Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers
Abstract
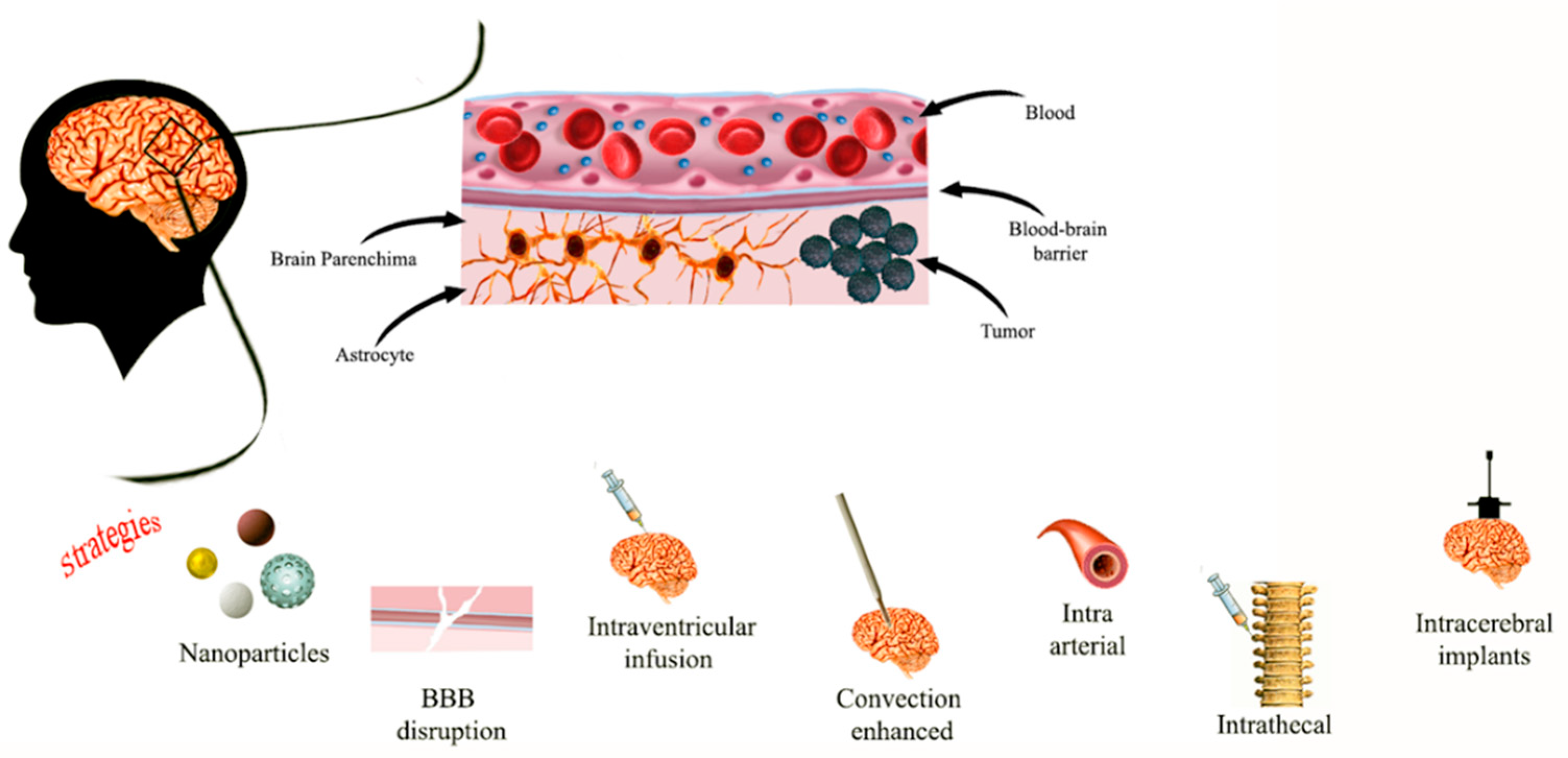
1. Introduction
2. Inorganic Nano-Systems for Pediatric Brain Cancer Diagnosis and Therapy
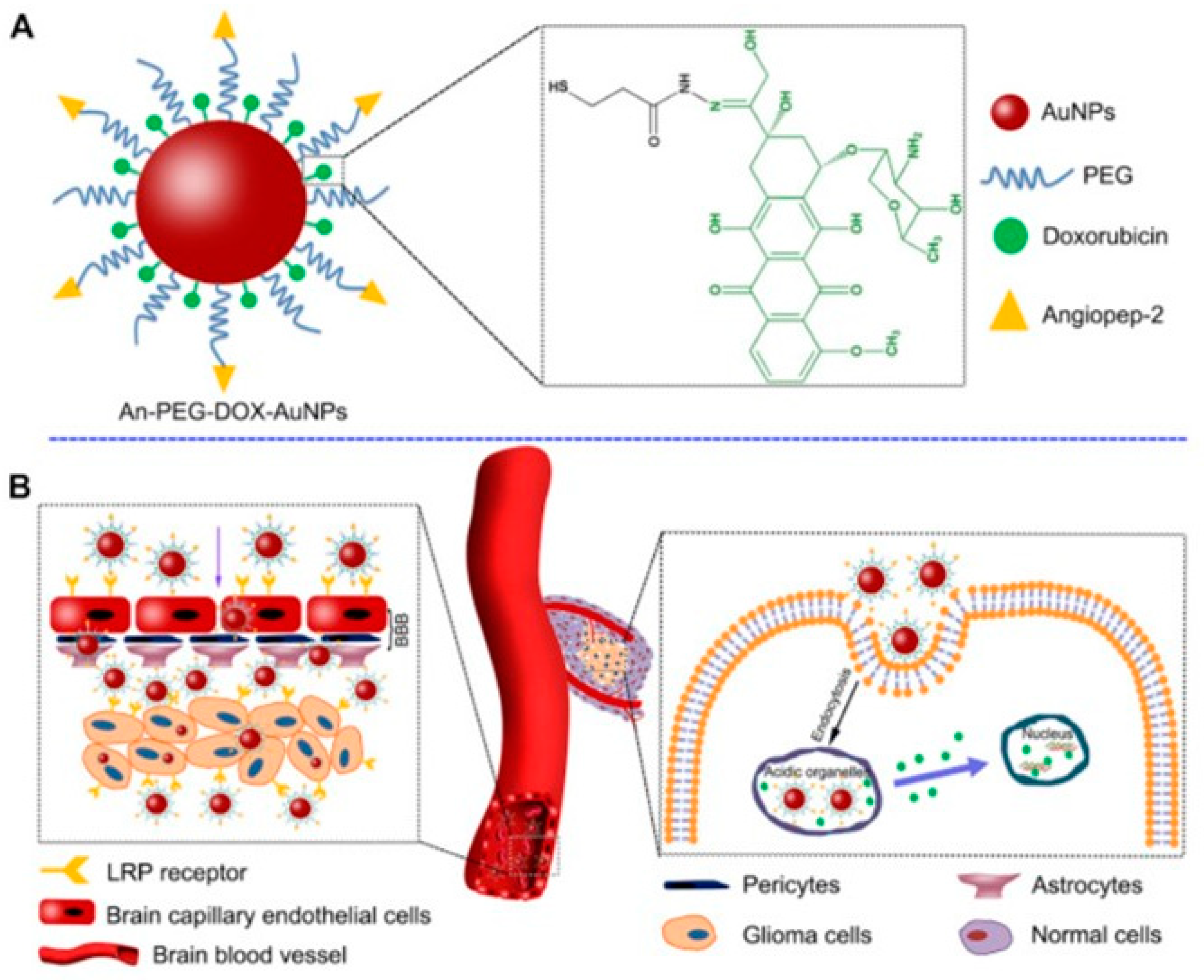
2.1. Gold Nanoparticles
2.2. Silver Nanoparticles
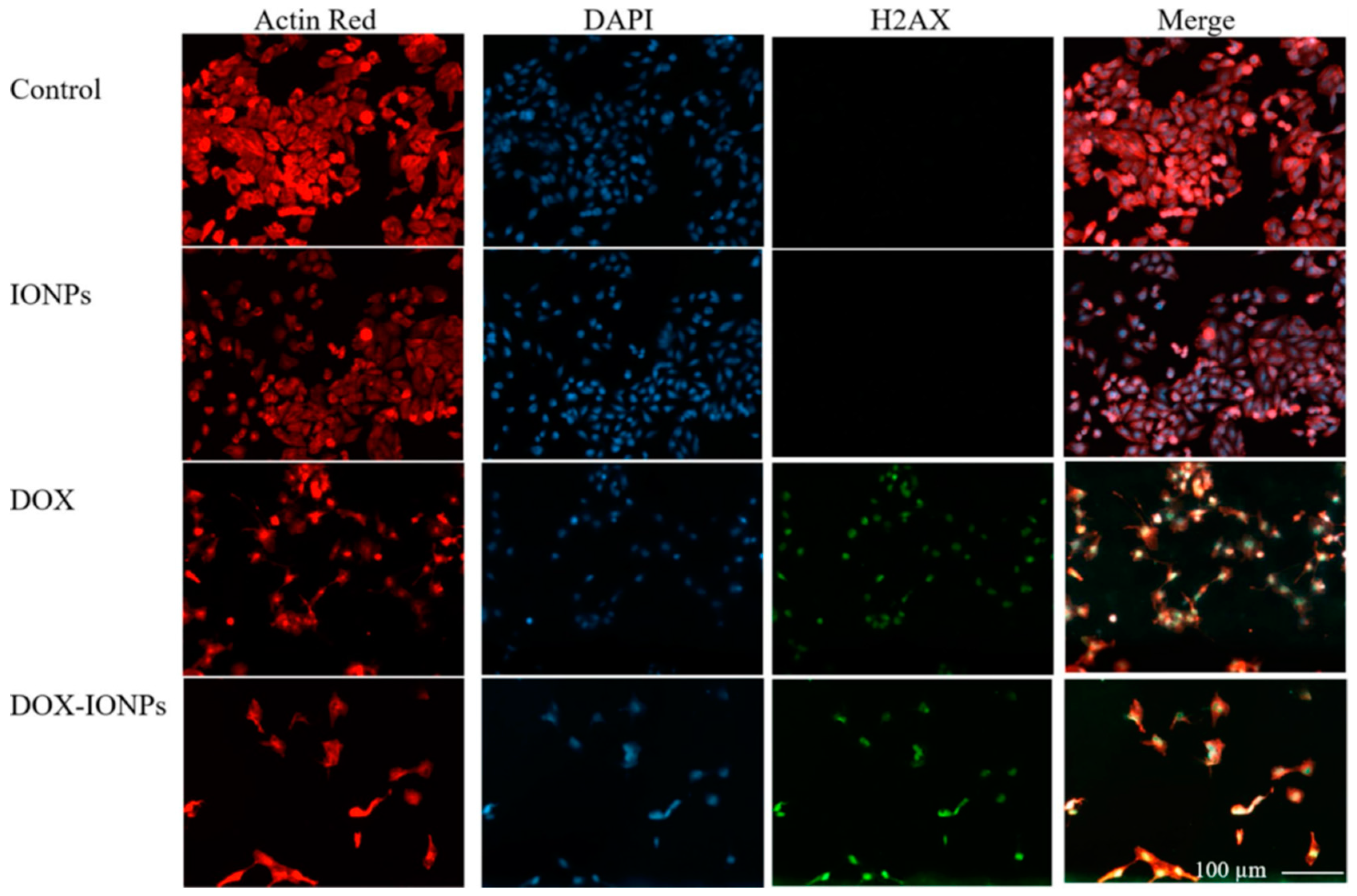
2.3. Iron and Zinc Oxide NPs
3. Lipid Based Nanoparticles in Pediatric Brain Tumors Treatment
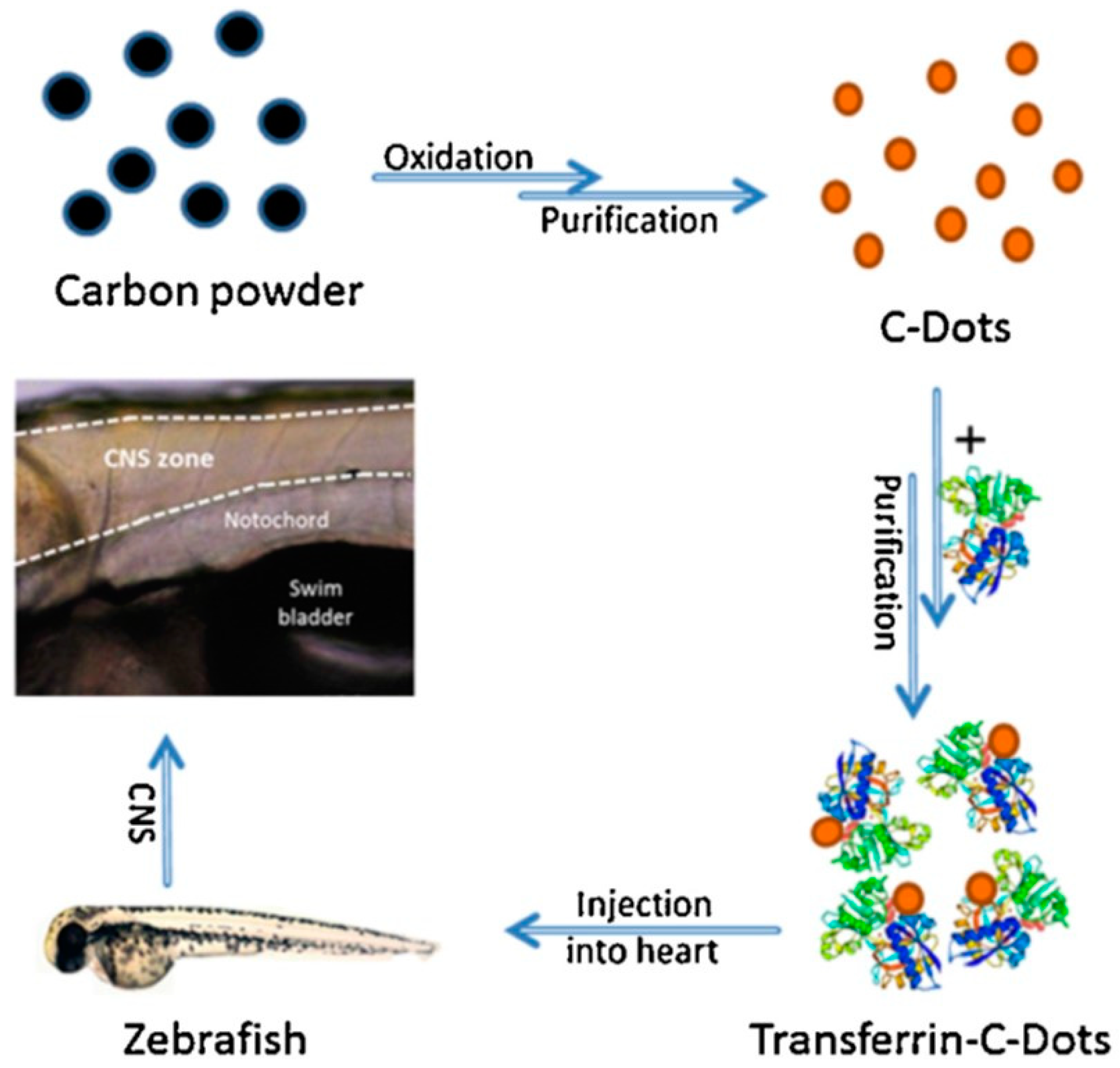
4. Therapeutic Carbon Dots in Pediatric Glioma Diagnosis and Treatment
5. Polymeric NPs as Diagnostic Tools and Therapeutic Nanocarriers in Pediatric Brain Malignancies
6. Dendrimers in Diagnosis and Treatment of Brain Cancer in Children
7. Nanoparticles in Immunotherapy for Pediatric Brain Tumors
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mueller, S.; Chang, S. Pediatric brain tumors: Current treatment strategies and future therapeutic approaches. Neurotherapeutics 2009, 6, 570–586. [Google Scholar] [CrossRef]
- Fleming, A.J.; Chi, S.N. Brain tumors in children. Curr. Probl. Pediatr. Adolesc. Health Care 2012, 42, 80–103. [Google Scholar] [CrossRef]
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef]
- Pollack, I.F.; Jakacki, R.I. Childhood brain tumors: Epidemiology, current management and future directions. Nat. Rev. Neurol. 2011, 7, 495–506. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Janagam, D.R.; Lowe, T.L. Overcoming the blood-brain barrier in chemotherapy treatment of pediatric brain tumors. Pharm. Res. 2014, 31, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.E. Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.-F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharm. 2003, 192, 1–11. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: Obstacles or opportunities for drug delivery? Curr. Pharm. Des. 2014, 20, 1422–1449. [Google Scholar] [CrossRef]
- Pardridge, W.M. Brain Drug Targeting and Gene Technologies. Jpn. J. Pharmacol. 2001, 87, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.; Gardberg, M.; Ek, P.; Frantzén, J.; Bobacka, J.; Minn, H. Gadolinium retention in gliomas and adjacent normal brain tissue: Association with tumor contrast enhancement and linear/macrocyclic agents. Neuroradiology 2019, 61, 535–544. [Google Scholar] [CrossRef]
- Castillo, M. History and Evolution of Brain Tumor Imaging: Insights through Radiology. Radiology 2014, 273, S111–S125. [Google Scholar] [CrossRef] [PubMed]
- Cha, S. Update on brain tumor imaging: From anatomy to physiology. AJNR Am. J. Neuroradiol. 2006, 27, 475–487. [Google Scholar] [PubMed]
- Karschnia, P.; Vogelbaum, M.A.; van den Bent, M.; Cahill, D.P.; Bello, L.; Narita, Y.; Berger, M.S.; Weller, M.; Tonn, J.-C. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur. J. Cancer 2021, 149, 23–33. [Google Scholar] [CrossRef]
