Video Capsule Endoscopy Optimal Timing in Overt Obscure Gastrointestinal Bleeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. VCE Procedure and Findings
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
3.2. Video Capsule Endoscopy Findings
3.3. Primary Outcome
3.4. Secondary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Gastroenterological Association. American Gastroenterological Association medical position statement: Evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology 2000, 118, 197–201. [Google Scholar] [CrossRef]
- Lewis, B.S. Small intestinal bleeding. Gastroenterol. Clin. N. Am. 1994, 23, 67–91. [Google Scholar] [CrossRef]
- Shim, K.N.; Moon, J.S.; Chang, N.K.; Hyuk, J.; Kim, J.H.; Min, B.H.; Jeon, S.R.; Kim, J.-O.; Choi, M.-G. Guideline for capsule endoscopy: Obscure gastrointestinal bleeding. Clin. Endosc. 2013, 46, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.S.; Gerson, L.; Das, A.; Lewis, B. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology 2007, 133, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Spada, C.; Eliakim, R.; Keuchel, M.; May, A.; Mulder, C.J.; Rondonotti, E.; Adler, S.N.; Albert, J.; Baltes, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015, 47, 352–376. [Google Scholar] [CrossRef] [Green Version]
- Gerson, L.B.; Fidler, J.L.; Cave, D.R.; Leighton, J.A. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am. J. Gastroenterol. 2015, 110, 1265–1287. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, R.; Xu, C.; Li, Z.-S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest. Endosc. 2010, 71, 280–286. [Google Scholar] [CrossRef]
- Song, J.H.; Hong, S.N.; Chang, D.K.; Jeon, S.R.; Kim, J.-O.; Kim, J.; Lee, B.-I.; Choi, M.-G.; Kim, K.O.; Yang, D.-H.; et al. The etiology of potential small-bowel bleeding depending on patient’s age and gender. United Eur. Gastroenterol. J. 2018, 6, 1169–1178. [Google Scholar] [CrossRef]
- Rondonotti, E.; Villa, F.; Mulder, C.J.; Jacobs, M.A.; de Franchis, R. Small bowel capsule endoscopy in 2007: Indications, risks and limitations. World J. Gastroenterol. 2007, 13, 6140–6149. [Google Scholar] [CrossRef]
- Enns, R.A.; Hookey, L.; Armstrong, D.; Bernstein, C.N.; Heitman, S.J.; Teshima, C.; Leontiadis, G.I.; Tse, F.; Sadowski, D. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 2017, 152, 497–514. [Google Scholar] [CrossRef] [Green Version]
- Pennazio, M.; Santucci, R.; Rondonotti, E.; Abbiati, C.; Beccari, G.; Rossini, F.P.; de Franchis, R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: Report of 100 consecutive cases. Gastroenterology 2004, 126, 643–653. [Google Scholar] [CrossRef]
- Bresci, G.; Parisi, G.; Bertoni, M.; Tumino, E.; Capria, A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: Usefulness of early use. J. Gastroenterol. 2005, 40, 256–259. [Google Scholar] [CrossRef]
