The Prevalence of MRI-Defined Sacroiliitis and Classification of Spondyloarthritis in Patients with Acute Anterior Uveitis: A Longitudinal Single-Centre Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Imaging
2.4. Diagnosis
2.5. Laboratory Analysis
2.6. Statistics
3. Results
3.1. Patients Characteristics
3.2. MRI of Sacroiliac Joints
3.3. Differences between AAU Patients with and without MRI Defined Sacroiliitis
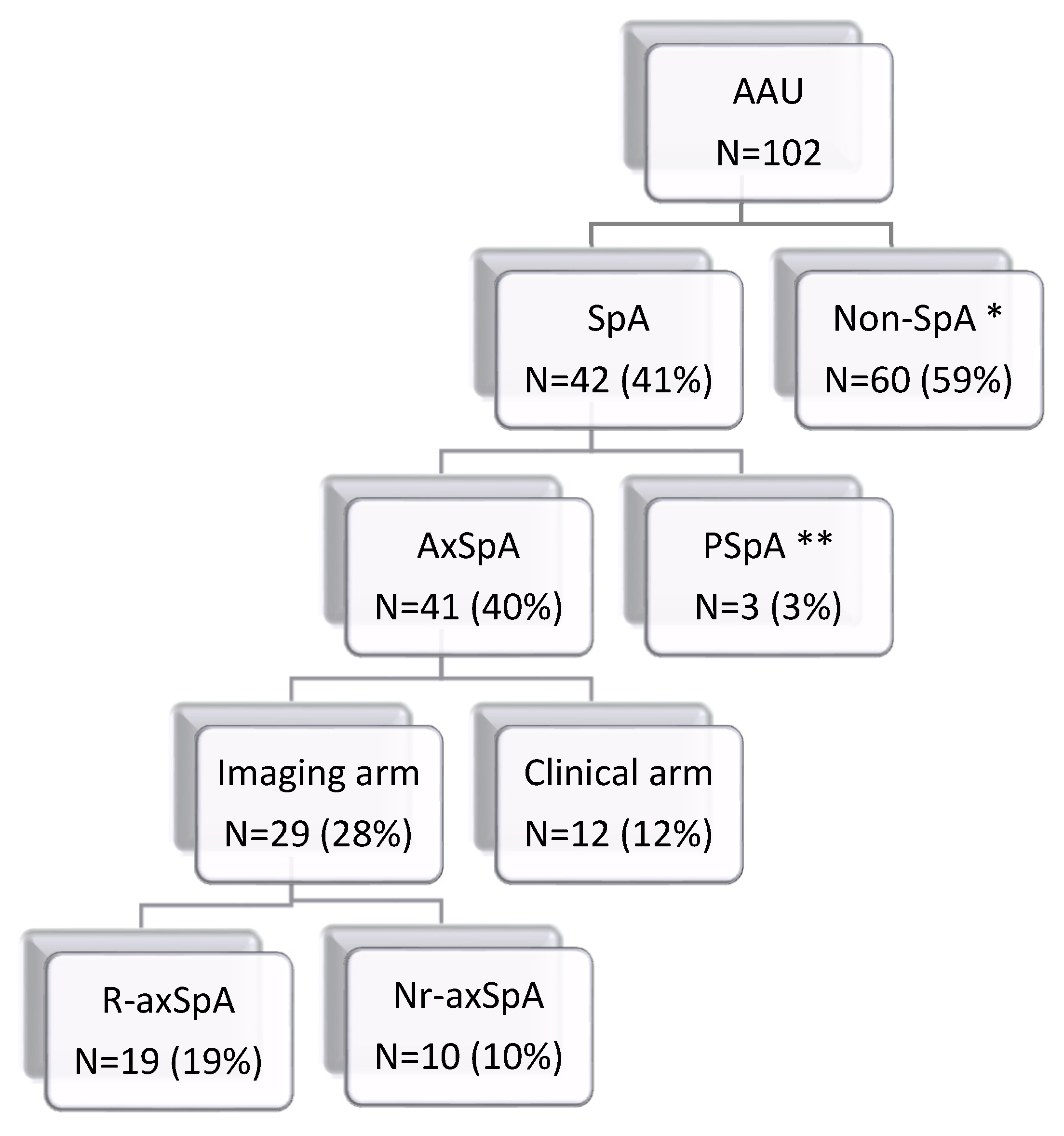
3.4. Fulfilment of the ASAS Classification Criteria for SpA (Regardless of Patient’s Age)
3.5. Clinical Diagnosis of SpA
3.6. Follow-Up Examination after Two Years
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantini, F.; Nannini, C.; Cassarà, E.; Kaloudi, O.; Niccoli, L. Uveitis in Spondyloarthritis: An Overview. J. Rheumatol. Suppl. 2015, 93, 27–29. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; He, C.; Li, D.; Tong, W.; Zou, Y.; Xu, W. Role of HLA-B27 in the pathogenesis of ankylosing spondylitis (Review). Mol. Med. Rep. 2017, 15, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, K.; Forsblad-D’Elia, H.; Deminger, A.; Klingberg, E.; Dehlin, M.; Exarchou, S.; Lindström, U.; Askling, J.; Jacobsson, L.T.H. Incidence of extra-articular manifestations in ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis: Results from a national register-based cohort study. Rheumatology 2020, 60, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Zeboulon, N.; Dougados, M.; Gossec, L. Prevalence and characteristics of uveitis in the spondyloarthropathies: A systematic literature review. Ann. Rheum. Dis. 2008, 67, 955–959. [Google Scholar] [CrossRef]
- Robinson, P.; Claushuis, T.A.M.; Cortes, A.; Martin, T.M.; Evans, D.; Leo, P.; Mukhopadhyay, P.; Bradbury, L.A.; Cremin, K.; Harris, J.; et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 2015, 67, 140–151. [Google Scholar] [CrossRef]
- D’Ambrosio, E.M.; La Cava, M.; Tortorella, P.; Gharbiya, M.; Campanella, M.; Iannetti, L. Clinical Features and Complications of the HLA-B27-associated Acute Anterior Uveitis: A Metanalysis. Semin. Ophthalmol. 2017, 32, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Khmelinskii, N.; Regel, A.; Baraliakos, X. The Role of Imaging in Diagnosing Axial Spondyloarthritis. Front. Med. 2018, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Rudwaleit, M.; Haibel, H.; Listing, J.; Märker-Hermann, E.; Zeidler, H.; Braun, J.; Sieper, J. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 2011, 70, 1369–1374. [Google Scholar] [CrossRef]
- Sykes, M.P.; Hamilton, L.; Jones, C.; Gaffney, K. Prevalence of axial spondyloarthritis in patients with acute anterior uveitis: A cross-sectional study utilising MRI. RMD Open 2018, 4, e000553. [Google Scholar] [CrossRef]
