Investigation of Therapeutic Response Markers for Acupuncture in Parkinson’s Disease: An Exploratory Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition of Clinical Data
2.2. Quality Test of Blood Samples
2.3. Acquisition of RNA Sequencing (RNA-Seq) Data
2.4. Transcriptomic Analysis
2.5. Comparison of PD Biomarkers
2.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Selection of a Representative Patient with PD Based on Clinical Outcome Changes following Acupuncture
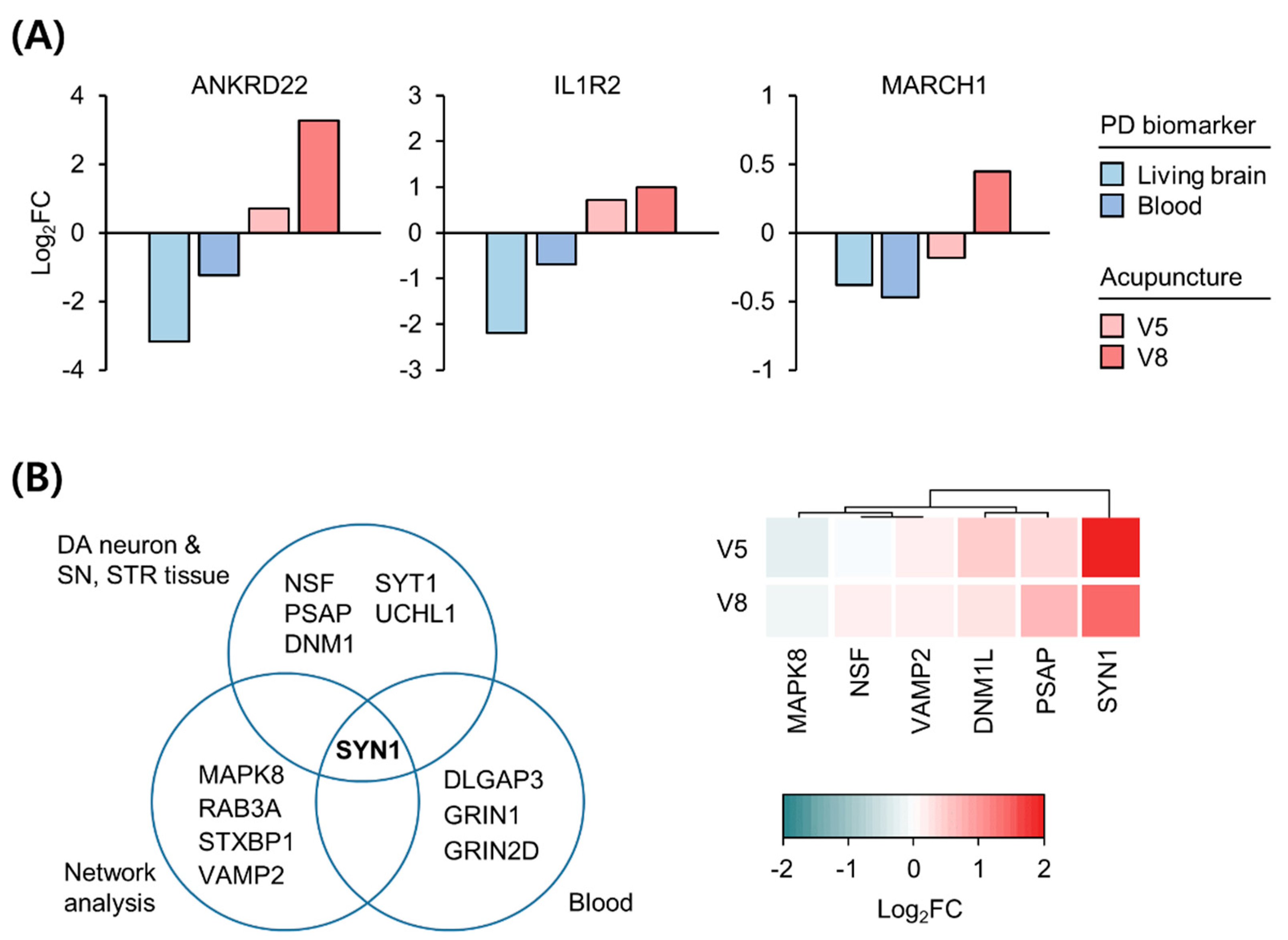
3.2. Transcriptomic Alterations following Acupuncture
3.3. Expression Changes in Biomarkers in the Blood and Brain of Patients with PD following Acupuncture
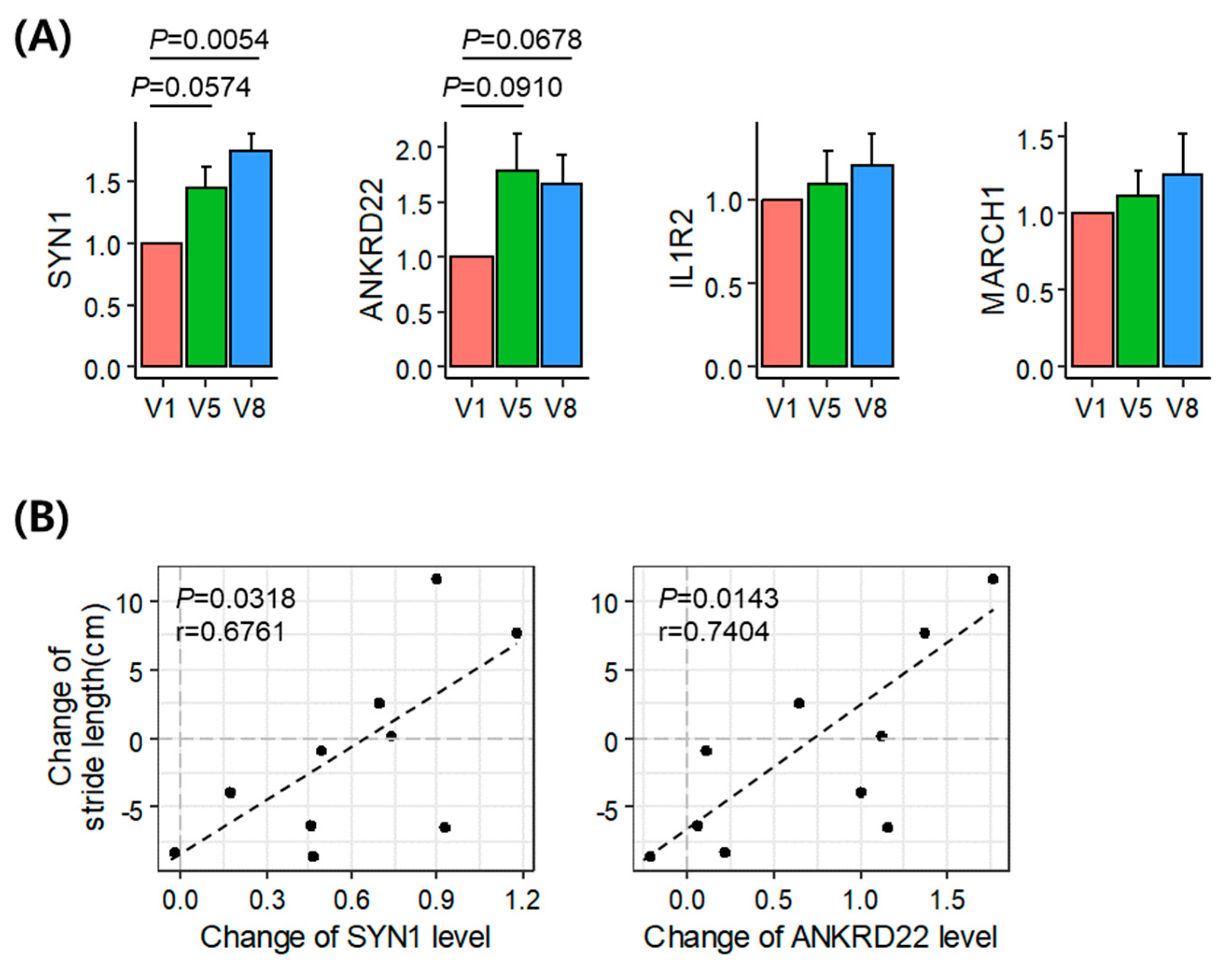
3.4. Validation of the Identified Acupuncture Therapy Response Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Doo, K.H.; Lee, J.H.; Cho, S.Y.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Kim, H.; Park, H.J.; Park, S.U. A prospective open-label study of combined treatment for idiopathic Parkinson’s disease using acupuncture and bee venom acupuncture as an adjunctive treatment. J. Altern Complement. Med. 2015, 21, 598–603. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Lei, H.; Schwenk, M.; Sherman, S.J.; Esternberg, E.; Mohler, J.; Najafi, B. Does integrative medicine enhance balance in aging adults? Proof of concept for the benefit of electroacupuncture therapy in Parkinson’s disease. Gerontology 2015, 61, 3–14. [Google Scholar] [CrossRef]
- Lei, H.; Toosizadeh, N.; Schwenk, M.; Sherman, S.; Karp, S.; Sternberget, E.; Najafi, B. A pilot clinical trial to objectively assess the efficacy of electroacupuncture on gait in patients with Parkinson’s disease using body worn sensors. PLoS ONE 2016, 11, e0155613. [Google Scholar] [CrossRef]