- Hirono, S.; Ozaki, K.; Kobayashi, M.; Hara, A.; Yamaki, T.; Matsutani, T.; Iwadate, Y. Oncological and functional outcomes of supratotal resection of IDH1 wild-type glioblastoma based on 11C-methionine PET: A retrospective, single-center study. Sci. Rep. 2021, 11, 14554. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA Evaluating the Risk of Brain Deposits with Repeated Use of Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging (MRI); Center for Drug Evaluation and Research: Beltsville, MD, USA, 2017. [Google Scholar]
- EMA. EMA’s Final Opinion Confirms Restrictions on Use of Linear Gadolinium Agents in Body Scans; EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Grabrucker, A.M.; Chhabra, R.; Belletti, D.; Forni, F.; Vandelli, M.A.; Ruozi, B.; Tosi, G. Nanoparticles as Blood–Brain Barrier Permeable CNS Targeted Drug Delivery Systems; Springer: Berlin, Germany, 2013; Volume 10. [Google Scholar]
- Zhang, T.T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Dong, S.; Lee, R.J.; Yang, D.; Zhang, H.; Teng, L. Cell-Penetrating Peptide and Transferrin Co-Modified Liposomes for Targeted Therapy of Glioma. Molecules 2019, 24, 3540. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.V.; Roney, C.A.; Antich, P.P.; Bonte, F.J.; Raghu, A.V.; Aminabhavi, T.M. Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Monteagudo, S.; Ceña, V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine 2016, 11, 833–849. [Google Scholar] [CrossRef]
- Li, C.; Wallace, S. Polymer-drug conjugates: Recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008, 60, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly(amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sulis Sato, S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Pendiuk Gonçalves, J.; Fraga da Cruz, A.; Ribeiro de Barros, H.; Santana Borges, B.; Almeida Soares de Medeiros, L.C.; Soares, M.J.; Padovan Dos Santos, M.; Grassi, M.T.; Chandra, A.; Del Mercato, L.L.; et al. Beyond gold nanoparticles cytotoxicity: Potential to impair metastasis hallmarks. Eur. J. Pharm. Biopharm. 2020, 157, 221–232. [Google Scholar] [CrossRef]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zanzonico, P.; Zhou, Z.; Donzelli, M.; Lyashchenko, S.K.; Haque, S.; Thakur, S.B.; Cheung, N.-K.V.; et al. A phase I study of convection enhanced delivery (CED) of 124I-8H9 radio-labeled monoclonal antibody in children with diffuse intrinsic pontine glioma (DIPG). J. Clin. Oncol. 2017, 35, 2010. [Google Scholar] [CrossRef]