- Goenka, M.K. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J. Gastroenterol. 2011, 17, 774–778. [Google Scholar] [CrossRef]
- Leung, W.K.; Ho, S.S.M.; Suen, B.-Y.; Lai, L.H.; Yu, S.; Ng, E.K.-W.; Ng, S.S.M.; Chiu, P.; Sung, J.J.Y.; Chan, F.K.; et al. Capsule endoscopy or angiography in patients with acute overt obscure gastrointestinal bleeding: A prospective randomized study with long-term follow-up. Am. J. Gastroenterol. 2012, 107, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Delvaux, M.; Fassler, I.; Gay, G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: Validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy 2004, 36, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Sahota, A.; Shah, A. Does capsule endoscopy improve outcomes in obscure gastrointestinal bleeding? Randomized trial versus dedicated small bowel radiography. Gastroenterology 2010, 138, 1673–1680.e1. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.; Figueiredo, P.; Lopes, S.; Freire, P.; Lérias, C.; Gouveia, H.; Leitão, M.C. Urgent capsule endoscopy is useful in severe obscure-overt gastrointestinal bleeding. Dig. Endosc. 2009, 21, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Keum, B.; Chun, H.J.; Yoo, I.K.; Lee, J.M.; Lee, J.S.; Nam, S.J.; Choi, H.S.; Kim, E.S.; Seo, Y.S.; et al. Efficacy and implications of a 48-h cutoff for video capsule endoscopy application in overt obscure gastrointestinal bleeding. Endosc. Int. Open 2015, 3, E334–E338. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Marshall, C.; Chaudhuri, B.; Okoli, C.; Foley, A.; Person, S.D.; Bhattacharya, K.; Cave, D. Timing of video capsule endoscopy relative to overt obscure GI bleeding: Implications from a retrospective study. Gastrointest. Endosc. 2013, 77, 761–766. [Google Scholar] [CrossRef]
- Lecleire, S.; Iwanicki-Caron, I.; Di-Fiore, A.; Elie, C.; Alhameedi, R.; Ramirez, S.; Hervé, S.; Ben-Soussan, E.; Ducrotté, P.; Antonietti, M. Yield and impact of emergency capsule enteroscopy in severe obscure-overt gastrointestinal bleeding. Endoscopy 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Uchida, G.; Nakamura, M.; Yamamura, T.; Furukawa, K.; Kawashima, H.; Honda, T.; Ishigami, M.; Fujishiro, M. Systematic review and meta-analysis of the diagnostic and therapeutic yield of small bowel endoscopy in patients with overt small bowel bleeding. Dig. Endosc. 2021, 33, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, P.; Liatsos, C.; Gralnek, I.M.; Kalantzis, C.; Giannakoulopoulou, E.; Alexandrakis, G.; Tsibouris, P.; Kalafatis, E.; Kalantzis, N. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest. Endosc. 2007, 66, 1174–1181. [Google Scholar] [CrossRef] [PubMed]


