Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT
Abstract
:1. Introduction
2. Materials and Methods
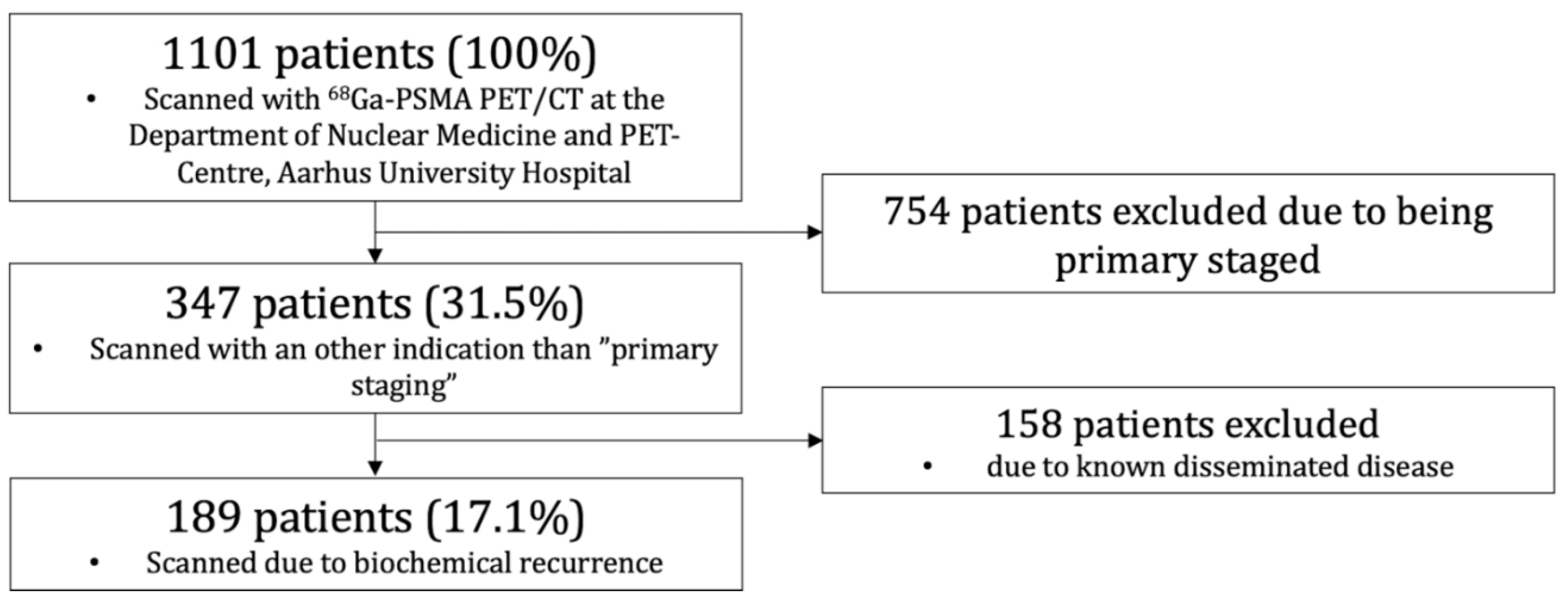
2.1. Patient Population
2.2. 68Ga-PSMA PET/CT
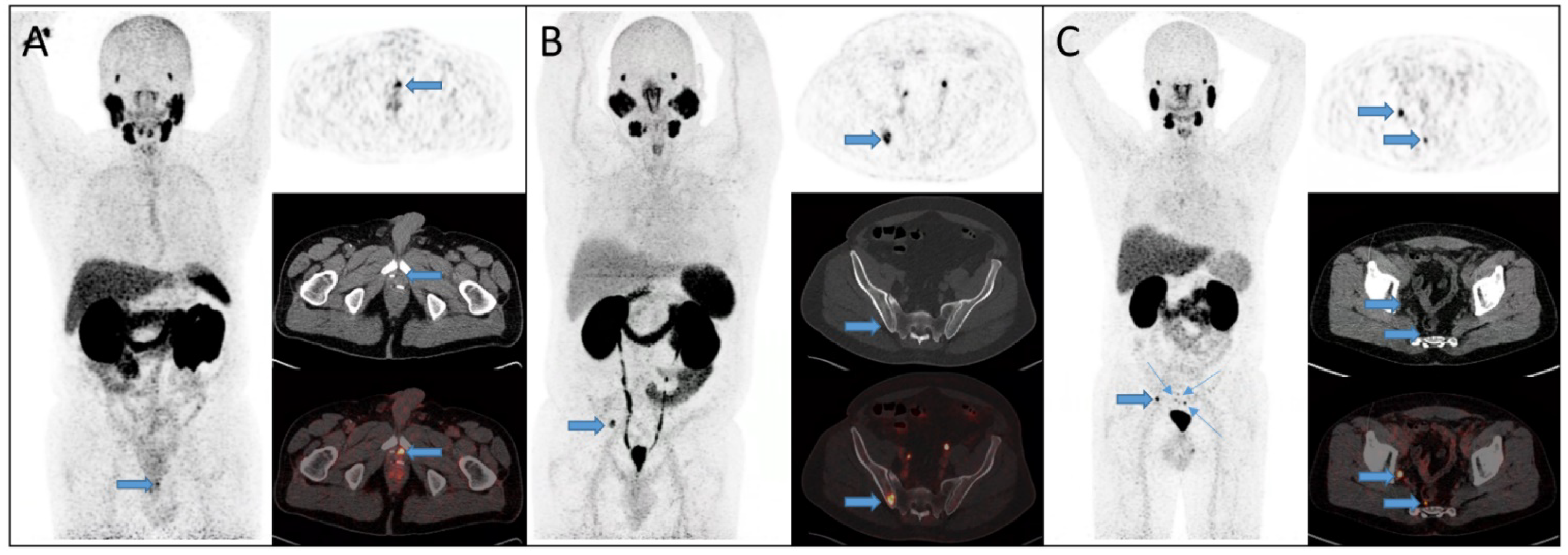
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Summary
3.2. 68Ga-PSMA PET/CT Detection Rate and Tumor Characteristics during Primary Staging
3.3. 68Ga-PSMA PET/CT Detection Rate in Patients Treated with RP
3.4. Primary Staged Using 68Ga-PSMA PET/CT vs. Other
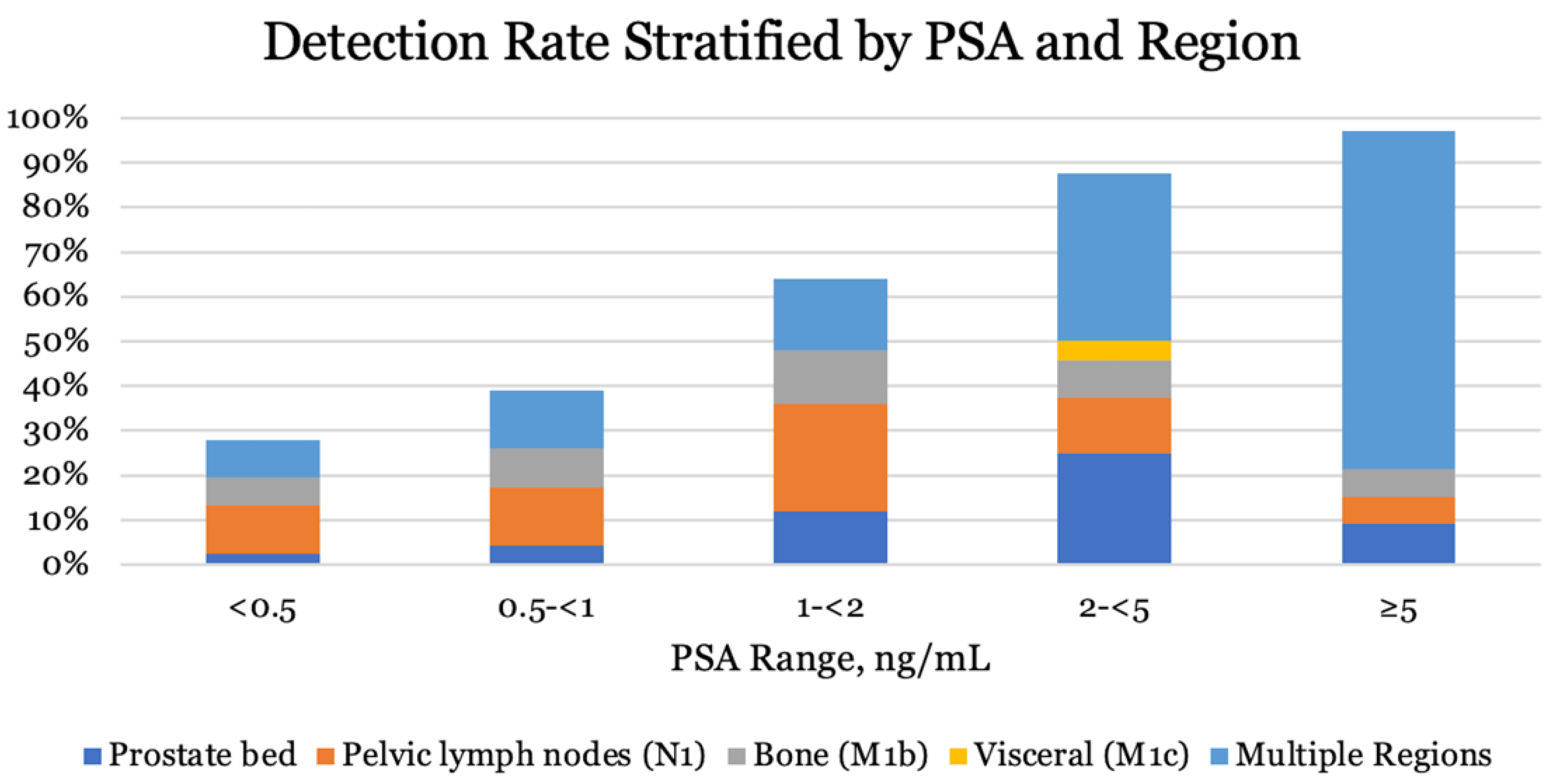
3.5. PSA-Level at Recurrent Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; de Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. Prostate Cancer. 2020. Available online: https://uroweb.org/guideline/prostate-cancer/#6_3 (accessed on 16 November 2021).
- Lütje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef] [Green Version]
- De Visschere, P.J.L.; Standaert, C.; Fütterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; Maurer, T.; Hadaschik, B.A.; Lecouvet, F.E.; Giannarini, G.; et al. A Systematic Review on the Role of Imaging in Early Recurrent Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef]
- Zhou, J.; Gou, Z.; Wu, R.; Yuan, Y.; Yu, G.; Zhao, Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skelet. Radiol. 2019, 48, 1915–1924. [Google Scholar] [CrossRef]
- Janssen, J.C.; Meißner, S.; Woythal, N.; Prasad, V.; Brenner, W.; Diederichs, G.; Hamm, B.; Makowski, M.R. Comparison of hybrid (68)Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. Eur. Radiol. 2018, 28, 610–619. [Google Scholar] [CrossRef]