- Negron, K.; Khalasawi, N.; Suk, J.S. Strategies to Enhance the Distribution of Therapeutic Nanoparticles in the Brain by Convection Enhanced Delivery. In Nanotherapy for Brain Tumor Drug Delivery; Humana: New York, NY, USA, 2021; Volume 163. [Google Scholar]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.; Lee, J.; Kotov, N.A. Gold nanoparticles enhance the anti-leukemia action of a 6-mercaptopurine chemotherapeutic agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yang, W.; Chen, X.; Gao, J.; Gong, X.; Wang, H.; Duan, Y.; Wei, D.; Chang, J. Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J. Mater. Chem. B 2019, 7, 4734–4750. [Google Scholar] [CrossRef]
- Luly, K.M.; Choi, J.; Rui, Y.; Green, J.J.; Jackson, E.M. Safety considerations for nanoparticle gene delivery in pediatric brain tumors. Nanomedicine 2020, 15, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Merkel, T.J.; Luthi, A.J.; Patel, P.C.; Cutler, J.I.; et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, H.; Li, H. Silencing of DNA repair sensitizes pediatric brain tumor cells to γ-irradiation using gold nanoparticles. Environ. Toxicol. Pharm. 2017, 53, 40–45. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Bredlau, A.L.; Dixit, S.; Chen, C.; Broome, A.-M. Nanotechnology Applications for Diffuse Intrinsic Pontine Glioma. Curr. Neuropharmacol. 2017, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef]
- Salazar-García, S.; García Rodrigo, J.F.; Martínez-Castañon, G.-A.; Ruiz-Rodríguez, V.; Portales-Pérez, D.; González, C. Silver nanoparticles (AgNPs) and zinc chloride (ZnCl2) exposure order determines the toxicity in C6 rat glioma cells. J. Nanoparticle Res. 2020, 22, 253. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Wahab, R.; Kaushik, N.K.; Verma, A.K.; Mishra, A.; Hwang, I.H.; Yang, Y.B.; Shin, H.S.; Kim, Y.S. Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa, and normal HEK cells. J. Biol. Inorg. Chem. 2011, 16, 431–442. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.; Yoshida, S.; Chen, C.; Barisone, G.; Diaz, E.; Li, Y.; Beckett, L.; Chung, J.; Antony, R.; Nolta, J.; et al. Novel targeted therapy for neuroblastoma: Silencing the MXD3 gene using siRNA. Pediatr Res. 2017, 82, 527–535. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.A.; Hall, D.E.; Kim, G.; Koo, Y.E.; Woolliscroft, M.J.; Sugai, J.V.; et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef]
- Infante, P.; Malfanti, A.; Quaglio, D.; Balducci, S.; De Martin, S.; Bufalieri, F.; Mastrotto, F.; Basili, I.; Garofalo, M.; Lospinoso Severini, L.; et al. Glabrescione B delivery by self-assembling micelles efficiently inhibits tumor growth in preclinical models of Hedgehog-dependent medulloblastoma. Cancer Lett 2021, 499, 220–231. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dey, A.; Malhotra, A.; Liu, J.; Ahn, S.I.; Sei, Y.J.; Kenney, A.M.; MacDonald, T.J.; Kim, Y. Engineered biomimetic nanoparticle for dual targeting of the cancer stem-like cell population in sonic hedgehog medulloblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 24205–24212. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Bastiat, G.; Lagarce, F. Gemcitabine and glioblastoma: Challenges and current perspectives. Drug Discov. Today 2018, 23, 416–423. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Zhou, Y.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Vanni, S.; Graham, R.M.; Leblanc, R.M. Pediatric glioblastoma target-specific efficient delivery of gemcitabine across the blood–brain barrier via carbon nitride dots. Nanoscale 2020, 12, 7927–7938. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.L.; Leblanc, R.M. Crossing the blood-brain-barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymer 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthc. Mater. 2019, 8, e1801359. [Google Scholar] [CrossRef]
- Choi, J.; Rui, Y.; Kim, J.; Gorelick, N.; Wilson, D.R.; Kozielski, K.; Mangraviti, A.; Sankey, E.; Brem, H.; Tyler, B.; et al. Nonviral polymeric nanoparticles for gene therapy in pediatric CNS malignancies. Nanomedicine 2020, 23, 102115. [Google Scholar] [CrossRef]
- Lopez-Bertoni, H.; Kozielski, K.L.; Rui, Y.; Lal, B.; Vaughan, H.; Wilson, D.R.; Mihelson, N.; Eberhart, C.G.; Laterra, J.; Green, J.J. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018, 18, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Mignani, S.; Majoral, J.-P. Dendrimers as macromolecular tools to tackle from colon to brain tumor types: A concise overview. New J. Chem. 2013, 37, 3337–3357. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fan, J.; Wang, Z.; Zeng, X.; Sun, Y.; Song, P.; Ju, D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials 2014, 35, 7588–7597. [Google Scholar] [CrossRef] [PubMed]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef]
- Cheng, X.; Murphy, W.; Recek, N.; Yan, D.; Cvelbar, U.; Vesel, A.; Mozetic, M.; Canady, M.D.J.; Keidar, M.; Sherman, J. Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapy. J. Phys. D Appl. Phys. 2014, 47, 335402. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef]
- Setua, S.; Ouberai, M.; Piccirillo, S.G.; Watts, C.; Welland, M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale 2014, 6, 10865–10873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meyers, J.D.; Agnes, R.S.; Doane, T.L.; Kenney, M.E.; Broome, A.M.; Burda, C.; Basilion, J.P. Addressing brain tumors with targeted gold nanoparticles: A new gold standard for hydrophobic drug delivery? Small 2011, 7, 2301–2306. [Google Scholar] [CrossRef]