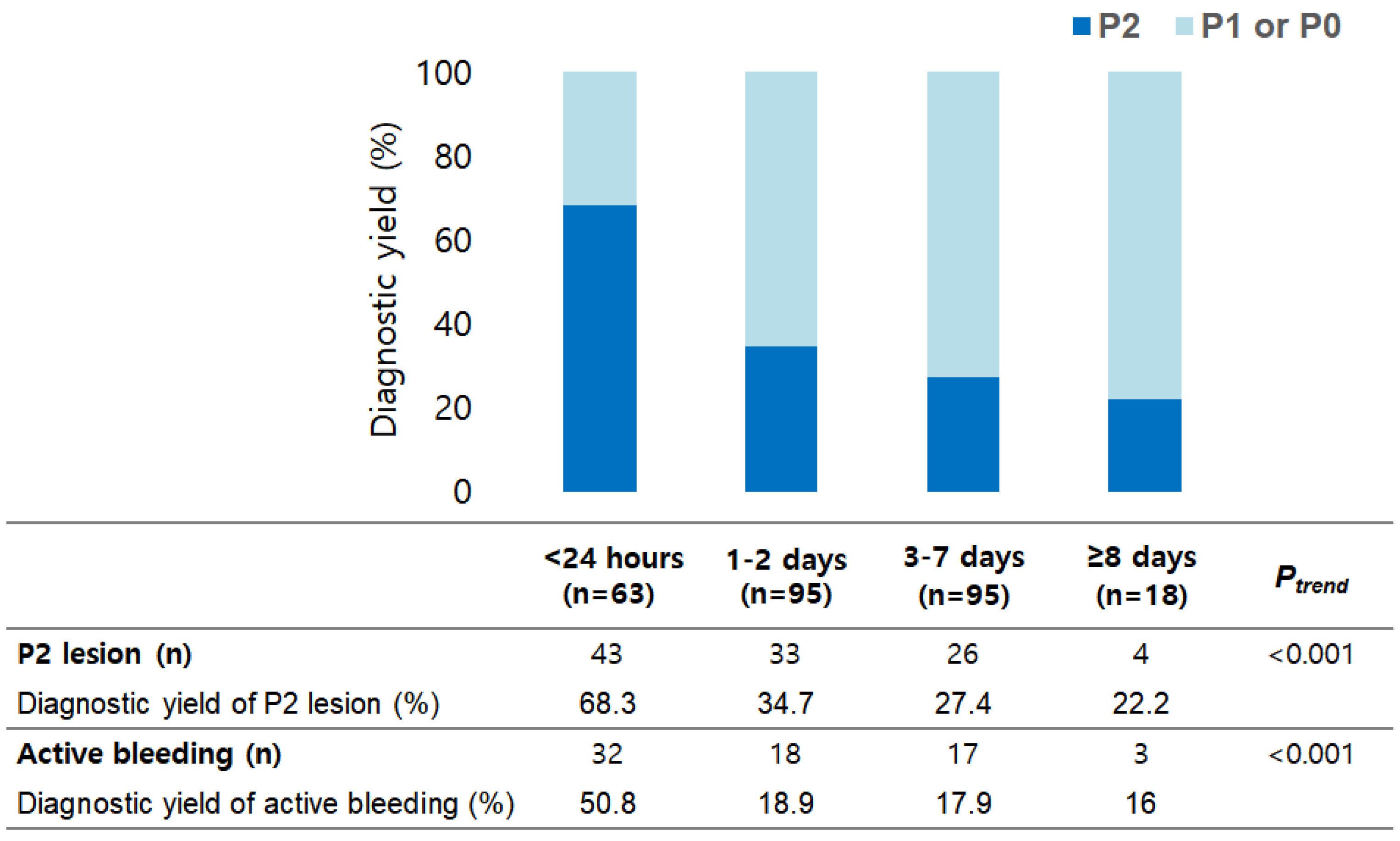
| Total (n = 271) | P0 or P1 Lesion (n = 165) | P2 Lesion (n = 106) | p | |
|---|---|---|---|---|
| Age (years) | 61.5 ± 15.6 | 62.2 ± 15.1 | 60.3 ± 16.3 | 0.338 |
| Sex, male | 178 (65.7) | 113 (68.5) | 65 (61.3) | 0.240 |
| Type of VCE, miroCam® | 187 (68.6) | 112 (67.9) | 74 (69.8) | 0.789 |
| Interval between VCE and last overt OGIB (days) | 3.0 ± 5.7 | 3.1 ± 3.4 | 2.7 ± 8.1 | 0.569 |
| Underlying liver cirrhosis | 23 (8.5) | 14 (8.5) | 9 (8.5) | 1.000 |
| Underlying ESRD | 13 (4.8) | 9 (5.5) | 4 (3.8) | 0.772 |
| Underlying coronary artery disease | 34 (12.5) | 19 (11.5) | 15 (14.2) | 0.575 |
| History of abdominal surgery | 39 (14.4) | 22 (13.3) | 17 (16.0) | 0.596 |
| Use of antiplatelet drug | 96 (35.4) | 57 (34.5) | 39 (36.8) | 0.795 |
| Use of anticoagulant drug | 34 (12.5) | 26 (15.8) | 8 (7.5) | 0.059 |
| Use of NSAIDs | 23 (8.5) | 13 (7.9) | 10 (9.4) | 0.661 |
| Hemoglobin (g/dL) | 9.5 ± 2.2 | 9.4 ± 2.2 | 9.7 ± 2.3 | 0.334 |
| Platelet (/mm3) | 202.4 ± 90.0 | 198.3 ± 92.9 | 208.6 ± 85.4 | 0.361 |
| PT (%) | 83.9 ± 18.3 | 81.9 ± 18.9 | 86.8 ± 17.1 | 0.039 |
| Transfusion requirement of pRBC | 3.2 ± 5.6 | 2.7 ± 3.1 | 4.1 ± 8.0 | 0.096 |
| Number of GI bleeding episodes | 1.6 ± 1.5 | 1.6 ± 1.7 | 1.4 ± 0.9 | 0.290 |
| Hospital days | 8.6 ± 15.8 | 6.7 ± 6.8 | 11.5 ± 23.5 | 0.040 |
| <24 h (n = 63) | 1–2 Days (n = 95) | 3–7 Days (n = 95) | ≥8 Days (n = 18) | p | |
|---|---|---|---|---|---|
| Age (years) | 62.7 ± 13.5 | 59.5 ± 16.9 | 62.1 ± 14.2 | 63.8 ± 21.4 | 0.488 |
| Sex, male | 40 (63.5) | 61 (64.2) | 65 (68.4) | 12 (66.7) | 0.908 |
| Type of VCE, miroCam® | 41 (65.1) | 66 (69.5) | 64 (67.4) | 15 (83.3) | 0.517 |
| Underlying liver cirrhosis | 5 (7.9) | 8 (8.4) | 10 (10.5) | 0 (0.0) | 0.531 |
| Underlying ESRD | 1 (1.6) | 3 (3.2) | 8 (8.4) | 1 (5.6) | 0.192 |
| Underlying coronary artery disease | 5 (7.9) | 9 (9.5) | 16 (16.8) | 4 (22.2) | 0.160 |
| History of abdominal surgery | 15 (23.8) | 11 (11.6) | 10 (10.5) | 3 (16.7) | 0.095 |
| Use of antiplatelet drug | 17 (27.0) | 29 (30.5) | 41 (43.2) | 9 (50.0) | 0.068 |
| Use of anticoagulant drug | 5 (7.9) | 13 (13.7) | 14 (14.7) | 2 (11.1) | 0.619 |
| Use of NSAIDs | 5 (7.9) | 9 (9.5) | 7 (7.4) | 2 (11.1) | 0.928 |
| Hemoglobin (g/dL) | 9.7 ± 2.1 | 9.8 ± 2.2 | 9.3 ± 2.3 | 9.0 ± 2.4 | 0.366 |
| Platelet (/mm3) | 187.1 ± 67.2 | 199.3 ± 106.8 | 203.4 ± 78.5 | 266.6 ± 98.4 | 0.010 |
| PT (%) | 84.5 ± 16.1 | 84.5 ± 19.4 | 82.6 ± 20.0 | 86.8 ± 10.1 | 0.816 |
| Transfusion requirement of pRBC | 5.4 ± 10.0 | 2.3 ± 2.9 | 2.8 ± 3.2 | 2.9 ± 3.1 | 0.007 |
| Number of GI bleeding episodes | 1.5 ± 1.0 | 1.7 ± 2.1 | 1.4 ± 0.8 | 1.4 ± 0.9 | 0.499 |
| Hospital days | 13.9 ± 29.6 | 5.5 ± 6.0 | 8.2 ± 8.1 | 7.7 ± 5.5 | 0.012 |
| Re bleeding events | 18 (28.6) | 22 (23.2) | 18 (18.9) | 5 (27.8) | 0.533 |
