Use of Physical Accessibility Modelling in Diagnostic Network Optimization: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Publication Selection
2.4. Charting the Data
3. Results
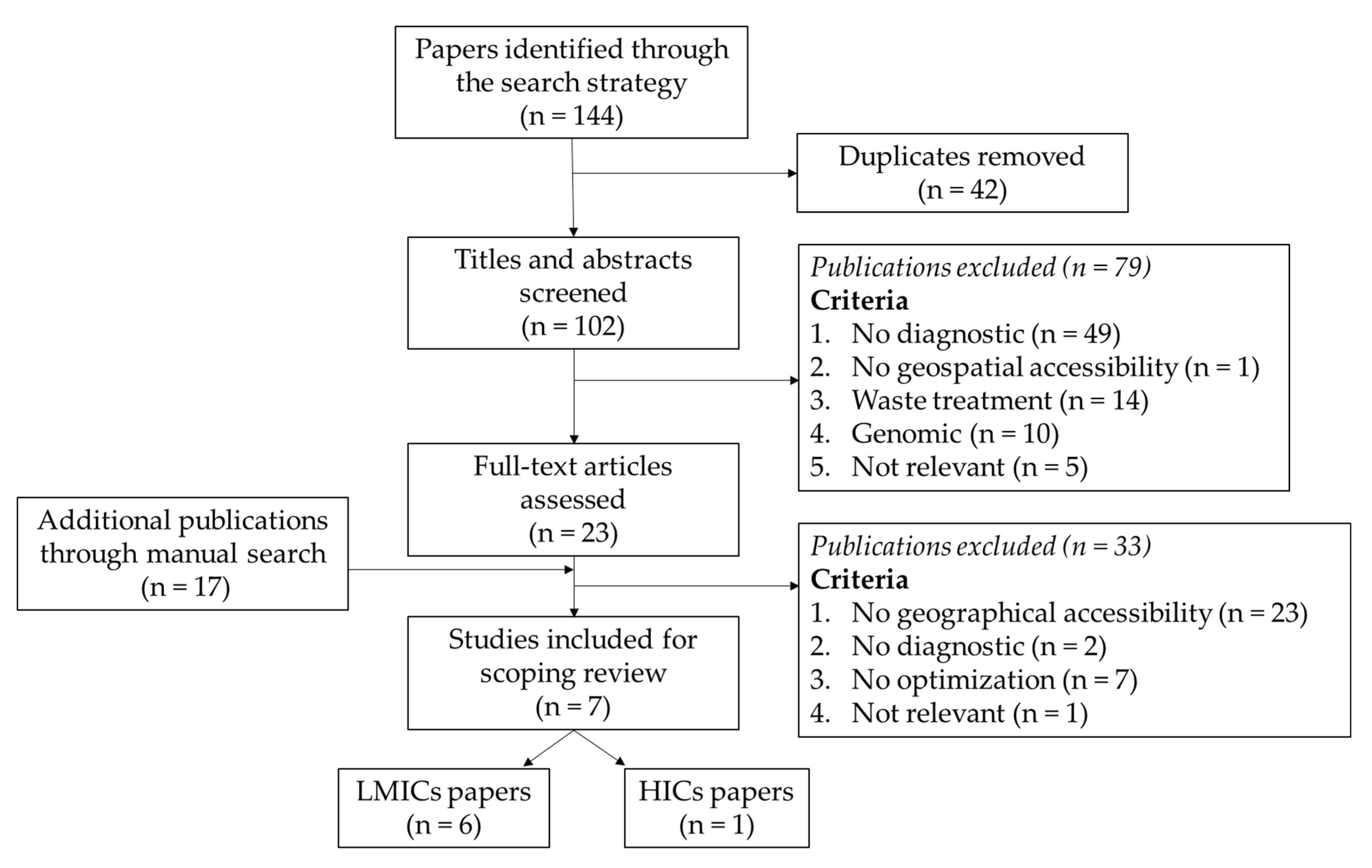
3.1. Overview of the Literature Search
3.2. Disease and Associated Diagnostics
3.3. Geographical Patterns of Studies
3.4. Accessibility Measures
3.5. Referral System
3.6. Optimization Goal
3.7. Data Used
4. Discussion
4.1. Limitations of the Study
4.2. Conclusions and Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghebreyesus, T.A. All roads lead to universal health coverage. Lancet Glob. Health 2017, 5, e839–e840. [Google Scholar] [CrossRef]
- Pai, M.; Kohli, M. Essential Diagnostics: A Key Element of Universal Health Coverage. Dr. Sulaiman Al Habib Med. J. 2019, 1, 3–7. [Google Scholar] [CrossRef]
- McNerney, R.; Sollis, K.; Peeling, R.W. Improving access to new diagnostics through harmonised regulation: Priorities for action. Afr. J. Lab. Med. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Yadav, H.; Shah, D.; Sayed, S.; Horton, S.; Schroeder, L.F. Availability of essential diagnostics in ten low-income and middle-income countries: Results from national health facility surveys. Lancet Glob. Health 2021, 9, e1553–e1560. [Google Scholar] [CrossRef]
- Fleming, K.A.; Horton, S.; Wilson, M.L.; Atun, R.; DeStigter, K.; Flanigan, J.; Sayed, S.; Adam, P.; Aguilar, B.; Andronikou, S.; et al. The Lancet Commission on diagnostics: Transforming access to diagnostics. Lancet 2021, 398, 1997–2050. [Google Scholar] [CrossRef]
- Nichols, K.; Girdwood, S.J.; Inglis, A.; Ondoa, P.; Sy, K.T.L.; Benade, M.; Tusiime, A.B.; Kao, K.; Carmona, S.; Albert, H.; et al. Bringing Data Analytics to the Design of Optimized Diagnostic Networks in Low- and Middle-Income Countries: Process, Terms and Definitions. Diagnostics 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.E.; Girdwood, S.J.; Crompton, T.; Stewart-Isherwood, L.; Berrie, L.; Chimhamhiwa, D.; Moyo, C.; Kuehnle, J.; Stevens, W.; Rosen, S. Monitoring viral load for the last mile: What will it cost? J. Int. AIDS Soc. 2019, 22, e25337. [Google Scholar] [CrossRef]