- Chung, Y.; Liao, H.; Lin, K.; Lin, Y.; Chou, C.; Chen, C.; Tsai, C. Prevalence of spondyloarthritis in 504 Chinese patients with HLA-B27-associated acute anterior uveitis. Scand. J. Rheumatol. 2009, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Juanola, X.; Loza Santamaría, E.; Cordero-Coma, M. Description and Prevalence of Spondyloarthritis in Patients with Anterior Uveitis: The SENTINEL Interdisciplinary Collaborative Project. Ophthalmology 2016, 123, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.L.; Maksymowych, W.P.; Lambert, R.G.W.; Muccioli, C.; Fernandes, A.R.C.; Pinheiro, M.M. Sacroiliac Joint Magnetic Resonance Imaging in Asymptomatic Patients with Recurrent Acute Anterior Uveitis: A Proof-of-concept Study. J. Rheumatol. 2017, 44, 1833–1840. [Google Scholar] [CrossRef]
- Jones, S.D.; Calin, A.; Steiner, A. An update on the Bath Ankylosing Spondylitis Disease Activity and Functional Indices (BASDAI, BASFI): Excellent Cronbach’s alpha scores. J. Rheumatol. 1996, 23, 407. [Google Scholar]
- Van Der Heijde, D.; Lie, E.; Kvien, T.K.; Sieper, J.; Van den Bosch, F.; Listing, J.; Braun, J.; Landewé, R.; for the Assessment of SpondyloArthritis International Society (ASAS). ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 1811–1818. [Google Scholar] [CrossRef]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Dougados, M.; Hermann, K.-G.; Landewé, R.; Maksymowych, W.; et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 2009, 68 (Suppl. 2), ii1–ii44. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Lambert, R.G.; Østergaard, M.; Pedersen, S.J.; Machado, P.M.; Weber, U.; Bennett, A.N.; Braun, J.; Burgos-Vargas, R.; De Hooge, M.; et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: An update of definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 2019, 78, 1550–1558. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Jurik, A.G.; Hermann, K.-G.; Landewé, R.; Van Der Heijde, D.; Baraliakos, X.; Marzo-Ortega, H.; Ostergaard, M.; Braun, J.; Sieper, J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: A consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 2009, 68, 1520–1527. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Braun, J.; Sieper, J. Assessment of SpondyloArthritis international S. ASAS classification criteria for axial spondyloarthritis. Z. Rheumatol. 2009, 68, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Van Der Heijde, D.; Landewe, R.; Akkoç, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; de Hooge, M.; Rudwaleit, M.; Sieper, J.; van Gaalen, F.; Reijnierse, M.; Landewé, R.; Huizinga, T.; Van Der Heijde, D. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: Results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann. Rheum. Dis. 2013, 72, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, A.; Jurik, A.G.; Lund, C.; Schiøttz-Christensen, B. The incidence of bone marrow oedema at the sacroiliac joints in a non-rheumatological population—A retrospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 590. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Van Der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G.; et al. The European Spondylarthropathy Study Group Preliminary Criteria for the Classification of Spondylarthropathy. Arthritis Rheum. 1991, 34, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; O’Rourke, M.; Ramasamy, P.; Murphy, C.C.; FitzGerald, O. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: The DUET (Dublin Uveitis Evaluation Tool). Ann. Rheum. Dis. 2015, 74, 1990–1995. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | AAU Patients (n = 102) | HS (n = 39) | p |
|---|---|---|---|
| Gender, f (%)/m (%) | 50 (49)/52 (51) | 19 (49)/20 (51) | 1.000 |
| Age (years), median (IQR) | 40 (32–45) | 39 (34–46) | 0.815 |
| Smokers, n (%) | 32 (31) | 13 (33) | 0.685 |
| AxSpA in FDR, n (%) | 6 (6) | 0 (0) | 0.187 |
| EMS in FDR, n (%) | 17 (17) | 0 (0) | 0.003 |
| Play sports regularly, n (%) | 34 (33) | 23 (59) | 0.007 |
| Spine injury, n (%) | 5 (5) | 0 (0) | 0.322 |
| Physically demanding occupation, n (%) | 17 (17) | ||
| Age of AAU onset (years), median (IQR) | 34 (27–42) | ||
| AAU disease duration (years), median (IQR) | 2 (0–7) | ||
| AAU relapse (n), median (IQR) | 2 (1–5) | ||
| Both eyes involvement, n (%) | 33 (32) | ||
| HLA-B27, n (%) | 77 (75) | 2 (5) | <0.0001 |
| BP, n (%) | 73 (72) | 27 (70) | 0.685 |
| IBP, n (%) | 21 (21) | 5 (13) | 0.340 |
| BASDAI, median (IQR) | 1 (0.23–2.06) | 0.3 (0–1.5) | 0.023 |
| ASDAS-CRP, median (IQR) | 1.04 (0.69–1.78) | 0.69 (0.64–1.22) | 0.039 |
| VAS (mm), median (IQR) | 0 (0–20) | ||
| CRP (mg/L), median (IQR) | 1.78 (1.83–4.85) | 1.13 (0.47–2.02) | 0.014 |
| Metrology: | |||
| Schober test (cm), median (IQR) | 5 (4–6) | ||
| Chin-chest test (cm), median (IQR) | 0 (0–0) | ||
| Chest expansion test (cm), median (IQR) | 5 (3–6) | ||
| Occiput to wall test (cm), median (IQR) | 0 (0–0) | ||
| BME, n (%) | 52 (51) | 11 (28) | 0.022 |
| Sacroiliitis by X-ray *, n (%) | 20 (57) | ||
| Sacroiliitis by MRI **, n (%) | 35 (34) | 0 (0) | <0.0001 |
| Characteristics | MRI Positive (n = 35) | MRI Negative (n = 67) | p |
|---|---|---|---|
| Gender, f (%)/m (%) | 13 (37)/22 (63) | 37 (55)/30 (45) | 0.098 |
| Age (years), median (IQR) | 40 (32–44) | 40 (32–46) | 0.657 |
| BMI | 24.2 (22.1–27.2) | 24.9 (22.2–27.7) | 0.640 |
| Smokers, n (%) | 12 (34) | 20 (30) | 0.659 |
| AxSpA in FDR, n (%) | 3 (9) | 3 (5) | 0.406 |
| EMS in FDR, n (%) | 4 (11) | 13 (19) | 0.406 |
| Play sports regularly, n (%) | 12 (34) | 22 (33) | 0.659 |
| Spine injury, n (%) | 2 (6) | 3 (5) | >0.9999 |
