Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, R.A. Regression of the male reproductive tract in the female embryo is regulated by the orphan nuclear receptor COUP-TFII. Biol. Reprod. 2017, 97, 517–518. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, A.; Behringer, R.R. Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 2003, 4, 969–980. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, H.H. A tale of two tracts: History, current advances, and future directions of research on sexual differentiation of reproductive tractsdagger. Biol. Reprod. 2019, 101, 602–616. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, N.; Woo, H.Y.; Lee, E.J.; Do, S.I.; Na, K.; Kim, H.S. Atypical mesonephric hyperplasia of the uterus harbors pathogenic mutation of kirsten rat sarcoma 2 viral oncogene homolog (KRAS) and gain of chromosome 1q. Cancer Genom. Proteom. 2020, 17, 813–826. [Google Scholar] [CrossRef]
- Ugwu, A.O.; Haruna, M.; Okunade, K.S.; Ohazurike, E.; Anorlu, R.I.; Banjo, A.A.F. Primary vaginal adenocarcinoma of intestinal-type: Case report of a rare gynaecological tumour. Oxf. Med. Case Rep. 2019, 2019, omz088. [Google Scholar] [CrossRef]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef]
- Morrell, L.H.; Matthews, K.J.; Chafe, W.E. Primary adenocarcinoma of intestinal type arising from a vaginal mass: A case report. J. Low. Genit. Tract Dis. 2015, 19, e52–e54. [Google Scholar] [CrossRef]
- Adams, T.S.; Cuello, M.A. Cancer of the vagina. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 14–21. [Google Scholar] [CrossRef]
- Tatsumi, K.; Schlappe, B.; Everett, E.N.; Gibson, P.C.; Mount, S.L. Primary vaginal mucinous adenocarcinoma of intestinal type, associated with intestinal metaplasia of Skene ducts in a diethylstilbestrol-exposed woman. Am. J. Clin. Pathol. 2015, 144, 790–795. [Google Scholar] [CrossRef][Green Version]
- Novak, E.; Woodruff, J.D.; Novak, E.R. Probable mesonephric origin of certain female genital tumors. Am. J. Obstet. Gynecol. 1954, 68, 1222–1242. [Google Scholar]
- Studdiford, W.E. Vaginal lesions of adenomatous origin. Am. J. Obstet. Gynecol. 1957, 73, 641–655. [Google Scholar] [CrossRef]
- Harris, R.E.; Daly, J.W. Primary mesonephric adenocarcinoma of the vagina. Am. J. Obstet. Gynecol. 1966, 95, 591–592. [Google Scholar] [CrossRef]
- Droegemueller, W.; Makowski, E.L.; Taylor, E.S. Vaginal mesonephric adenocarcinoma in two prepubertal children. Am. J. Dis. Child. 1970, 119, 168–170. [Google Scholar] [CrossRef]
- Shaaban, M.M. Primary adenocarcinoma of the vagina of mesonephric pattern. Report of a case and review of literature. Aust. N. Z. J. Obstet. Gynaecol. 1970, 10, 55–58. [Google Scholar] [CrossRef]
- Siegel, H.A.; Sagerman, R.; Berdon, W.E.; Wigger, H.J. Mesonephric adenocarcinoma of the vagina in a 7-month-old infant simulating sarcoma botryoides. Successful control with supervoltage radiotherapy. J. Pediatr. Surg. 1970, 5, 468–470. [Google Scholar] [CrossRef]
- Hinchey, W.W.; Silva, E.G.; Guarda, L.A.; Ordonez, N.G.; Wharton, J.T. Paravaginal Wolffian duct (mesonephros) adenocarcinoma: A light and electron microscopic study. Am. J. Clin. Pathol. 1983, 80, 539–544. [Google Scholar] [CrossRef]
- Bague, S.; Rodriguez, I.M.; Prat, J. Malignant mesonephric tumors of the female genital tract: A clinicopathologic study of 9 cases. Am. J. Surg. Pathol. 2004, 28, 601–607. [Google Scholar] [CrossRef]