- Vajrala, G.; Maricar, S.; Thammineni, P.R.; Raya, C.N.; Vidiyala, S.K.; Madigubba, S.; Panigrahi, M.K. The Effect of Gold Nanoparticles on Chemo and Radiotherapy of Brain Tumor Cells. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e530. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Testini, M.; Ratano, P.; Palamà, I.E. Hybrid Clustered Nanoparticles for Chemo-Antibacterial Combinatorial Cancer Therapy. Cancers 2019, 11, 1338. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Stephen, Z.R.; Wang, K.; Dayringer, C.J.; Sham, J.G.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Nanoparticle mediated silencing of DNA repair sensitizes pediatric brain tumor cells to γ-irradiation. Mol. Oncol 2015, 9, 1071–1080. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. Aaps. J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Orringer, D.A.; Koo, Y.E.; Chen, T.; Kopelman, R.; Sagher, O.; Philbert, M.A. Small solutions for big problems: The application of nanoparticles to brain tumor diagnosis and therapy. Clin. Pharm. 2009, 85, 531–534. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; D’Amone, S.; Arcadio, V.; Biasiucci, M.; Mezzi, A.; Cortese, B. Cell mechanotactic and cytotoxic response to zinc oxide nanorods depends on substrate stiffness. Toxicol. Res. 2016, 5, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Kemper, E.M.; Boogerd, W.; Thuis, I.; Beijnen, J.H.; van Tellingen, O. Modulation of the blood-brain barrier in oncology: Therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 2004, 30, 415–423. [Google Scholar] [CrossRef]
- Brasnjevic, I.; Steinbusch, H.W.; Schmitz, C.; Martinez-Martinez, P. Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. The targeted delivery of cancer drugs across the blood–brain barrier: Chemical modifications of drugs or drug-nanoparticles? Drug Discov. Today 2008, 13, 1099–1106. [Google Scholar] [CrossRef]
- Smith, M.W.; Gumbleton, M. Endocytosis at the blood-brain barrier: From basic understanding to drug delivery strategies. J. Drug Target. 2006, 14, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release Off. J. Control. Release Soc. 2011, 153, 198–205. [Google Scholar] [CrossRef]
- Bell, J.B.; Rink, J.S.; Eckerdt, F.; Clymer, J.; Goldman, S.; Thaxton, C.S.; Platanias, L.C. HDL nanoparticles targeting sonic hedgehog subtype medulloblastoma. Sci. Rep. 2018, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nogales, C.; Noguera, R.; Couvreur, P.; Blanco-Prieto, M.J. Therapeutic Opportunities in Neuroblastoma Using Nanotechnology. J. Pharm. Exp. 2019, 370, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Lu, Y.M.; Wang, H.; Liu, J.; Liao, M.H.; Hong, L.J.; Tao, R.R.; Ahmed, M.M.; Liu, P.; Liu, S.S.; et al. The effect of lipid nanoparticle PEGylation on neuroinflammatory response in mouse brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef]