- Zeng, B.Y.; Zhao, K. Effect of acupuncture on the motor and nonmotor symptoms in Parkinson’s disease—A review of clinical studies. CNS Neurosci. Ther. 2016, 22, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Sun, Y.; Wu, H.; Pei, J.; Zhang, J.; Zhang, Y.; Wang, L.; Li, B.; Wang, L.; Shi, J.; et al. Acupuncture promotes mTOR-independent autophagic clearance of aggregation-prone proteins in mouse brain. Sci. Rep. 2016, 6, 19714. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Lim, S. Acupuncture inhibits the increase in alpha-synuclein by modulating SGK1 in an MPTP induced parkinsonism mouse model. Am. J. Chin. Med. 2019, 47, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Borrageiro, G.; Haylett, W.; Seedat, S.; Kuivaniemi, H.; Bardien, S. A review of genome-wide transcriptomics studies in Parkinson’s disease. Eur. J. Neurosci. 2018, 47, 1–16. [Google Scholar] [CrossRef]
- Courtney, E.; Kornfeld, S.; Janitz, K.; Janitz, M. Transcriptome profiling in neurodegenerative disease. J. Neurosci. Methods 2010, 193, 189–202. [Google Scholar] [CrossRef]
- Green, H.; Zhang, X.; Tiklova, K.; Volakakis, N.; Brodin, L.; Berg, L.; Greengard, P.; Perlmann, T.; Svenningsson, P. Alterations of p11 in brain tissue and peripheral blood leukocytes in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 2735–2740. [Google Scholar] [CrossRef]
- Benoit, S.M.; Xu, H.; Schmid, S.; Alexandrova, R.; Kaur, G.; Thiruvahindrapuram, B.; Pereira, S.L.; Jog, M.; Hebb, M.O. Expanding the search for genetic biomarkers of Parkinson’s disease into the living brain. Neurobiol. Dis. 2020, 140, 104872. [Google Scholar] [CrossRef]
