Immune Checkpoint Inhibitors in Advanced NSCLC: [18F]FDG PET/CT as a Troubleshooter in Treatment Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject
2.2. [18F]FDG PET/CT Examination and Analysis
2.3. Response Evaluation
2.4. Statistical Analysis
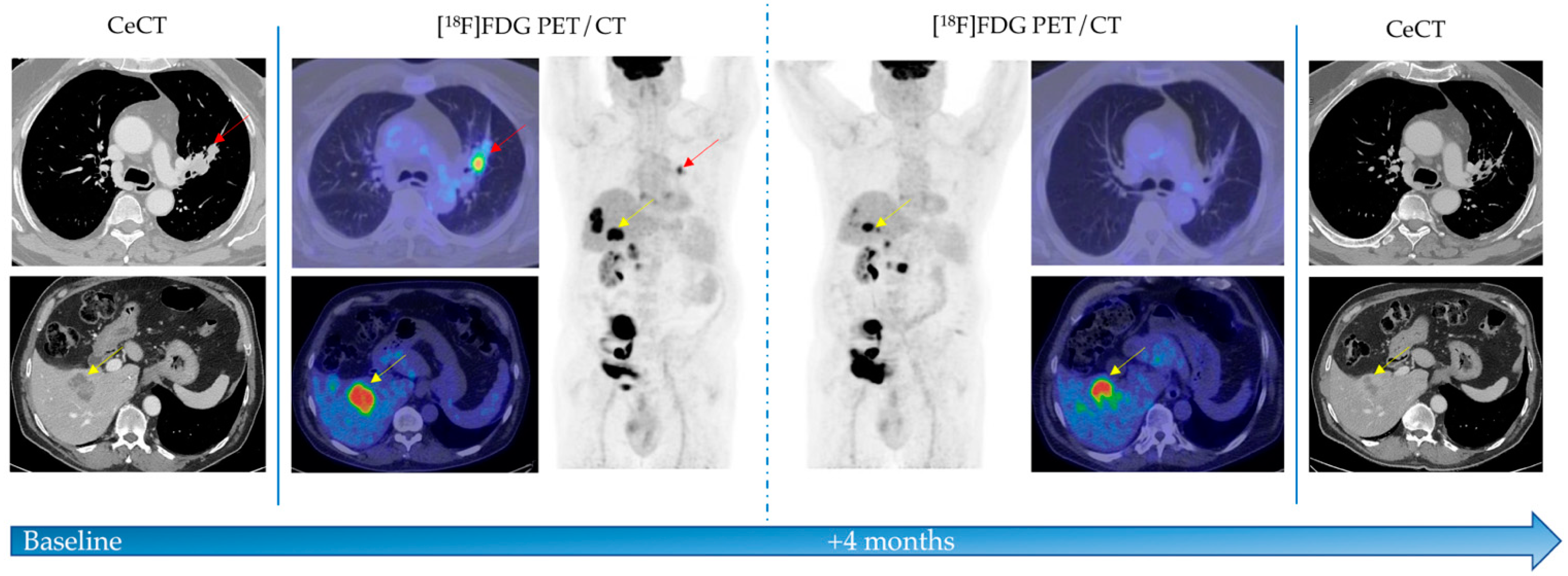
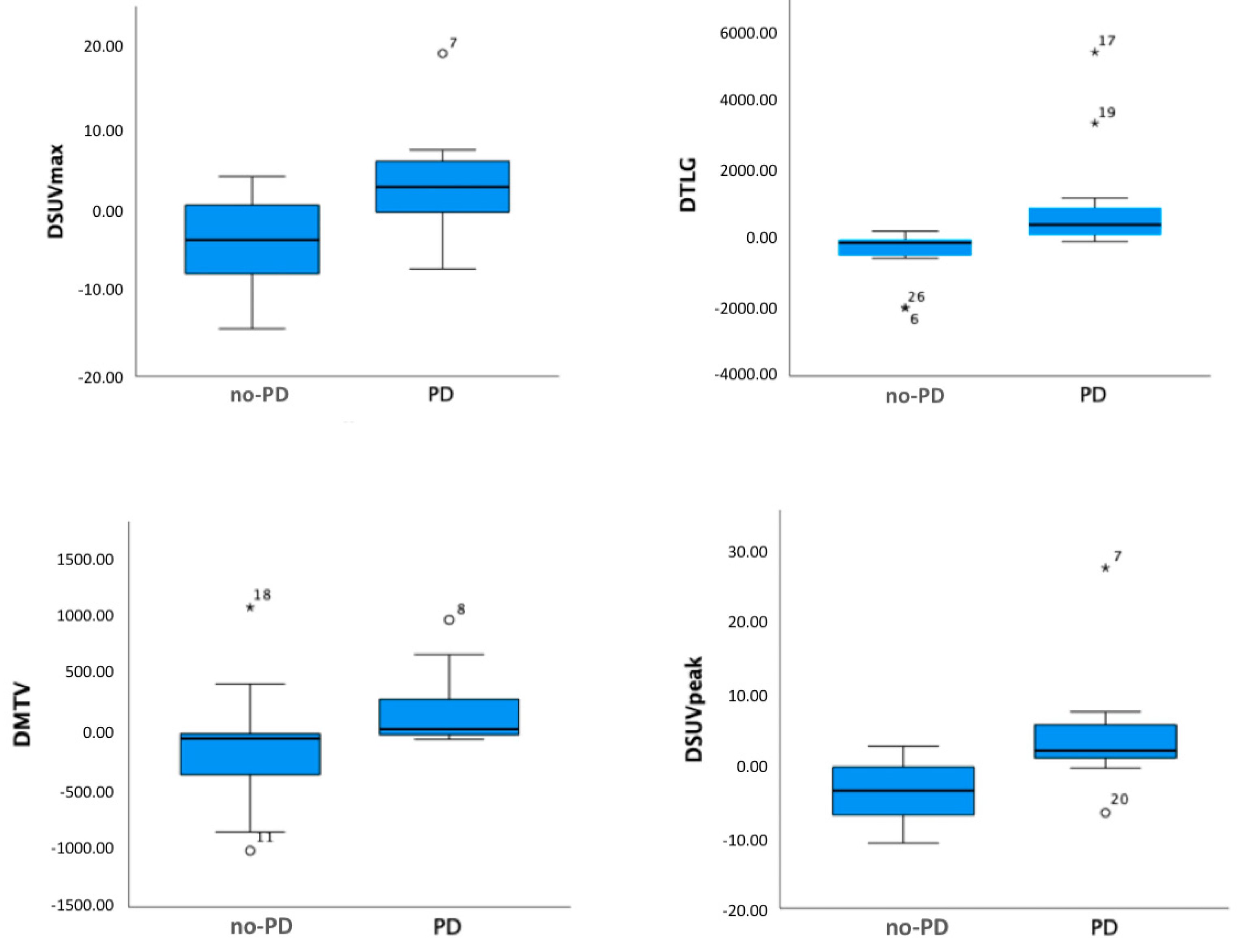
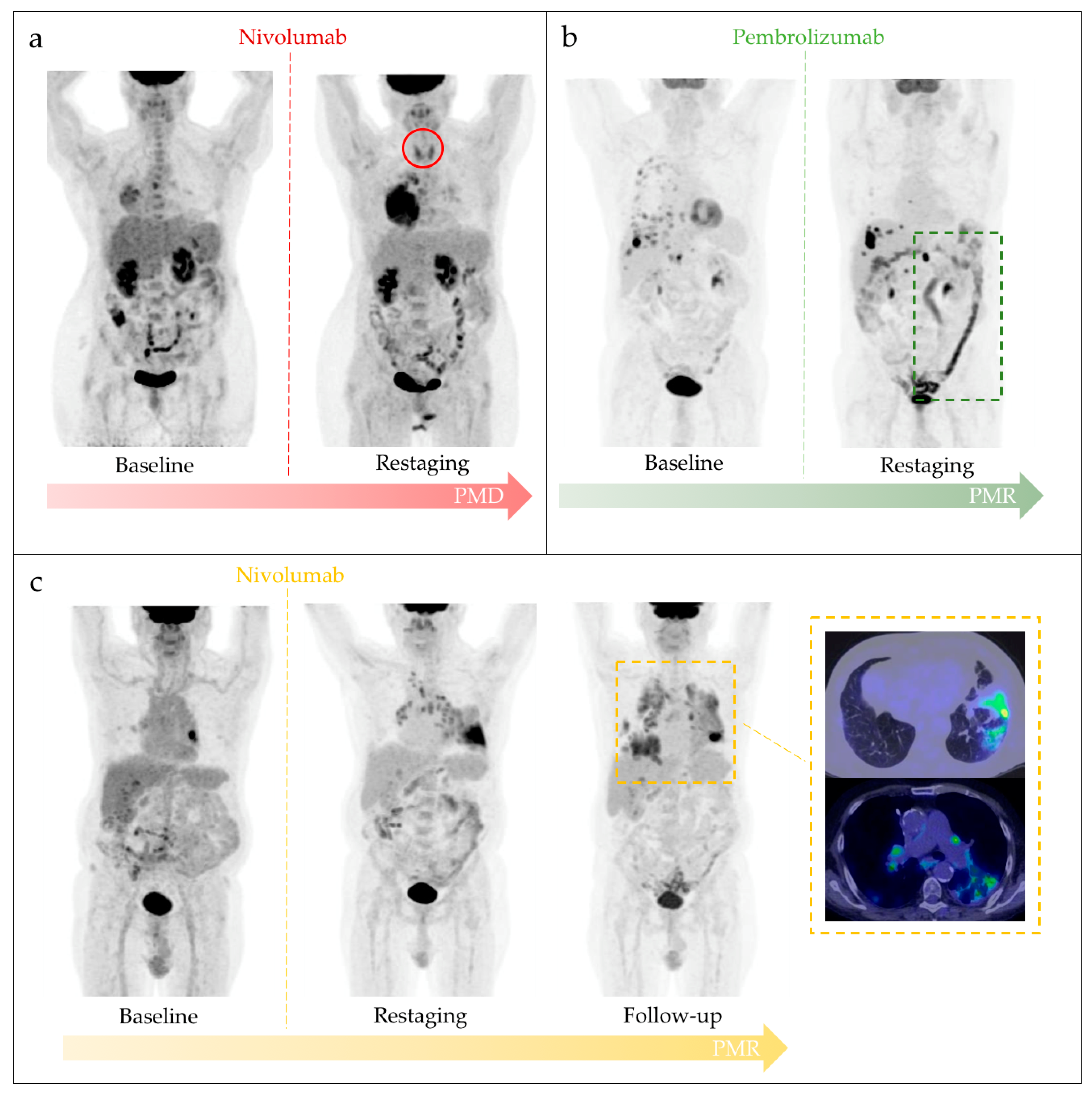
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, J.U.; Yoon, H.K. Potential Predictive Value of Change in Inflammatory Cytokines Levels Subsequent to Initiation of Immune Checkpoint Inhibitor in Patients with Advanced Non-Small Cell Lung Cancer. Cytokine 2021, 138, 155363. [Google Scholar] [CrossRef]
- Sardaro, A.; Ferrari, C.; Carbonara, R.; Altini, C.; Lavelli, V.; Rubini, G. Synergism between Immunotherapy and Radiotherapy in Esophageal Cancer: An Overview of Current Knowledge and Future Perspectives. Cancer Biother. Radiopharm. 2021, 36, 123–132. [Google Scholar] [CrossRef]
- Weinmann, S.C.; Pisetsky, D.S. Mechanisms of Immune-Related Adverse Events during the Treatment of Cancer with Immune Checkpoint Inhibitors. Rheumatology 2019, 58, vii59–vii67. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Chuang, H.H.; Gambhire, D.; Kairemo, K. Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives. Diagnostics 2017, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, L.; Sepulcri, M.; Pasello, G. PET/CT and the Response to Immunotherapy in Lung Cancer. Curr. Radiopharm. 2020, 13, 177. [Google Scholar] [CrossRef]