| <24 h (n = 63) | 1–2 Days (n = 95) | 3–7 Days (n = 95) | ≥ 8 Days (n = 18) | p | |
|---|---|---|---|---|---|
| Therapeutic intervention | 25 (39.7) | 19 (20.0) | 20 (21.1) | 2 (11.1) | 0.010 |
| -Surgery | 3 | 4 | 7 | 0 | |
| -Endoscopy | 21 | 15 | 12 | 2 | |
| -Embolization | 1 | 0 | 1 | 0 | |
| Conservative management | 38 (60.3) | 76 (80.0) | 75 (78.9) | 16 (88.9) | |
| -Medication | 10 | 19 | 18 | 4 | |
| -Close observation | 28 | 57 | 57 | 12 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Timing of VCE | ||||
| <24 h | 4.23 (1.34–13.34) | 0.014 | 4.99 (1.47–16.89) | 0.010 |
| 1–2 days | 1.38 (0.45–4.22) | 0.568 | 1.63 (0.51–5.23) | 0.414 |
| 3–7 days | 1.14 (0.37–3.50) | 0.816 | 1.24 (0.39–3.94) | 0.720 |
| ≥8 days | 1 | 0.001 | 1 | 0.001 |
| Age (years) | 0.99 (0.98–1.01) | 0.337 | 0.99 (0.97–1.01) | 0.283 |
| Sex, male | 0.73 (0.44–1.22) | 0.226 | 0.64 (0.36–1.13) | 0.123 |
| Underlying liver cirrhosis | 1.00 (0.42–2.40) | 0.999 | 1.09 (0.40–2.92) | 0.872 |
| Underlying ESRD | 0.68 (0.20–2.27) | 0.530 | 1.27 (0.34–4.83) | 0.722 |
| Underlying coronaryartery disease | 1.27 (0.61–2.62) | 0.523 | 1.50 (0.61–3.71) | 0.380 |
| History of abdominalsurgery | 1.24 (0.63–2.47) | 0.536 | 0.89 (0.40–1.94) | 0.761 |
| Use of antiplatelet drug | 1.10 (0.66–1.83) | 0.706 | 1.27 (0.63–2.58) | 0.506 |
| Use of anticoagulant drug | 0.44 (0.19–1.00) | 0.051 | 0.46 (0.18–1.21) | 0.115 |
| Use of NSAIDs | 1.22 (0.51–2.89) | 0.654 | 1.01 (0.39–2.60) | 0.991 |
| Transfusion requirementof pRBC | 1.05 (1.00–1.11) | 0.076 | 1.03 (0.97–1.09) | 0.296 |
| Number of GI bleedingepisodes | 0.89 (0.71–1.12) | 0.309 | 0.88 (0.68–1.13) | 0.319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.H.; Kim, J.E.; Chung, H.H.; Hong, S.N.; Kim, H.; Kim, E.R.; Chang, D.K.; Kim, Y.-H. Video Capsule Endoscopy Optimal Timing in Overt Obscure Gastrointestinal Bleeding. Diagnostics 2022, 12, 154. https://doi.org/10.3390/diagnostics12010154
Song JH, Kim JE, Chung HH, Hong SN, Kim H, Kim ER, Chang DK, Kim Y-H. Video Capsule Endoscopy Optimal Timing in Overt Obscure Gastrointestinal Bleeding. Diagnostics. 2022; 12(1):154. https://doi.org/10.3390/diagnostics12010154
Chicago/Turabian StyleSong, Joo Hye, Ji Eun Kim, Hwe Hoon Chung, Sung Noh Hong, Heejung Kim, Eun Ran Kim, Dong Kyung Chang, and Young-Ho Kim. 2022. "Video Capsule Endoscopy Optimal Timing in Overt Obscure Gastrointestinal Bleeding" Diagnostics 12, no. 1: 154. https://doi.org/10.3390/diagnostics12010154
APA StyleSong, J. H., Kim, J. E., Chung, H. H., Hong, S. N., Kim, H., Kim, E. R., Chang, D. K., & Kim, Y.-H. (2022). Video Capsule Endoscopy Optimal Timing in Overt Obscure Gastrointestinal Bleeding. Diagnostics, 12(1), 154. https://doi.org/10.3390/diagnostics12010154