- Schwenck, J.; Rempp, H.; Reischl, G.; Kruck, S.; Stenzl, A.; Nikolaou, K.; Pfannenberg, C.; la Fougere, C. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [PubMed]
- Beresford, M.J.; Gillatt, D.; Benson, R.J.; Ajithkumar, T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of (68)Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.A.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Hope, T.A.; Eiber, M.; Armstrong, W.R.; Juarez, R.; Murthy, V.; Lawhn-Heath, C.; Behr, S.C.; Zhang, L.; Barbato, F.; Ceci, F.; et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021, 7, 1635–1642. [Google Scholar] [CrossRef]
- Mei, R.; Farolfi, A.; Castellucci, P.; Nanni, C.; Zanoni, L.; Fanti, S. PET/CT Variants and Pitfalls in Prostate Cancer: What You Might See on PET and Should Never Forget. Semin. Nucl. Med. 2021, 51, 621–632. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Franzese, E.; Falco, S.; Laterza, M.M.; Montella, L.; Facchini, S.; Liguori, C.; Coppola, P.; Diessa, Y.; Berretta, M.; Pisconti, S.; et al. The use of (68)Ga prostate-specific membrane antigen PET-CT in prostate cancer: Diagnostic challenges and therapeutic opportunities. Future Sci. OA 2021, 7, Fso705. [Google Scholar] [CrossRef]
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.; Kaufman, R.P.; Kaur, P., Jr.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V.S. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar]