- Frega, S.; Maso, A.D.; Pasello, G.; Cuppari, L.; Bonanno, L.; Conte, P.; Evangelista, L. Novel Nuclear Medicine Imaging Applications in Immuno-Oncology. Cancers 2020, 12, 1303. [Google Scholar] [CrossRef]
- Grizzi, F.; Castello, A.; Lopci, E. Is It Time to Change Our Vision of Tumor Metabolism Prior to Immunotherapy? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1072–1075. [Google Scholar] [CrossRef]
- Aide, N.; de Pontdeville, M.; Lopci, E. Evaluating Response to Immunotherapy with 18 F-FDG PET/CT: Where Do We Stand? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1019–1021. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Toyokawa, G.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Wakasu, S.; Haro, A.; Osoegawa, A.; Tagawa, T.; et al. 18F-FDG Uptake in PET/CT Is a Potential Predictive Biomarker of Response to Anti-PD-1 Antibody Therapy in Non-Small Cell Lung Cancer. Sci. Rep. 2019, 9, 13362. [Google Scholar] [CrossRef] [Green Version]
- Chardin, D.; Paquet, M.; Schiappa, R.; Darcourt, J.; Bailleux, C.; Poudenx, M.; Sciazza, A.; Ilie, M.; Benzaquen, J.; Martin, N.; et al. Original Research: Baseline Metabolic Tumor Volume as a Strong Predictive and Prognostic Biomarker in Patients with Non-Small Cell Lung Cancer Treated with PD1 Inhibitors: A Prospective Study. J. Immunother. Cancer 2020, 8, 645. [Google Scholar] [CrossRef]
- Costa, L.B.; Queiroz, M.A.; Barbosa, F.G.; Nunes, R.F.; Zaniboni, E.C.; Ruiz, M.M.; Jardim, D.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. Radiographics 2020, 41, 120–143. [Google Scholar] [CrossRef]