- McNall, R.Y.; Nowicki, P.D.; Miller, B.; Billups, C.A.; Liu, T.; Daw, N.C. Adenocarcinoma of the cervix and vagina in pediatric patients. Pediatr. Blood Cancer 2004, 43, 289–294. [Google Scholar] [CrossRef]
- Ersahin, C.; Huang, M.; Potkul, R.K.; Hammadeh, R.; Salhadar, A. Mesonephric adenocarcinoma of the vagina with a 3-year follow-up. Gynecol. Oncol. 2005, 99, 757–760. [Google Scholar] [CrossRef]
- Bifulco, G.; Mandato, V.D.; Mignogna, C.; Giampaolino, P.; Di Spiezio Sardo, A.; De Cecio, R.; De Rosa, G.; Piccoli, R.; Radice, L.; Nappi, C. A case of mesonephric adenocarcinoma of the vagina with a 1-year follow-up. Int. J. Gynecol. Cancer 2008, 18, 1127–1131. [Google Scholar] [CrossRef]
- Roma, A.A. Mesonephric carcinosarcoma involving uterine cervix and vagina: Report of 2 cases with immunohistochemical positivity for PAX2, PAX8, and GATA-3. Int. J. Gynecol. Pathol. 2014, 33, 624–629. [Google Scholar] [CrossRef]
- Mueller, I.; Kametriser, G.; Jacobs, V.R.; Bogner, G.; Staudach, A.; Koch, H.; Wolfrum-Ristau, P.; Schausberger, C.; Fischer, T.; Sedlmayer, F. Mesonephric adenocarcinoma of the vagina: Diagnosis and multimodal treatment of a rare tumor and analysis of worldwide experience. Strahlenther. Onkol. 2016, 192, 668–671. [Google Scholar] [CrossRef]
- Shoeir, S.; Balachandran, A.A.; Wang, J.; Sultan, A.H. Mesonephric adenocarcinoma of the vagina masquerading as a suburethral cyst. BMJ Case Rep. 2018, 2018, bcr2018224758. [Google Scholar] [CrossRef]
- Kim, H.; Na, K.; Bae, G.E.; Kim, H.S. Mesonephric-like adenocarcinoma of the uterine corpus: Comprehensive immunohistochemical analyses using markers for mesonephric, endometrioid and serous tumors. Diagnostics 2021, 11, 2042. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Prives, C. Mutant p53: One name, many proteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef]
- Slovackova, J.; Grochova, D.; Navratilova, J.; Smarda, J.; Smardova, J. Transactivation by temperature-dependent p53 mutants in yeast and human cells. Cell Cycle 2010, 9, 2141–2148. [Google Scholar] [CrossRef][Green Version]
- Blecharz, P.; Reinfuss, M.; Rys, J.; Jakubowicz, J.; Skotnicki, P.; Wysocki, W. Radiotherapy for carcinoma of the vagina. Immunocytochemical and cytofluorometric analysis of prognostic factors. Strahlenther. Onkol. 2013, 189, 394–400. [Google Scholar] [CrossRef]
- Hellman, K.; Johansson, H.; Andersson, S.; Pettersson, F.; Auer, G. Prognostic significance of cell cycle- and invasion-related molecular markers and genomic instability in primary carcinoma of the vagina. Int. J. Gynecol. Cancer 2013, 23, 41–51. [Google Scholar] [CrossRef]
- Alonso, I.; Felix, A.; Torne, A.; Fuste, V.; del Pino, M.; Castillo, P.; Balasch, J.; Pahisa, J.; Rios, J.; Ordi, J. Human papillomavirus as a favorable prognostic biomarker in squamous cell carcinomas of the vagina. Gynecol. Oncol. 2012, 125, 194–199. [Google Scholar] [CrossRef]
- Koyamatsu, Y.; Yokoyama, M.; Nakao, Y.; Fukuda, K.; Saito, T.; Matsukuma, K.; Iwasaka, T. A comparative analysis of human papillomavirus types 16 and 18 and expression of p53 gene and Ki-67 in cervical, vaginal, and vulvar carcinomas. Gynecol. Oncol. 2003, 90, 547–551. [Google Scholar] [CrossRef]