- Klingenberg, S.; Jochumsen, M.R.; Ulhøi, B.P.; Fredsøe, J.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. (68)Ga-PSMA PET/CT for primary NM staging of high-risk prostate cancer. J. Nucl. Med. 2020, 62, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Sörensen, J.; Tolbod, L.P.; Pedersen, B.G.; Frøkiær, J.; Borre, M.; Bouchelouche, K. Potential synergy between PSMA uptake and tumour blood flow for prediction of human prostate cancer aggressiveness. EJNMMI Res. 2021, 11, 12. [Google Scholar] [CrossRef]
- Vinsensia, M.; Chyoke, P.L.; Hadaschik, B.; Holland-Letz, T.; Moltz, J.; Kopka, K.; Rauscher, I.; Mier, W.; Schwaiger, M.; Haberkorn, U.; et al. (68)Ga-PSMA PET/CT and Volumetric Morphology of PET-Positive Lymph Nodes Stratified by Tumor Differentiation of Prostate Cancer. J. Nucl. Med. 2017, 58, 1949–1955. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauscher, I.; Düwel, C.; Haller, B.; Rischpler, C.; Heck, M.M.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. Efficacy, Predictive Factors, and Prediction Nomograms for (68)Ga-labeled Prostate-Specific Membrane Antigen-Ligand Positron-Emission Tomography/Computed Tomography in Early Biochemical Recurrent Prostate Cancer After Radical Prostatectomy. Eur. Urol. 2018, 73, 656–661. [Google Scholar] [CrossRef]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid ⁶⁸Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einspieler, I.; Rauscher, I.; Düwel, C.; Krönke, M.; Rischpler, C.; Habl, G.; Dewes, S.; Ott, A.; Wester, H.-J.; Schwaiger, M.; et al. Detection Efficacy of Hybrid (68)Ga-PSMA Ligand PET/CT in Prostate Cancer Patients with Biochemical Recurrence after Primary Radiation Therapy Defined by Phoenix Criteria. J. Nucl. Med. 2017, 58, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | All Patients, n = 189 | Positive Scans, n = 103 | Negative Scans, n = 86 |