- Girdwood, S.J.; Nichols, B.E.; Moyo, C.; Crompton, T.; Chimhamhiwa, D.; Rosen, S. Optimizing viral load testing access for the last mile: Geospatial cost model for point of care instrument placement. PLoS ONE 2019, 14, e0221586. [Google Scholar] [CrossRef]
- Ray, N.; Ebener, S. AccessMod 3.0: Computing Geographic Coverage and Accessibility to Health Care Services Using Anisotropic Movement of Patients|SpringerLink. Available online: https://link.springer.com/article/10.1186/1476-072X-7-63 (accessed on 29 October 2020).
- Ouma, P.; Macharia, P.M.; Okiro, E.; Alegana, V. Methods of Measuring Spatial Accessibility to Health Care in Uganda. In Practicing Health Geography: The African Context; Makanga, P.T., Ed.; Global Perspectives on Health Geography; Springer International Publishing: Cham, Switzerland, 2021; pp. 77–90. ISBN 978-3-030-63471-1. [Google Scholar]
- Huerta Munoz, U.; Källestål, C. Geographical accessibility and spatial coverage modeling of the primary health care network in the Western Province of Rwanda. Int. J. Health Geogr. 2012, 11, 40. [Google Scholar] [CrossRef]
- Kim, C.; Tappis, H.; McDaniel, P.; Soroush, M.S.; Fried, B.; Weinberger, M.; Trogdon, J.G.; Lich, K.H.; Delamater, P.L. National and subnational estimates of coverage and travel time to emergency obstetric care in Afghanistan: Modeling of spatial accessibility. Health Place 2020, 66, 102452. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Monet, J.-P.; Brun, M.; Bindaoudou, I.A.-K.; Daoudou, I.; Schaaf, M.; Agbigbi, Y.; Ray, N. National optimisation of accessibility to emergency obstetrical and neonatal care in Togo: A geospatial analysis. BMJ Open 2021, 11, e045891. [Google Scholar] [CrossRef]
- Oliphant, N.P.; Ray, N.; Bensaid, K.; Ouedraogo, A.; Gali, A.Y.; Habi, O.; Maazou, I.; Panciera, R.; Muñiz, M.; Sy, Z.; et al. Optimising geographical accessibility to primary health care: A geospatial analysis of community health posts and community health workers in Niger. BMJ Glob. Health 2021, 6, e005238. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Excel 2019; Microsoft: Redmond, WA, USA, 2019.