- da Cruz, M.T.; Simões, S.; de Lima, M.C. Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp. Neurol. 2004, 187, 65–75. [Google Scholar] [CrossRef]
- Yang, J.; Lee, T.-I.; Lee, J.; Lim, E.-K.; Hyung, W.; Lee, C.-H.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; et al. Synthesis of Ultrasensitive Magnetic Resonance Contrast Agents for Cancer Imaging Using PEG-Fatty Acid. Chem. Mater. 2007, 19, 3870–3876. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.-C.; Huang, H.; Feng, J.-J.; Chen, J.-R.; Wang, A.-J. Facile synthesis of water-soluble and biocompatible fluorescent nitrogen-doped carbon dots for cell imaging. Analyst 2014, 139, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, H.; Sharma, A.; Singh, J.; Alajangi, H.K.; Kumar, S.; Singla, N.; Kaur, I.P.; Barnwal, R.P. Carbon Based Nanodots in Early Diagnosis of Cancer. Front. Chem. 2021, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, L.; Li, Q.; Cao, Y.; Chen, D.; Du, Q.; Yang, X.; Huang, D.; Pei, R.; Chen, X.; et al. NIR-laser-triggered gadolinium-doped carbon dots for magnetic resonance imaging, drug delivery and combined photothermal chemotherapy for triple negative breast cancer. J. Nanobiotechnol. 2021, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Yang, J.; Wu, F.-G. On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 2018, 134, 232–243. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid Interface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; De Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Palamà, I.E. Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics 2018, 10, 52. [Google Scholar] [CrossRef]
- Bernal, G.M.; LaRiviere, M.J.; Mansour, N.; Pytel, P.; Cahill, K.E.; Voce, D.J.; Kang, S.; Spretz, R.; Welp, U.; Noriega, S.E.; et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 149–157. [Google Scholar] [CrossRef]
- Perumal, V.; Sivakumar, P.M.; Zarrabi, A.; Muthupandian, S.; Vijayaraghavalu, S.; Sahoo, K.; Das, A.; Das, S.; Payyappilly, S.S.; Das, S. Near infra-red polymeric nanoparticle based optical imaging in Cancer diagnosis. J. Photochem. Photobiol. B: Biol. 2019, 199, 111630. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, E.J.; Lee, Y.K.; Kim, K.; Kwon, I.C.; Lee, K.Y. Optical Imaging and Gene Therapy with Neuroblastoma-Targeting Polymeric Nanoparticles for Potential Theranostic Applications. Small 2016, 12, 1201–1211. [Google Scholar] [CrossRef]
- Gupta, A.; Wang, S.; Pera, P.; Rao, K.V.R.; Patel, N.; Ohulchanskyy, T.Y.; Missert, J.; Morgan, J.; Koo-Lee, Y.-E.; Kopelman, R.; et al. Multifunctional nanoplatforms for fluorescence imaging and photodynamic therapy developed by post-loading photosensitizer and fluorophore to polyacrylamide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 941–950. [Google Scholar] [CrossRef]