- Skomedal, H.; Kristensen, G.; Helland, A.; Nesland, J.M.; Kooi, S.; Borresen, A.L.; Holm, R. TP53 gene mutations and protein accumulation in primary vaginal carcinomas. Br. J. Cancer 1995, 72, 129–133. [Google Scholar] [CrossRef][Green Version]
- Rasmussen, C.L.; Bertoli, H.K.; Sand, F.L.; Kjaer, A.K.; Thomsen, L.T.; Kjaer, S.K. The prognostic significance of HPV, p16, and p53 protein expression in vaginal cancer: A systematic review. Acta Obstet. Gynecol. Scand. 2021, 100, 2144–2156. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Martelotto, L.G.; De Filippo, M.R.; Piscuglio, S.; Ng, C.K.; Hussein, Y.R.; Reis-Filho, J.S.; Soslow, R.A.; Weigelt, B. TP53 mutational spectrum in endometrioid and serous endometrial cancers. Int. J. Gynecol. Pathol. 2016, 35, 289–300. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Dahoud, W.; Vanderbilt, C.M.; Chiang, S.; Murali, R.; Rios-Doria, E.V.; Alektiar, K.M.; Aghajanian, C.; Abu-Rustum, N.R.; Ladanyi, M.; et al. Clinicopathologic and genomic analysis of TP53-mutated endometrial carcinomas. Clin. Cancer Res. 2021, 27, 2613–2623. [Google Scholar] [CrossRef]
- Jung, H.; Bae, G.E.; Kim, H.M.; Kim, H.S. Clinicopathological and molecular differences between gastric-type mucinous carcinoma and usual-type endocervical adenocarcinoma of the uterine cervix. Cancer Genom. Proteom. 2020, 17, 627–641. [Google Scholar] [CrossRef]
- Li, V.D.; Li, K.H.; Li, J.T. TP53 mutations as potential prognostic markers for specific cancers: Analysis of data from The Cancer Genome Atlas and the International Agency for Research on Cancer TP53 Database. J. Cancer Res. Clin. Oncol. 2019, 145, 625–636. [Google Scholar] [CrossRef]
- Choi, S.; Na, K.; Kim, S.W.; Kim, H.S. Dedifferentiated mesonephric-like adenocarcinoma of the uterine corpus. Anticancer Res. 2021, 41, 2719–2726. [Google Scholar] [CrossRef]
- Meixner, E.; Arians, N.; Bougatf, N.; Hoeltgen, L.; Konig, L.; Lang, K.; Domschke, C.; Wallwiener, M.; Lischalk, J.W.; Kommoss, F.K.F.; et al. Vaginal cancer treated with curative radiotherapy with or without concomitant chemotherapy: Oncologic outcomes and prognostic factors. Tumori 2021, 3008916211056369. [Google Scholar] [CrossRef]
- Stock, R.G.; Chen, A.S.; Seski, J. A 30-year experience in the management of primary carcinoma of the vagina: Analysis of prognostic factors and treatment modalities. Gynecol. Oncol. 1995, 56, 45–52. [Google Scholar] [CrossRef]
- Kanayama, N.; Isohashi, F.; Yoshioka, Y.; Baek, S.; Chatani, M.; Kotsuma, T.; Tanaka, E.; Yoshida, K.; Seo, Y.; Suzuki, O.; et al. Definitive radiotherapy for primary vaginal cancer: Correlation between treatment patterns and recurrence rate. J. Radiat. Res. 2015, 56, 346–353. [Google Scholar] [CrossRef]
- Orton, A.; Boothe, D.; Williams, N.; Buchmiller, T.; Huang, Y.J.; Suneja, G.; Poppe, M.; Gaffney, D. Brachytherapy improves survival in primary vaginal cancer. Gynecol. Oncol. 2016, 141, 501–506. [Google Scholar] [CrossRef]
- Yang, J.; Delara, R.; Magrina, J.; Magtibay, P.; Langstraat, C.; Dinh, T.; Karlin, N.; Vora, S.A.; Butler, K. Management and outcomes of primary vaginal cancer. Gynecol. Oncol. 2020, 159, 456–463. [Google Scholar] [CrossRef]