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.M.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, S.; An, J.; Choi, J.D.; Seol, I.C.; Park, G.; Lee, S.H.; Moon, Y.; Kang, W.; Jung, E.S.; et al. Gait disturbance improvement and cerebral cortex rearrangement by acupuncture in Parkinson’s disease: A pilot assessor-blinded, randomized, controlled, parallel-group trial. Neurorehabil. Neural Repair. 2020, 34, 1111–1123. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, H.; Jung, I.; Yoo, H. Acupuncture for improving gait disturbance in Parkinson’s disease: A study protocol for a pi-lot randomized controlled trial. Eur. J. Integr. Med. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; Prieto, C.; Sierra, M.; Sánchez-Juan, P.; González-Aramburu, I.; Sánchez-Quintana, C.; Berciano, J.; Combarros, O.; Sainz, J. Identification of candidate genes for Parkinson’s disease through blood transcriptome analysis in LRRK2-G2019S carriers, idiopathic cases, and controls. Neurobiol. Aging 2015, 36, 1105–1109. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Miller, R.M.; Kiser, G.L.; Kaysser-Kranich, T.M.; Lockner, R.J.; Palaniappan, C.; Federoff, H.J. Robust dysregulation of gene expression in substantia nigra and striatum in Parkinson’s disease. Neurobiol. Dis. 2006, 21, 305–313. [Google Scholar] [CrossRef]
- Simunovic, F.; Yi, M.; Wang, Y.; Macey, L.; Brown, L.T.; Krichevsky, A.M.; Andersen, S.L.; Stephens, R.M.; Benes, F.M.; Sonntag, K.C. Gene expression profiling of substantia nigra dopamine neurons: Further insights into Parkinson’s disease pathology. Brain 2009, 132, 1795–1809. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bonchev, D. A network view on Parkinson’s disease. Comput. Struct. Biotechnol. J. 2013, 7, e201304004. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Bian, H.; Li, X. Gene expression profiling predicts pathways and genes associated with Parkinson’s disease. Neurol. Sci. 2016, 37, 73–79. [Google Scholar] [CrossRef]
- Kim, A.; Lee, S.Y.; Seo, C.S.; Chung, S.K. Prunellae Spica extract suppresses teratoma formation of pluripotent stem cells through p53-mediated apoptosis. Nutrients 2020, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; Prieto, C.; Sierra, M.; Sánchez-Juan, P.; González-Aramburu, I.; Sánchez-Quintana, C.; Berciano, J.; Combarros, O.; Sainz, J. Comparative blood transcriptome analysis in idiopathic and LRRK2 G2019S-associated Parkinson’s disease. Neurobiol. Aging 2016, 38, 214.e1–214.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Litvak, V.; Gilbertson, T.; Kühn, A.; Lu, C.S.; Lee, S.T.; Tsai, C.H.; Tisch, S.; Limousin, P.; Hariz, M.; et al. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp. Neurol. 2007, 205, 214–221. [Google Scholar] [CrossRef]
- Timmermann, L.; Florin, E. Parkinson’s disease and pathological oscillatory activity: Is the beta band the bad guy? - New lessons learned from low-frequency deep brain stimulation. Exp. Neurol. 2012, 233, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Pahan, K. Ankyrin repeat and BTB/POZ domain containing protein-2 inhibits the aggregation of alpha-synuclein: Implications for Parkinson’s disease. FEBS Lett. 2013, 587, 3567–3574. [Google Scholar] [CrossRef]
- Nussbaum, R.L. Putting the parkin into Parkinson’s. Nature 1998, 392, 544–545. [Google Scholar] [CrossRef]