- Kuupiel, D.; Adu, K.M.; Apiribu, F.; Bawontuo, V.; Adogboba, D.A.; Ali, K.T.; Mashamba-Thompson, T.P. Geographic accessibility to public health facilities providing tuberculosis testing services at point-of-care in the upper east region, Ghana. BMC Public Health 2019, 19, 718. [Google Scholar] [CrossRef]
- Nichols, B.E.; Girdwood, S.J.; Crompton, T.; Stewart-Isherwood, L.; Berrie, L.; Chimhamhiwa, D.; Moyo, C.; Kuehnle, J.; Stevens, W.; Rosen, S. Impact of a borderless sample transport network for scaling up viral load monitoring: Results of a geospatial optimization model for Zambia. J. Int. AIDS Soc. 2018, 21, e25206. [Google Scholar] [CrossRef]
- Albert, H.; Purcell, R.; Wang, Y.Y.; Kao, K.; Mareka, M.; Katz, Z.; Maama, B.L.; Mots’oane, T. Designing an optimized diagnostic network to improve access to TB diagnosis and treatment in Lesotho. PLoS ONE 2020, 15, e0233620. [Google Scholar] [CrossRef]
- Glencross, D.K.; Coetzee, L.M.; Cassim, N. An Integrated Tiered Service Delivery Model (ITSDM) Based on Local CD4 Testing Demands Can Improve Turn-Around Times and Save Costs whilst Ensuring Accessible and Scalable CD4 Services across a National Programme. PLoS ONE 2014, 9, e114727. [Google Scholar] [CrossRef]
- Albert, H.; Sartorius, B.; Bessell, P.; De Souza, D.; Rupani, S.; Gonzalez, K.; Kayembe, S.; Ndung’u, J.; Pullan, R.; Makana, D.P.; et al. Developing strategies for onchocerciasis elimination mapping and surveillance through the diagnostic network optimization approach. Front. Trop. Dis. 2021, 2, 26. [Google Scholar] [CrossRef]
- Lamas-Fernandez, C.; Hayward, G.; Moore, M.; Monks, T. A mathematical model for designing networks of C-Reactive Protein point of care testing. PLoS ONE 2019, 14, e0222676. [Google Scholar]
- OpenStreetMap Fundation OpenStreetMap. Available online: https://www.openstreetmap.org/ (accessed on 28 October 2021).