- Wang, Q.; Kumar, V.; Lin, F.; Sethi, B.; Coulter, D.W.; McGuire, T.R.; Mahato, R.I. ApoE mimetic peptide targeted nanoparticles carrying a BRD4 inhibitor for treating Medulloblastoma in mice. J. Control Release 2020, 323, 463–474. [Google Scholar] [CrossRef]
- Jayasundara, S.; Ali, M. Dendrimer-based Nanoparticles for Targeted Brain Tumor Imaging. J. Text. Sci. Eng. 2017, 8, 444. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.H.; Van Antwerp, M.E.; Chen, X.; Baker, J.J.R. Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst 2009, 134, 1373–1379. [Google Scholar] [CrossRef]
- Ruan, S.; Qin, L.; Xiao, W.; Hu, C.; Zhou, Y.; Wang, R.; Sun, X.; Yu, W.; He, Q.; Gao, H. Acid-Responsive Transferrin Dissociation and GLUT Mediated Exocytosis for Increased Blood–Brain Barrier Transcytosis and Programmed Glioma Targeting Delivery. Adv. Funct. Mater. 2018, 28, 1802227. [Google Scholar] [CrossRef]
- Bors, L.A.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, M.R. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.B.; Madsen, P.J.; Hegde, M.; Ahmed, N.; Cole, K.A.; Maris, J.M.; Resnick, A.C.; Storm, P.B.; Waanders, A.J. Immunotherapy for pediatric brain tumors: Past and present. Neuro Oncol. 2019, 21, 1226–1238. [Google Scholar] [CrossRef]
- Wedekind, M.F.; Denton, N.L.; Chen, C.Y.; Cripe, T.P. Pediatric Cancer Immunotherapy: Opportunities and Challenges. Paediatr. Drugs 2018, 20, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; De Leon, G.; Pham, C.; Grippin, A.; Kemeny, H.; Chua, J.; Huang, J.; Sampson, J.H.; Sanchez-Perez, L.; Flores, C.; et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. Oncoimmunology 2016, 6, e1256527. [Google Scholar] [CrossRef]
- Sayour, E.; Mendez-Gomez, H.; Grippin, A.; De Leon, G.; Stover, B.; Flores, C.; Pham, C.; Mitchell, D. MBRS-02. Personalized Immunotherapy with Translatable RNA Nanoparticles Targeting Medulloblastoma. Neuro-Oncology 2018, 20, i128–i129. [Google Scholar] [CrossRef]
- Mendez-Gomez, H.; McGuiness, J.; Grippin, A.; Weidert, F.; Carrera-Justiz, S.; Mitchell, D.; Sayour, E. IMMU-13. Customizable Multi-Lamellar RNA-Nanoparticles for Pediatric Glioma. Neuro-Oncology 2021, 23, i29–i30. [Google Scholar] [CrossRef]
- Lenzen, A.; Cole, L.; Lauing, K.L.; Zhai, L.; Ladomersky, E.; Lulla, R.R.; Hashizume, R.; Stegh, A.; Wainwright, D.A. IMMU-24. Immunotherapeutic Nanotechnology Targeting Ido1 for Pediatric Diffuse Intrinsic Pontine Glioma. Neuro-Oncology 2018, 20, i103. [Google Scholar] [CrossRef][Green Version]


| Nanoparticle | Use | Advantages | Disadvantages | In Vitro/In Vivo Models |