- Lin, G.; Lee, P.T.; Chen, K.; Mao, D.; Tan, K.L.; Zuo, Z.; Lin, W.W.; Wang, L.; Bellen, H.J. Phospholipase PLA2G6, a parkinsonism-associated gene, affects Vps26 and Vps35, retromer function, and ceramide levels, similar to α-synuclein gain. Cell Metab. 2018, 28, 605–618.e6. [Google Scholar] [CrossRef]
- Weber, T.; Stephan, R.; Moreno, E.; Pielage, J. The ankyrin repeat domain controls presynaptic localization of Drosophila ankyrin2 and is essential for synaptic stability. Front. Cell Dev. Biol. 2019, 7, 148. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef]
- Marte, A.; Russo, I.; Rebosio, C.; Valente, P.; Belluzzi, E.; Pischedda, F.; Montani, C.; Lavarello, C.; Petretto, A.; Fedele, E.; et al. Leucine-rich repeat kinase 2 phosphorylation on synapsin I regulates glutamate release at pre-synaptic sites. J. Neurochem. 2019, 150, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Doo, A.R.; Park, J.Y.; Bae, H.; Chae, Y.; Shim, I.; Lee, H.; Moon, W.; Lee, H.; Park, H.J. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS ONE 2011, 6, e27566. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.; Shi, J.; Rochester, L.; Del Din, S.; Pantall, A. Entropy of real-world gait in Parkinson’s disease determined from wearable sensors as a digital marker of altered ambulatory behavior. Sensors 2020, 20, 2631. [Google Scholar] [CrossRef] [PubMed]
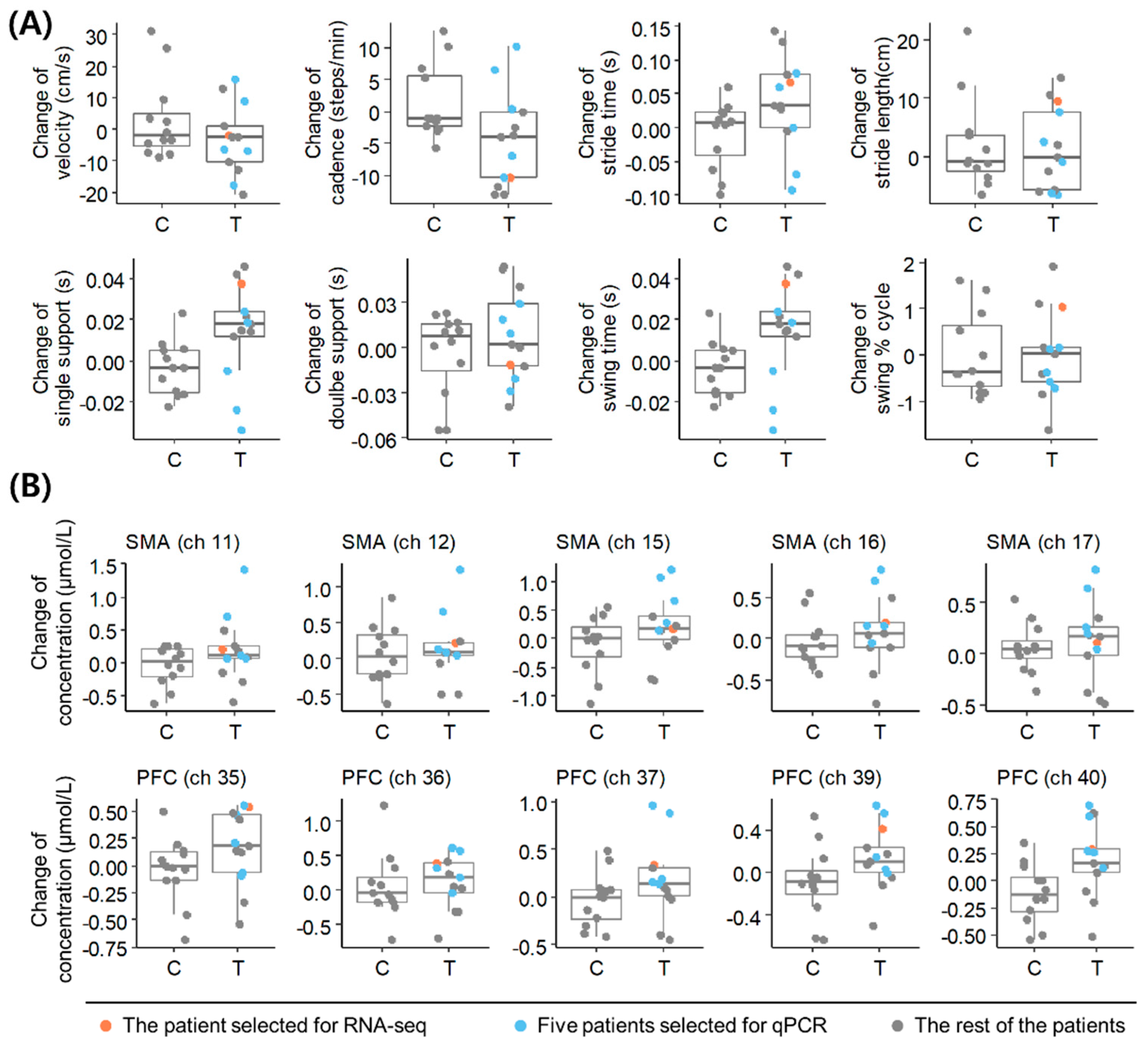

| Case | Age | Sex | Age at Onset (Years) | Disease Duration (Years) | Hoehn and Yahr Scale | Velocity (cm/s) | Cadence (Steps/min) | Stride Time (s) | Stride Length (cm) | Single Support Time (s) | Double Support Time (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T02 * | 69 | M | 64 | 5 | 3 | 155.433 | 135.567 | 0.885 | 138.270 | 0.360 | 0.162 |