- Liberini, V.; Laudicella, R.; Capozza, M.; Huellner, M.W.; Burger, I.A.; Baldari, S.; Terreno, E.; Deandreis, D. The Future of Cancer Diagnosis, Treatment and Surveillance: A Systemic Review on Immunotherapy and Immuno-PET Radiotracers. Molecules 2021, 26, 2201. [Google Scholar] [CrossRef]
- Ranieri, G.; Marech, I.; Asabella, A.N.; di Palo, A.; Porcelli, M.; Lavelli, V.; Rubini, G.; Ferrari, C.; Gadaleta, C.D. Tyrosine-Kinase Inhibitors Therapies with Mainly Anti-Angiogenic Activity in Advanced Renal Cell Carcinoma: Value of PET/CT in Response Evaluation. Int. J. Mol. Sci. 2017, 18, 1937. [Google Scholar] [CrossRef] [Green Version]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279. [Google Scholar] [CrossRef]
- Evangelista, L.; Cuppari, L.; Menis, J.; Bonanno, L.; Reccia, P.; Frega, S.; Pasello, G. 18F-FDG PET/CT in Non-Small-Cell Lung Cancer Patients: A Potential Predictive Biomarker of Response to Immunotherapy. Nucl. Med. Commun. 2019, 40, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Monaco, L.; Gemelli, M.; Gotuzzo, I.; Bauckneht, M.; Crivellaro, C.; Genova, C.; Cortinovis, D.; Zullo, L.; Ammoni, L.C.; Bernasconi, D.P.; et al. Metabolic Parameters as Biomarkers of Response to Immunotherapy and Prognosis in Non-Small Cell Lung Cancer (NSCLC): A Real World Experience. Cancers 2021, 13, 1634. [Google Scholar] [CrossRef] [PubMed]
- Polverari, G.; Ceci, F.; Bertaglia, V.; Reale, M.L.; Rampado, O.; Gallio, E.; Passera, R.; Liberini, V.; Scapoli, P.; Arena, V.; et al. 18F-FDG Pet Parameters and Radiomics Features Analysis in Advanced Nsclc Treated with Immunotherapy as Predictors of Therapy Response and Survival. Cancers 2020, 12, 1163. [Google Scholar] [CrossRef]