|---|---|---|---|
| Age at scan, median (range), y | 69.2 (50.9–80.5) | 68.1 (52.3–80.5) | 69.7 (50.9–79.2) |
| PSA before treatment, median (range), ng/mL | 10.5 (0.9–183) | 10.8 (3.2–183) | 10.3 (0.9–138) |
| Unavailable (n) | 5 | 3 | 2 |
| Initial therapy (n) | |||
| RP | 153 | 68 | 85 |
| RT | 36 | 35 | 1 |
| Characteristic | All Patients, n = 189 | PET Positive Results, n, (%) | χ2 |
|---|---|---|---|
| PSA before treatment, ng/mL | p = 0.948 | ||
| <10 | 86 | 46 (53.5) | |
| 10–20 | 57 | 32 (56.1) | |
| >20 | 41 | 22 (53.7) | |
| Unavailable (n) | 5 | - | |
| cT-stage, prior treatment | p = 0.667 | ||
| <cT2a | 70 | 35 (50) | |
| cT2b | 10 | 6 (60) | |
| >cT2c | 38 | 22 (57.9) | |
| Unknown | 71 | - | |
| ISUP Grade Group, prior treatment | p = 0.832 | ||
| 1 | 30 | 18 (60) | |
| 2 | 58 | 30 (51.7) | |
| 3 | 31 | 17 (54.8) | |
| 4 | 45 | 26 (57.8) | |
| 5 | 20 | 9 (45) | |
| Unknown | 5 | - | |
| Risk stratification (D’Amico) | p = 0.629 | ||
| Low | 18 | 9 (50) | |
| Intermediate | 70 | 41 (58.6) | |
| High | 95 | 49 (51.6) | |
| Unknown | 6 | - |
| All RP Patients, n = 153 | PET-Positive Results, n, (%) | χ2 | |
|---|---|---|---|
| ISUP Grade Group, prostatectomy | p = 0.504 | ||
| 1 | 10 | 7 (70) | |
| 2 | 55 | 22 (40) | |
| 3 | 38 | 18 (47.4) | |
| 4 | 22 | 9 (40.9) | |
| 5 | 23 | 10 (43.5) | |
| Unknown | 5 | - | |
| Surgical margins | p = 0.577 | ||
| Positive | 48 | 20 (41.7) | |
| Negative | 101 | 47 (46.5) | |
| Unknown | 4 | - | |
| pT-stage | p = 0.173 | ||
| <pT2a | 5 | 1 (20) | |
| pT2b | 2 | 2 (100) | |