- Elrod, J.K.; Fortenberry, J.L. The hub-and-spoke organization design: An avenue for serving patients well. BMC Health Serv. Res. 2017, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Markham, F.; Doran, B. Equity, discrimination and remote policy: Investigating the centralization of remote service delivery in the Northern Territory. Appl. Geogr. 2015, 58, 105–115. [Google Scholar] [CrossRef]
- Fonjungo, P.N.; Boeras, D.I.; Zeh, C.; Alexander, H.; Parekh, B.S.; Nkengasong, J.N. Access and Quality of HIV-Related Point-of-Care Diagnostic Testing in Global Health Programs. Clin. Infect. Dis. 2016, 62, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Standley, C.J.; Muhayangabo, R.; Bah, M.S.; Barry, A.M.; Bile, E.; Fischer, J.E.; Heegaard, W.; Koivogui, L.; Lakiss, S.K.; Sorrell, E.M.; et al. Creating a National Specimen Referral System in Guinea: Lessons from Initial Development and Implementation. Front. Public Health 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Louis, F.J.; Osborne, A.J.; Elias, V.J.; Buteau, J.; Boncy, J.; Elong, A.; Dismer, A.; Sasi, V.; Domercant, J.W.; Lauture, D.; et al. Specimen Referral Network to Rapidly Scale-Up CD4 Testing: The Hub and Spoke Model for Haiti. J. AIDS Clin. Res. 2015, 6, 1000488. [Google Scholar] [CrossRef]
- Kiyaga, C.; Sendagire, H.; Joseph, E.; McConnell, I.; Grosz, J.; Narayan, V.; Esiru, G.; Elyanu, P.; Akol, Z.; Kirungi, W.; et al. Uganda’s New National Laboratory Sample Transport System: A Successful Model for Improving Access to Diagnostic Services for Early Infant HIV Diagnosis and Other Programs. PLoS ONE 2013, 8, e78609. [Google Scholar] [CrossRef]
- Kebede, Y.; Fonjungo, P.N.; Tibesso, G.; Shrivastava, R.; Nkengasong, J.N.; Kenyon, T.; Kebede, A.; Gadde, R.; Ayana, G. Improved Specimen-Referral System and Increased Access to Quality Laboratory Services in Ethiopia: The Role of the Public-Private Partnership. J. Infect. Dis. 2016, 213, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. Using the Hub and Spoke Model of Telemental Health to Expand the Reach of Community Based Care in the United States. Community Ment. Health J. 2021, 57, 49–56. [Google Scholar] [CrossRef]
- Day, S.C.; Day, G.; Keller, M.; Touchett, H.; Amspoker, A.B.; Martin, L.; Lindsay, J.A. Personalized implementation of video telehealth for rural veterans (PIVOT-R). mHealth 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Roberts, R.; McKay, R. Older people’s mental health in rural areas: Converting policy into service development, service access and a sustainable workforce. Aust. J. Rural Health 2019, 27, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.L.; Player, H.; Hite, S.; Satyananda, V.; Stacey, J.; Sun, V.; Jones, V.; Hayter, J. Feasibility of Remote Occupational Therapy Services via Telemedicine in a Breast Cancer Recovery Program. Am. J. Occup. Ther. 2021, 75, 7502205030p1–7502205030p9. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.W.; Saidi, S.; Musa, A.; Kithyoma, V.; Kumar, P. “Even though I am alone, I feel that we are many”—An appreciative inquiry study of asynchronous, provider-to-provider teleconsultations in Turkana, Kenya. PLoS ONE 2020, 15, e0238806. [Google Scholar] [CrossRef] [PubMed]
- Tobgay, T.; Samdrup, P.; Jamtsho, T.; Mannion, K.; Ortega, L.; Khamsiriwatchara, A.; Price, R.N.; Thriemer, K.; Kaewkungwal, J. Performance and user acceptance of the Bhutan febrile and malaria information system: Report from a pilot study. Malar. J. 2016, 15, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferguson, W.J.; Kemp, K.; Kost, G. Using a geographic information system to enhance patient access to point-of-care diagnostics in a limited-resource setting. Int. J. Health Geogr. 2016, 15, 10. [Google Scholar] [CrossRef]
- Hierink, F.; Rodrigues, N.; Muñiz, M.; Panciera, R.; Ray, N. Modelling geographical accessibility to support disaster response and rehabilitation of a healthcare system: An impact analysis of Cyclones Idai and Kenneth in Mozambique. BMJ Open 2020, 10, e039138. [Google Scholar] [CrossRef] [PubMed]
- Macharia, P.M.; Ray, N.; Giorgi, E.; Okiro, E.A.; Snow, R.W. Defining service catchment areas in low-resource settings. BMJ Glob. Health 2021, 6, e006381. [Google Scholar] [CrossRef] [PubMed]
- Delamater, P.L.; Messina, J.P.; Shortridge, A.M.; Grady, S.C. Measuring geographic access to health care: Raster and network-based methods. Int. J. Health Geogr. 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Neutens, T. Accessibility, equity and health care: Review and research directions for transport geographers. J. Transp. Geogr. 2015, 43, 14–27. [Google Scholar] [CrossRef]
- Stevens, F.R.; Gaughan, A.E.; Linard, C.; Tatem, A.J. Disaggregating Census Data for Population Mapping Using Random Forests with Remotely-Sensed and Ancillary Data. PLoS ONE 2015, 10, e0107042. [Google Scholar] [CrossRef]
- Purcell, R.; Albert, H. Building an Open Access Software Tool to Allow Countries to Design Patient-Centered and Cost-Effective Diagnostic Networks—ppt download 2019. Available online: https://slideplayer.com/slide/17812219 (accessed on 27 October 2021).