- Fukunaga, M.; Takahashi, H.; Yasuda, M. Mesonephric adenocarcinoma of the uterine cervix: A case report with immunohistochemical and ultrastructural studies. Pathol. Res. Pract. 2008, 204, 671–676. [Google Scholar] [CrossRef]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; Lindeman, N.; MacConaill, L.; Hirsch, M.; Dal Cin, P.; Gorman, M.; Barletta, J.A.; Nucci, M.R.; et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Kir, G.; Seneldir, H.; Kiran, G. A case of mesonephric adenocarcinoma of the uterine cervix mimicking an endometrial clear cell carcinoma in the curettage specimen. J. Obstet. Gynaecol. 2016, 36, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.S.; Schultheis, A.M.; Ho, C.; Wang, L.; DeLair, D.F.; Weigelt, B.; Gardner, G.; Lichtman, S.M.; Hameed, M.; Park, K.J. Mixed mesonephric adenocarcinoma and high-grade neuroendocrine carcinoma of the uterine cervix: Case description of a previously unreported entity with insights into its molecular pathogenesis. Int. J. Gynecol. Pathol. 2017, 36, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, N.; Redroban, L.; Galarza, D. Mesonephric adenocarcinoma of the cervix: A case report with a three-year follow-up, lung metastases, and next-generation sequencing analysis. Diagn. Pathol. 2019, 14, 71. [Google Scholar] [CrossRef]
- Skala, S.L.; Gregg, P.A.; Orr, J.W., Jr.; Udager, A.M.; Brown, N.A.; Cho, K.R. Cervical mesonephric adenocarcinoma with novel FGFR2 mutation. Int. J. Gynecol. Pathol. 2020, 39, 452–455. [Google Scholar] [CrossRef]
- Lin, D.I.; Shah, N.; Tse, J.Y.; Killian, J.K.; Hemmerich, A.; Edgerly, C.; Haberberger, J.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; et al. Molecular profiling of mesonephric and mesonephric-like carcinomas of cervical, endometrial and ovarian origin. Gynecol. Oncol. Rep. 2020, 34, 100652. [Google Scholar] [CrossRef]
- Marani, C.; Akaev, I.; Yeoh, C.C.; Walsh, E.; Rahimi, S. Cervical malignant mixed mesonephric tumour: A case report with local recurrence after six-years and next-generation sequencing analysis with particular reference to the ataxia telangiectasia mutated gene. Exp. Ther. Med. 2021, 21, 394. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q.; Shen, Y. Mesonephric adenocarcinomas in female genital tract: A case series. Medicine 2021, 100, e27174. [Google Scholar] [CrossRef]
- da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Da Cruz Paula, A.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef]
- Ordi, J.; Nogales, F.F.; Palacin, A.; Marquez, M.; Pahisa, J.; Vanrell, J.A.; Cardesa, A. Mesonephric adenocarcinoma of the uterine corpus: CD10 expression as evidence of mesonephric differentiation. Am. J. Surg. Pathol. 2001, 25, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Marmol, M.; Rey, V.; Ordi, J.; Pahissa, J.; Rovirosa, A.; Gascon, P.; Mellado, B. Activity of chemotherapy with carboplatin plus paclitaxel in a recurrent mesonephric adenocarcinoma of the uterine corpus. Gynecol. Oncol. 2003, 90, 458–461. [Google Scholar] [CrossRef]
- Wani, Y.; Notohara, K.; Tsukayama, C. Mesonephric adenocarcinoma of the uterine corpus: A case report and review of the literature. Int. J. Gynecol. Pathol. 2008, 27, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, C.; Qi, J.I.; Qu, P. Mesonephric carcinoma of the uterine corpus: A report of two cases. Oncol. Lett. 2016, 11, 335–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.S.; Nam, J.H.; Kim, G.E.; Choi, Y.D.; Choi, C.; Park, C.S. Mesonephric adenocarcinoma of the uterine corpus: A case report and diagnostic pitfall. Int. J. Surg. Pathol. 2016, 24, 153–158. [Google Scholar] [CrossRef]