| >pT2c | 145 | 64 (44.1) | |
| Unknown | 1 | - | |
| Stratification | n | PET-Positive Results, n, (%) | χ2 |
|---|---|---|---|
| All patients | 189 | 103 (54.5) | p < 0.01 |
| PSA ng/mL | |||
| <0.5 | 82 | 23 (28) | |
| 0.5 ≤ 1 | 23 | 9 (39) | |
| 1 ≤ 2 | 25 | 16 (64) | |
| 2 ≤ 5 | 24 | 21 (87.5) | |
| ≥5 | 33 | 32 (97) | |
| Unknown | 2 | - |
| Stratification | n | PET-Positive Results, n, (%) | χ2 |
|---|---|---|---|
| All patients | 153 | 68 (44.4) | p < 0.01 |
| PSA ng/mL | |||
| <0.5 | 79 | 21 (26.6) | |
| 0.5 ≤ 1 | 22 | 8 (36.4) | |
| 1 ≤ 2 | 23 | 14 (60.9) | |
| 2 ≤ 5 | 12 | 9 (75) | |
| ≥5 | 16 | 15 (93.8) | |
| Unknown | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, M.T.; Jochumsen, M.R.; Klingenberg, S.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT. Diagnostics 2022, 12, 195. https://doi.org/10.3390/diagnostics12010195
Christensen MT, Jochumsen MR, Klingenberg S, Sørensen KD, Borre M, Bouchelouche K. Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT. Diagnostics. 2022; 12(1):195. https://doi.org/10.3390/diagnostics12010195
Chicago/Turabian StyleChristensen, Mads T., Mads R. Jochumsen, Søren Klingenberg, Karina D. Sørensen, Michael Borre, and Kirsten Bouchelouche. 2022. "Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT" Diagnostics 12, no. 1: 195. https://doi.org/10.3390/diagnostics12010195
APA StyleChristensen, M. T., Jochumsen, M. R., Klingenberg, S., Sørensen, K. D., Borre, M., & Bouchelouche, K. (2022). Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT. Diagnostics, 12(1), 195. https://doi.org/10.3390/diagnostics12010195