- Llamasoft Inc., FIND, GHSC-PSM Llamasoft: OptiDx. Available online: https://www.finddx.org/dno/dx-network-opt/ (accessed on 27 October 2021).
- Underwood, T. Diagnostic Network Optimization: A Network Analytics Approach to Design Patient-Centred and Cost-Efficient Diagnostic Systems 2021. Available online: https://www.finddx.org/wp-content/uploads/2021/11/Guide-to-Diagnostic-Network-Optimization_15.11.2021.pdf (accessed on 30 November 2021).
| Ref. | Country | Disease | Accessibility Model | Referral System | Diagnostic Network Optimization Strategies |
|---|---|---|---|---|---|
| [18] | Ghana (sub-national) | TB | Distance: along the road network; Travel time: Costdistance, average 20 km/h motorized tricycle | - | 51 additional TB testing health facilities located <10 km from population: MapInfo and SQL query. Only study interested in population accessibility. |
| [20] | Lesotho (national) | TB | Travel distance: along the road network or using a distance adjustment factor | Sample | Diagnostic network scenarios modelled using Supply Chain Guru software. The optimized scenario is the lowest overall cost solution that meets all constraints. |
| [8] | Zambia (national) | HIV VL | Travel time: ArcGIS Network Analyst tool, Salesman Problem | Sample | ArcGIS Location-Allocation function, maximizing ART POC facilities coverage and Geospatial model that minimizes driving time and minimizes overall costs. |
| [19] | Zambia (national) | HIV VL | Travel time: ArcGIS Network Analyst tool, Salesman Problem | Sample | ArcGIS Location-Allocation function, and geospatial model that maximized the Sample Transport Network, while minimizing the transport cost. Two sample transportation scenarios: district-bounded and borderless scenarios. |
| [21] | South Africa (national) | HIV | Distance: Euclidean distance (<100 km) | Sample | Integrated tiered service delivery model that ensure CD4 testing are accessible at health facilities within 24–48 h local turnaround time and contain test costs. |
| [23] | UK (sub-national) | Lower Respiratory Tract Infections | Distance: along the road network, Open-Source Routing Machine | Population | ArcGIS Location-Allocation function. Mathematical model allocates C-reactive protein testing location to minimize the overall travel and ensuring that patients never have to travel more than a predefined maximum distance. |
| [22] | DRC and Angola (sub-national) | Onchocerciasis | Travel distance: along the road network or using a distance adjustment factor | Sample | DNO can help evaluate alternate sampling strategies to bring opportunities for overall cost savings. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chênes, C.; Albert, H.; Kao, K.; Ray, N. Use of Physical Accessibility Modelling in Diagnostic Network Optimization: A Review. Diagnostics 2022, 12, 103. https://doi.org/10.3390/diagnostics12010103
Chênes C, Albert H, Kao K, Ray N. Use of Physical Accessibility Modelling in Diagnostic Network Optimization: A Review. Diagnostics. 2022; 12(1):103. https://doi.org/10.3390/diagnostics12010103
Chicago/Turabian StyleChênes, Camille, Heidi Albert, Kekeletso Kao, and Nicolas Ray. 2022. "Use of Physical Accessibility Modelling in Diagnostic Network Optimization: A Review" Diagnostics 12, no. 1: 103. https://doi.org/10.3390/diagnostics12010103
APA StyleChênes, C., Albert, H., Kao, K., & Ray, N. (2022). Use of Physical Accessibility Modelling in Diagnostic Network Optimization: A Review. Diagnostics, 12(1), 103. https://doi.org/10.3390/diagnostics12010103