| T04 | 60 | M | 56 | 4 | 2 | 133.200 | 121.900 | 0.986 | 131.248 | 0.373 | 0.250 |
| T09 | 75 | M | 73 | 2 | 2 | 103.500 | 104.567 | 1.143 | 119.055 | 0.441 | 0.261 |
| T11 | 59 | M | 38 | 21 | 1 | 146.500 | 134.567 | 0.888 | 130.540 | 0.346 | 0.195 |
| T12 | 70 | M | 63 | 7 | 1 | 102.800 | 118.000 | 1.018 | 104.502 | 0.381 | 0.262 |
| T13 | 57 | F | 51 | 6 | 1 | 96.333 | 108.400 | 1.106 | 106.441 | 0.421 | 0.269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-M.; Kim, A.; Park, G.; Kwon, O.; Park, S.; Yoo, H.; Jang, J.-H. Investigation of Therapeutic Response Markers for Acupuncture in Parkinson’s Disease: An Exploratory Pilot Study. Diagnostics 2021, 11, 1697. https://doi.org/10.3390/diagnostics11091697
Park S-M, Kim A, Park G, Kwon O, Park S, Yoo H, Jang J-H. Investigation of Therapeutic Response Markers for Acupuncture in Parkinson’s Disease: An Exploratory Pilot Study. Diagnostics. 2021; 11(9):1697. https://doi.org/10.3390/diagnostics11091697
Chicago/Turabian StylePark, Sang-Min, Aeyung Kim, Gunhyuk Park, Ojin Kwon, Sangsoo Park, Horyong Yoo, and Jung-Hee Jang. 2021. "Investigation of Therapeutic Response Markers for Acupuncture in Parkinson’s Disease: An Exploratory Pilot Study" Diagnostics 11, no. 9: 1697. https://doi.org/10.3390/diagnostics11091697
APA StylePark, S.-M., Kim, A., Park, G., Kwon, O., Park, S., Yoo, H., & Jang, J.-H. (2021). Investigation of Therapeutic Response Markers for Acupuncture in Parkinson’s Disease: An Exploratory Pilot Study. Diagnostics, 11(9), 1697. https://doi.org/10.3390/diagnostics11091697