- Mirkovic, J.; McFarland, M.; Garcia, E.; Sholl, L.M.; Lindeman, N.; MacConaill, L.; Dong, F.; Hirsch, M.; Nucci, M.R.; Quick, C.M.; et al. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital tract. Am. J. Surg. Pathol. 2018, 42, 227–233. [Google Scholar] [CrossRef]
- Ando, H.; Watanabe, Y.; Ogawa, M.; Tamura, H.; Deguchi, T.; Ikeda, K.; Fujitani, M.; Shioji, M.; Tsujie, T.; Doi, R.; et al. Mesonephric adenocarcinoma of the uterine corpus with intracystic growth completely confined to the myometrium: A case report and literature review. Diagn. Pathol. 2017, 12, 63. [Google Scholar] [CrossRef]
- Patel, V.; Kipp, B.; Schoolmeester, J.K. Corded and hyalinized mesonephric-like adenocarcinoma of the uterine corpus: Report of a case mimicking endometrioid carcinoma. Hum. Pathol. 2019, 86, 243–248. [Google Scholar] [CrossRef]
- Yano, M.; Shintani, D.; Katoh, T.; Hamada, M.; Ito, K.; Kozawa, E.; Hasegawa, K.; Yasuda, M. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Mullerian duct lineage: A case report. Diagn. Pathol. 2019, 14, 54. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Ambelil, M.; Conyers, J.; Zhu, H. Mesonephric adenocarcinoma of the uterine corpus: Report of 2 cases and review of the literature. Int. J. Gynecol. Pathol. 2019, 38, 224–229. [Google Scholar] [CrossRef]
- Kolin, D.L.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A combined morphologic and molecular approach to retrospectively identify KRAS-mutated mesonephric-like adenocarcinomas of the endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, H.; Zhu, X.; Yu, M.; Zhang, X. Mesonephric adenocarcinoma of the uterine corpus: A report on 2 cases with comparison to its cervical counterpart. Int. J. Gynecol. Pathol. 2020, 39, 546–551. [Google Scholar] [CrossRef]
- Horn, L.C.; Hohn, A.K.; Krucken, I.; Stiller, M.; Obeck, U.; Brambs, C.E. Mesonephric-like adenocarcinomas of the uterine corpus: Report of a case series and review of the literature indicating poor prognosis for this subtype of endometrial adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 971–983. [Google Scholar] [CrossRef]
- Chapel, D.B.; Joseph, N.M.; Krausz, T.; Lastra, R.R. An ovarian adenocarcinoma with combined low-grade serous and mesonephric morphologies suggests a Mullerian origin for some mesonephric carcinomas. Int. J. Gynecol. Pathol. 2018, 37, 448–459. [Google Scholar] [CrossRef]
- Seay, K.; Akanbi, T.; Bustamante, B.; Chaudhary, S.; Goldberg, G.L. Mesonephric-like adenocarcinoma of the ovary with co-existent endometriosis: A case report and review of the literature. Gynecol. Oncol. Rep. 2020, 34, 100657. [Google Scholar] [CrossRef]
- Dundr, P.; Gregova, M.; Nemejcova, K.; Bartu, M.; Hajkova, N.; Hojny, J.; Struzinska, I.; Fischerova, D. Ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor: A case report with complex morphological and molecular analysis. Diagn. Pathol. 2020, 15, 91. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Vosmikova, H.; Laco, J. Ovarian combined low-grade serous and mesonephric-like adenocarcinoma: Further evidence for a Mullerian origin of mesonephric-like adenocarcinoma. Int. J. Gynecol. Pathol. 2020, 39, 84–92. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, Y.; Xie, C. Mesonephric-like adenocarcinoma of the ovary: A case report and a review of the literature. Medicine 2020, 99, e23450. [Google Scholar] [CrossRef]