| Physically demanding occupation, n (%) | 9 (26) | 8 (12) | 0.096 |
| Age of AAU onset (years), median (IQR) | 33 (26–40) | 34 (28–44) | 0.182 |
| AAU disease duration (years), median (IQR) | 2 (0–8) | 1 (0–6) | 0.334 |
| AAU relapse (n), median (IQR) | 2 (1–5) | 2 (1–4) | 0.787 |
| Both eyes involvement, n (%) | 15 (43) | 18 (27) | 0.121 |
| HLA-B27, n (%) | 31 (89) | 46 (69) | 0.030 |
| BP, n (%) | 29 (83) | 44 (66) | 0.105 |
| IBP, n (%) | 12 (34) | 9 (13) | 0.020 |
| BASDAI, median (IQR) | 1.1 (0.25–2.26) | 1 (0.2–1.9) | 0.732 |
| ASDAS-CRP, median (IQR) | 1.46 (0.93–2.05) | 0.9 (0.64–1.53) | 0.006 |
| VAS (mm), median (IQR) | 0 (0–12) | 0 (0–20) | 0.480 |
| CRP (mg/L), median (IQR) | 4.43 (1.76–10.44) | 1.22 (0.65–2.92) | <0.0001 |
| Metrology: | |||
| Schober test (cm), mean (±SD) | 4.8 (±1.3) | 5 (±1.2) | 0.669 |
| Chin-chest test (cm), mean (±SD) | 0.4 (±1.0) | 0.4 (±1.0) | 0.530 |
| Chest expansion test (cm), mean (±SD) | 4.1 (±2.0) | 4.9 (±2.0) | 0.090 |
| Occiput to wall test (cm), mean (±SD) | 0.8 (±1.9) | 0.1 (±0.8) | 0.002 |
| BME, n (%) | 33 (94) | 19 (28) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubova, K.; Hasikova, L.; Mintalova, K.; Gregova, M.; Kasalicky, P.; Klimova, A.; Brichova, M.; Svozilkova, P.; Heissigerova, J.; Vencovsky, J.; et al. The Prevalence of MRI-Defined Sacroiliitis and Classification of Spondyloarthritis in Patients with Acute Anterior Uveitis: A Longitudinal Single-Centre Cohort Study. Diagnostics 2022, 12, 161. https://doi.org/10.3390/diagnostics12010161
Bubova K, Hasikova L, Mintalova K, Gregova M, Kasalicky P, Klimova A, Brichova M, Svozilkova P, Heissigerova J, Vencovsky J, et al. The Prevalence of MRI-Defined Sacroiliitis and Classification of Spondyloarthritis in Patients with Acute Anterior Uveitis: A Longitudinal Single-Centre Cohort Study. Diagnostics. 2022; 12(1):161. https://doi.org/10.3390/diagnostics12010161
Chicago/Turabian StyleBubova, Kristyna, Lenka Hasikova, Katerina Mintalova, Monika Gregova, Petr Kasalicky, Aneta Klimova, Michaela Brichova, Petra Svozilkova, Jarmila Heissigerova, Jiri Vencovsky, and et al. 2022. "The Prevalence of MRI-Defined Sacroiliitis and Classification of Spondyloarthritis in Patients with Acute Anterior Uveitis: A Longitudinal Single-Centre Cohort Study" Diagnostics 12, no. 1: 161. https://doi.org/10.3390/diagnostics12010161
APA StyleBubova, K., Hasikova, L., Mintalova, K., Gregova, M., Kasalicky, P., Klimova, A., Brichova, M., Svozilkova, P., Heissigerova, J., Vencovsky, J., Pavelka, K., & Senolt, L. (2022). The Prevalence of MRI-Defined Sacroiliitis and Classification of Spondyloarthritis in Patients with Acute Anterior Uveitis: A Longitudinal Single-Centre Cohort Study. Diagnostics, 12(1), 161. https://doi.org/10.3390/diagnostics12010161







