer, M.; Wild, T.; Nitz, W.R.; Volk, M.; Lenhart, M.; Feuerbach, S. Quantification of blood flow in the carotid arteries: Comparison of Doppler ultrasound and three different phase-contrast magnetic resonance imaging sequences. Investig. Radiol. 2001, 36, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; van der Riet, W.; Globits, S.; Crelier, G.; Salomonowitz, E. Accelerated phase-contrast MR imaging: Comparison of k-t BLAST with SENSE and Doppler ultrasound for velocity and flow measurements in the aorta. J. Magn. Reson. Imaging 2009, 29, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Heverhagen, J.T.; Hoppe, M.; Klose, K.J.; Wagner, H.J. Does the application of gadolinium-DTPA have an impact on magnetic resonance phase contrast velocity measurements? Results from an in vitro study. Eur. J. Radiol. 2002, 44, 65–69. [Google Scholar] [CrossRef]
- Bass, J.C.; Prince, M.R.; Londy, F.J.; Chenevert, T.L. Effect of gadolinium on phase-contrast MR angiography of the renal arteries. AJR Am. J. Roentgenol. 1997, 168, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Lucke-Wold, B.; Hosaka, K.; Dodd, W.; Motwani, K.; Laurent, D.; Martinez, M.; Hoh, B. Interleukin-6: Important mediator of vasospasm following subarachnoid hemorrhage. Curr. Neurovasc. Res. 2021. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef]
- Shankar, J.J.; Tan, I.Y.; Krings, T.; Terbrugge, K.; Agid, R. CT angiography for evaluation of cerebral vasospasm following acute subarachnoid haemorrhage. Neuroradiology 2012, 54, 197–203. [Google Scholar] [CrossRef]
- Wen, B.; Tian, S.; Cheng, J.; Li, Y.; Zhang, H.; Xue, K.; Zhang, Z.; Fan, Y.; Wu, B. Test-retest multisite reproducibility of neurovascular 4D flow MRI. J. Magn. Reson. Imaging 2019, 49, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Takagi, R.; Amano, Y.; Murai, Y.; Orita, E.; Matsumura, Y.; Kumita, S.-I. 4D flow MRI assessment of extracranial-intracranial bypass: Qualitative and quantitative evaluation of the hemodynamics. Neuroradiology 2016, 58, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.G.; Thrippleton, M.J.; Wardlaw, J.M.; Marshall, I. 4D flow MRI for non-invasive measurement of blood flow in the brain: A systematic review. J. Cereb. Blood Flow Metab. 2021, 41, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Krejza, J.; Mariak, Z.; Babikian, V.L. Importance of angle correction in the measurement of blood flow velocity with transcranial Doppler sonography. Am. J. Neuroradiol. 2001, 22, 1743–1747. [Google Scholar]
- Schnell, S.; Wu, C.; Ansari, S.A. Four-dimensional MRI flow examinations in cerebral and extracerebral vessels—Ready for clinical routine? Curr. Opin. Neurol. 2016, 29, 419–428. [Google Scholar] [CrossRef] [Green Version]

| TCD (cm/s) | 4D Flow MRI (cm/s) | Correlation | |||
|---|---|---|---|---|---|
| Right | Distal M1 | PSV | 66.22 | 57.29 | 0.49 * |
| EDV | 33.22 | 32.69 | 0.53 * | ||
| MV | 45.45 | 41.22 | 0.57 * | ||
| Mid M1 | PSV | 76.27 | 70.06 | 0.68 * | |
| EDV | 38.95 | 38.27 | 0.73 * | ||
| MV | 54.40 | 49.53 | 0.74 * | ||
| Proximal M1 | PSV | 77.36 | 73.10 | 0.69 * | |
| EDV | 37.59 | 40.98 | 0.73 * | ||
| MV | 53.59 | 51.69 | 0.72 * | ||
| Left | Distal M1 | PSV | 67.35 | 61.54 | 0.39 |
| EDV | 32.85 | 33.05 | 0.48 * | ||
| MV | 46.75 | 42.55 | 0.44 * | ||
| Mid M1 | PSV | 80.40 | 72.39 | 0.61 * | |
| EDV | 40.60 | 40.15 | 0.60 * | ||
| MV | 56.20 | 50.89 | 0.58 * | ||
| Proximal M1 | PSV | 82.57 | 74.54 | 0.67 * | |
| EDV | 41.68 | 40.12 | 0.81 * | ||
| MV | 58.05 | 51.59 | 0.78 * | ||
| Basilar artery | PSV | 56.18 | 57.23 | 0.17 | |
| EDV | 26.86 | 32.68 | 0.57 * | ||
| MV | 38.90 | 40.86 | 0.41 | ||
| TCD (cm/s) | 4D Flow MRI (cm/s) | p-Value | ||
|---|---|---|---|---|
| Distal | ΔPSV | 11.21 | 11.86 | 0.890 |
| ΔEDV | 5.84 | 5.87 | 0.981 | |
| ΔMV | 7.68 | 7.69 | 0.994 | |
| Mid | ΔPSV | 10.05 | 12.09 | 0.470 |
| ΔEDV | 5.05 | 4.04 | 0.435 | |
| ΔMV | 7.21 | 6.46 | 0.654 | |
| Proximal | ΔPSV | 10.94 | 7.69 | 0.124 |
| ΔEDV | 7.21 | 2.96 | 0.003 | |
| ΔMV | 8.89 | 4.05 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.-Y.; Kang, Y.; Lee, H.-J.; Hwang, M.; Baik, J.; Park, S. Intracranial Flow Velocity Quantification Using Non-Contrast Four-Dimensional Flow MRI: A Prospective Comparative Study with Transcranial Doppler Ultrasound. Diagnostics 2022, 12, 23. https://doi.org/10.3390/diagnostics12010023
Ha S-Y, Kang Y, Lee H-J, Hwang M, Baik J, Park S. Intracranial Flow Velocity Quantification Using Non-Contrast Four-Dimensional Flow MRI: A Prospective Comparative Study with Transcranial Doppler Ultrasound. Diagnostics. 2022; 12(1):23. https://doi.org/10.3390/diagnostics12010023
Chicago/Turabian StyleHa, Sam-Yeol, Yeonah Kang, Ho-Joon Lee, Moonjung Hwang, Jiyeon Baik, and Seongho Park. 2022. "Intracranial Flow Velocity Quantification Using Non-Contrast Four-Dimensional Flow MRI: A Prospective Comparative Study with Transcranial Doppler Ultrasound" Diagnostics 12, no. 1: 23. https://doi.org/10.3390/diagnostics12010023
APA StyleHa, S.-Y., Kang, Y., Lee, H.-J., Hwang, M., Baik, J., & Park, S. (2022). Intracranial Flow Velocity Quantification Using Non-Contrast Four-Dimensional Flow MRI: A Prospective Comparative Study with Transcranial Doppler Ultrasound. Diagnostics, 12(1), 23. https://doi.org/10.3390/diagnostics12010023