- Deolet, E.; Arora, I.; Van Dorpe, J.; Van der Meulen, J.; Desai, S.; Van Roy, N.; Kaur, B.; Van de Vijver, K.; McCluggage, W.G. Extrauterine mesonephric-like neoplasms: Expanding the morphologic spectrum. Am. J. Surg. Pathol. 2022, 46, 124–133. [Google Scholar] [CrossRef]
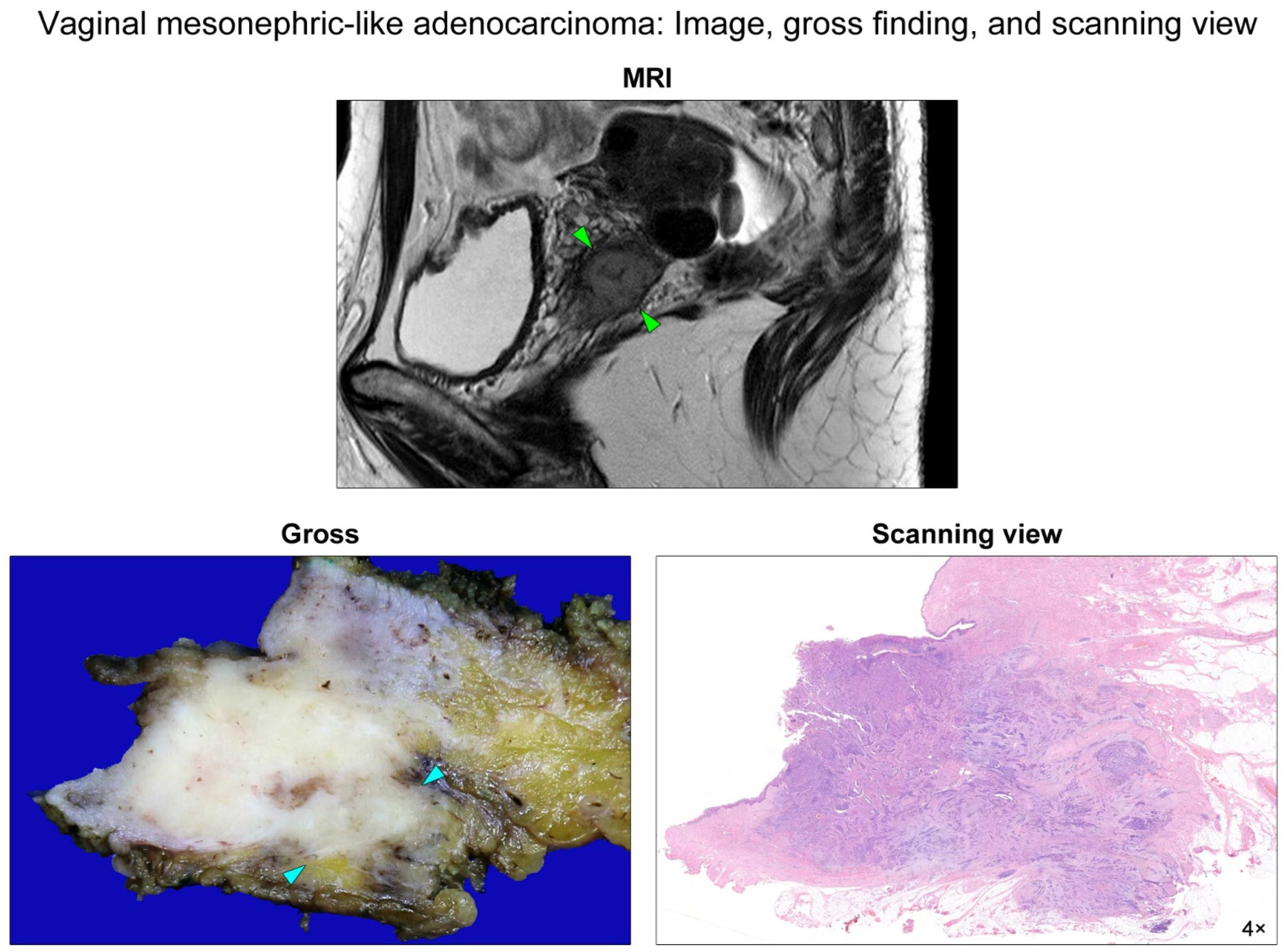
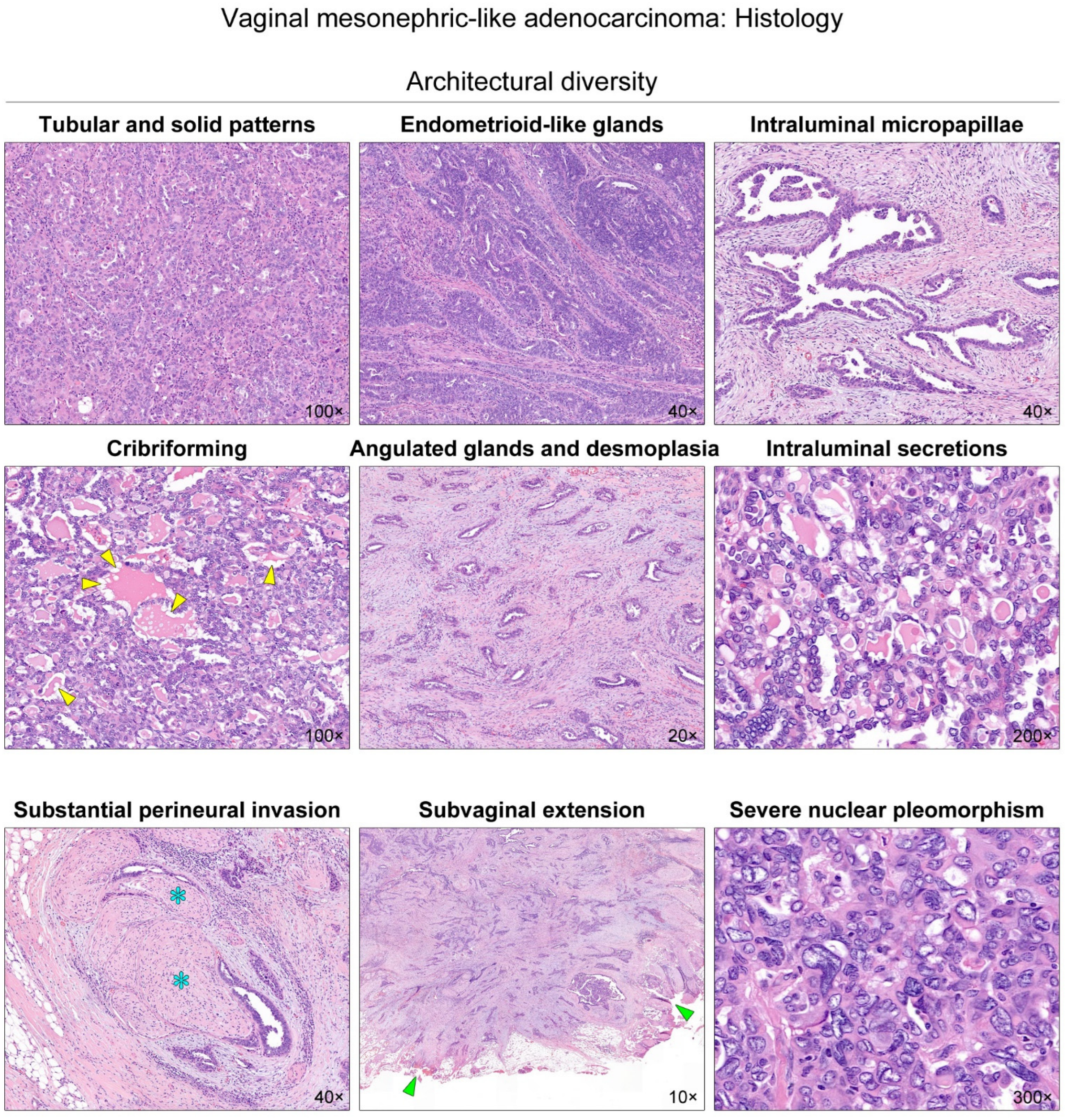

| Gene | Mutation Type | Sequence Change | Amino Acid Change | Variant Allele Frequency | Clinical Significance |
|---|---|---|---|---|---|
| KRAS | Missense | c.35G > A | p.G12D | 28% | Pathogenic |
| TP53 | Nonsense | c.856G > T | p.E286* | 39% | Pathogenic |
| Case No | Authors (Year Published) | Age | Presenting Symptom or Sign | Tumor Size | Treatment | Initial Stage | Postsurgical Treatment | Recurrence | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Novak et al. (1954) [10] | 42 years | NA | NA | Surgery | NA | NA | NA | NA |
| 2 | Novak et al. (1954) [10] | 13 years | NA | NA | NA | NA | NA | NA | Died shortly after |
| 3 | Novak et al. (1954) [10] | 51 years | NA | NA | Surgery (incomplete excision) | NA | NA | NA | NA |
| 4 | Novak et al. (1954) [10] | 21 years | NA | NA | NA | NA | NA | NA | NA |
| 5 | Studdiford (1957) [11] | 40 years | NA | NA | Radiation | NA | NA | No | NED (24 months) |
| 6 | Studdiford (1957) [11] | 16 years | NA | NA | Surgery (incomplete excision) | NA | Radiation | No | NED (60 months) |
| 7 | Studdiford (1957) [11] | 16 years | NA | NA | Radiation | NA | NA | Yes | Died 2 years later from widespread metastases |
| 8 | Studdiford (1957) [11] | 42 years | NA | NA | Radiation | NA | NA | No | NED (24 months) |
| 9 | Harris and Daly (1966) [12] | 61 years | Vaginal bleeding | 4 cm | Radiation | NA | NA | No | NED (12 months) |
| 10 | Droegemueller et al. (1970) [13] | 7.5 years | Vaginal spotting | NA | Surgery (en bloc mass excision) | NA | No | No | NED (53 months) |
| 11 | Droegemueller et al. (1970) [13] | 8 years | Vaginal bleeding | 6 cm | Radiation | III | NA | Yes | Deteriorated (metastases to the pelvis, abdomen, inguinal LN, and lungs) |
| 12 | Shaaban (1970) [14] | 26 years | Contact bleeding oncoitus or douching | 5 cm | Surgery (RH with vaginectomy) | III | No | No | NED (24 months) |
| 13 | Siegel et al. (1970) [15] | 7 months | Vaginal bleeding | NA | Radiation | III | NA | No | NED (24 months) |
| 14 | Hinchey et al. (1983) [16] | 29 years | Pelvic fullness | 6 cm | Surgery (mass excision with BSO) | NA | Radiation | No | NED (4 months) |
| 15 | Bague et al. (2004) [17] | 54 years | Enlarged uterus due to leiomyoma | 4 cm | Surgery (TH with vaginectomy and BSO) | II | No | Yes | AWD (103 months) |
| 16 | Bague et al. (2004) [17] | 38 years | Painful coitus | NA | Surgery (mass excision) | NA | NA | NA | NA |
| 17 | McNall et al. (2004) [18] | 13 years | Vaginal bleeding | 6 cm | Surgery (mass excision with partial Vaginectomy, BO, right iliac LNS, and left iliac LND) | III | CCRT | No | NED (55 months) |
| 18 | Ersahin et al. (2005) [19] | 55 years | Asymptomatic vaginal mass | 0.9 cm | Surgery (radical upper vaginectomy with BSO, pelvic LND, and para-aortic LNS) | III | CCRT | No | NED (36 months) |