|---|---|---|---|---|
| Au NPs |
|
| ||
| Ag NPs | Tumor therapy |
| Cytotoxicity in lung, stomach, breast, and endothelial cells [43] | C6 rat glioma cells [44] |
| Fe3O4 and ZnO NPs |
| Potential toxicity in complex biological systems (ZnO) [48] | ||
| Lipid based NPs |
|
| ||
| Carbon dots |
|
|
| |
| Polymeric NPs |
| |||
| Dendrimers |
|
| Neurotoxicity [68] |
| Phase | Intervention/Treatment | Recruitment Status | Last Update Posted | Ages Eligible for Study | ClinicalTrials.gov Identifier | Type of Cancer |
|---|---|---|---|---|---|---|
| Phase 1 | Irinotecan loaded liposomes | Recruiting | 18 September 2019 | 1 to 20 | NCT02013336 | NB |
| Phase 1 | Doxorubicin loaded liposomes | Recruiting | 25 September 2020 | Up to 30 years | NCT02536183 | NB |
| Phase 1 | Doxorubicin loaded liposomes | Completed | 28 April 2015 | Up to 21 years | NCT00019630 | BT |
| Phase 1 | Doxorubicin loaded liposomes | Withdrawn | 19 March 2019 | 1 year to 40 years | NCT02557854 | NB |
| Phase 1 | Cytarabine loaded liposomes | Unknown | 23 March 2010 | 1 year to 21 years | NCT00003073 | CNST |
| Phase 1 | Panobinostat Nanoparticle Formulation MTX110 | Completed | 15 October 2021 | 1 year to 17 years | NCT03566199 | DIPG |
| Phase 1 | Infusate with MTX110 and gadolinium | Recruiting | 8 December 2021 | 1 year to 17 years | NCT04264143 | DIPG, DP, TG, DMD |
| Not applicable | DSC-MRI with ferumoxytol (small iron particles) | Unknown | 1 February 2018 | 1 year to 17 years | NCT00978562 | BN |
| Phase 2 | Combidex (ultra-small iron oxide particle) as MRI contrast agent | Terminated | 16 May 2017 | 1 year to 17 years | NCT00659334 | BN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A.; Quintarelli, C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics 2022, 12, 173. https://doi.org/10.3390/diagnostics12010173
Guido C, Baldari C, Maiorano G, Mastronuzzi A, Carai A, Quintarelli C, De Angelis B, Cortese B, Gigli G, Palamà IE. Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics. 2022; 12(1):173. https://doi.org/10.3390/diagnostics12010173
Chicago/Turabian StyleGuido, Clara, Clara Baldari, Gabriele Maiorano, Angela Mastronuzzi, Andrea Carai, Concetta Quintarelli, Biagio De Angelis, Barbara Cortese, Giuseppe Gigli, and Ilaria Elena Palamà. 2022. "Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers" Diagnostics 12, no. 1: 173. https://doi.org/10.3390/diagnostics12010173
APA StyleGuido, C., Baldari, C., Maiorano, G., Mastronuzzi, A., Carai, A., Quintarelli, C., De Angelis, B., Cortese, B., Gigli, G., & Palamà, I. E. (2022). Nanoparticles for Diagnosis and Target Therapy in Pediatric Brain Cancers. Diagnostics, 12(1), 173. https://doi.org/10.3390/diagnostics12010173