| 19 | Bifulco et al. (2008) [20] | 58 years | Pelvic pain and vulvar pruritus | 14 cm | Surgery (radical mass excision with pelvic and para-aortic LND) | III | No | No | NED (12 months) |
| 20 | Roma (2014) [21] | 58 years | Vaginal bleeding | 5 cm | Surgery (pelvic exenteration) | III | NA | NA | NA |
| 21 | Mueller et al. (2016) [22] | 54 years | Vaginal bleeding | 2.5 cm | Surgery (mass excision) | II | CCRT | No | NED (48 months) |
| 22 | Shoeir et al. (2018) [23] | 63 years | Painless vaginal swelling | 3.1 cm | Surgery (mass excision) | I | Radiation | No | NED |
| 23 | Lee et al. (2021) (the present case) | 52 years | Vaginal bleeding | 2 cm | Surgery (radical resection with BSO and PLND) | II | SCRT | No | NED (10 months) |
| Case No | Authors (Year Published) | Preoperative Biopsy Diagnosis | Final Diagnosis | PAX8 | PAX2 | GATA3 | TTF1 | CD10 | ER | PR | PTEN | p16 | p53 | TP53 Mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 | Novak et al. (1954) [10] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5–8 | Studdiford (1957) [11] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 | Harris and Daly (1966) [12] | MA | MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10 | Droegemueller et al. (1970) [13] | Papillary MA | Papillary MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | Droegemueller et al. (1970) [13] | Clear cell MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 12 | Shaaban (1970) [14] | Variable-sized, irregular glands with intraluminal invaginations and marked cystic dilatation | MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13 | Siegel et al. (1970) [15] | MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 14 | Hinchey et al. (1983) [16] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 15 | Bague et al. (2004) [17] | NA | MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 16 | Bague et al. (2004) [17] | NA | MCS | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 17 | McNall et al. (2004) [18] | MA | MA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 18 | Ersahin et al. (2005) [19] | Infiltrating adenocarcinoma | MA | NA | NA | NA | NA | Negative | Negative | Negative | NA | NA | NA | NA |
| 19 | Bifulco et al. (2008) [20] | Moderately differentiated adenocarcinoma | MA | NA | NA | NA | NA | Positive | Negative | Negative | NA | NA | NA | NA |
| 20 | Roma (2014) [21] | MCS | MCS | Positive | Positive | Focal positive | NA | Focal positive | Negative | NA | NA | Negative | NA | NA |
| 21 | Mueller et al. (2016) [22] | Vaginal adenosis with microglandular hyperplasia | MA | NA | NA | NA | NA | Weak positive | Negative | Negative | NA | NA | NA | NA |
| 22 | Shoeir et al. (2018) [23] | NA | MA | Diffuse positive | NA | Negative | NA | Focal positive | Negative | Negative | NA | Focal positive | NA | NA |
| 23 | Lee et al. (2021) (the present case) | Poorly differentiated adenocarcinoma | MA | Diffuse strong positive | Diffuse strong positive | Focal moderate positive | Diffuse strong positive | Negative | Focal Weak positive | Negative | Noloss | Patchy positive | Mutation pattern (complete absence) | p.E286* |
| Organ | Authors (Year Published) | p53 Expression (Number of Examined Cases) | TP53 Mutation (Number of Mutant/Examined Cases) | Type of TP53 Mutation |
|---|---|---|---|---|
| Cervix | Fukunaga et al. (2008) [43] | Positive (1) * | NA | |
| Roma (2014) [21] | Wild-type pattern (1) | NA | ||
| Mirkovic et al. (2015) [44] | NA | 1/13 | NA | |
| Kir et al. (2016) [45] | Totally negative (1) * | NA | ||
| Cavalcanti et al. (2017) [46] | NA | 0/1 | ||
| Montalvo et al. (2019) [47] | Wild-type pattern (1) | 0/1 | ||
| Skala et al. (2020) [48] | NA | 0/1 | ||
| Kim et al. (2020) [4] | NA | 0/4 | ||
| Lin et al. (2020) [49] | NA | 1/10 | p.R280G | |
| Marani et al. (2021) [50] | Wild-type pattern (1) | 0/1 | ||
| Xie et al. (2021) [51] | Wild-type pattern (2) | NA | ||
| da Silva et al. (2021) [52] | Wild-type pattern (4) | 0/8 | ||
| Uterine corpus | Ordi et al. (2001) [53] | Wild-type pattern (1) | NA | |
| Montagut et al. (2003) [54] | Wild-type pattern (1) | NA | ||
| Wani et al. (2008) [55] | Wild-type pattern (weak and focal positivity) (1) | NA | ||
| Mirkovic et al. (2015) [44] | NA | 0/2 | ||
| Zhao et al. (2016) [56] | Negative (1) * | NA | ||
| Kim et al. (2016) [57] | Wild-type pattern (1) | NA | ||
| McFarland et al. (2016) [6]; Mirkovic et al. (2018) [58] | Wild-type pattern (4) | 0/3 | ||
| Ando et al. (2017) [59] | Wild-type pattern (1) | NA | ||
| Patel et al. (2019) [60] | NA | 0/1 | ||
| Yano et al. (2019) [61] | Wild-type pattern (1) | 0/1 | ||
| Zhang et al. (2019) [62] | Wild-type pattern (1) | NA | ||
| Na et al. (2019) [5] | Wild-type pattern (11) | 0/11 | ||
| Kolin et al. (2019) [63] | Wild-type pattern (3) | 0/4 | ||
| Liang et al. (2020) [64] | Wild-type pattern (2) | 0/2 | ||
| Horn et al. (2020) [65] | Wild-type pattern (4) | 0/4 | ||
| Xie et al. (2021) [51] | Wild-type pattern (5) | NA | ||
| da Silva et al. (2021) [52] | Wild-type pattern (6) | 1/13 | p.I254N | |
| Choi et al. (2021) [37] | Wild-type pattern (1) | 0/1 | ||
| Kim et al. (2021) [24] | Wild-type pattern (25) | 0/25 | ||
| Ovary | Mirkovic et al. (2015) [44] | NA | 0/1 | |
| McFarland et al. (2016) [6]; Mirkovic et al. (2018) [58] | Wild-type pattern (3) | 0/4 | ||
| Chapel et al. (2018) [66] | Wild-type pattern (1) | 0/1 | ||
| Seay et al. (2020) [67] | Wild-type pattern (1) | 0/1 | ||
| Dundr et al. (2020) [68] | Wild-type pattern (1) | 0/1 | ||
| McCluggage et al. (2020) [69] | Wild-type pattern (1) | 0/1 | ||
| Chen et al. (2020) [70] | Wild-type pattern (1) | NA | ||
| Xie et al. (2021) [51] | Wild-type pattern (2) | NA | ||
| Deolet et al. (2021) [71] | Wild-type pattern (4) | 0/5 | ||
| da Silva et al. (2021) [52] | Wild-type pattern (7) | 0/15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, H.; Kim, H.-S. Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation. Diagnostics 2022, 12, 119. https://doi.org/10.3390/diagnostics12010119
Lee H, Kim H, Kim H-S. Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation. Diagnostics. 2022; 12(1):119. https://doi.org/10.3390/diagnostics12010119
Chicago/Turabian StyleLee, Hyunjee, Hyunjin Kim, and Hyun-Soo Kim. 2022. "Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation" Diagnostics 12, no. 1: 119. https://doi.org/10.3390/diagnostics12010119
APA StyleLee, H., Kim, H., & Kim, H.-S. (2022). Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation. Diagnostics, 12(1), 119. https://doi.org/10.3390/diagnostics12010119