- Takada, K.; Toyokawa, G.; Okamoto, T.; Baba, S.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; et al. Metabolic Characteristics of Programmed Cell Death-Ligand 1-Expressing Lung Cancer on 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Cancer Med. 2017, 6, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L. The Prediction of Response to Immunotherapy in Non-Small Cell Lung Cancer Patients by 18F-FDG PET/CT. J. Thorac. Dis. 2019, 11, E221–E223. [Google Scholar] [CrossRef] [PubMed]
- Omori, S.; Kenmotsu, H.; Abe, M.; Watanabe, R.; Sugino, T.; Kobayashi, H.; Nakashima, K.; Wakuda, K.; Ono, A.; Taira, T.; et al. Changes in Programmed Death Ligand 1 Expression in Non-Small Cell Lung Cancer Patients Who Received Anticancer Treatments. Int. J. Clin. Oncol. 2018, 23, 1052–1059. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, C.; Zhong, W.-Z. Neoadjuvant Immunotherapy for Non–Small Cell Lung Cancer: State of the Art. Cancer Commun. 2021, 41, 287–302. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between Immune-Related Adverse Events and Efficacy in Non-Small Cell Lung Cancer Treated with Nivolumab. Lung Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villadolid, J.; Amin, A. Immune Checkpoint Inhibitors in Clinical Practice: Update on Management of Immune-Related Toxicities. Transl. Lung Cancer Res. 2015, 4, 560. [Google Scholar] [CrossRef]
- Grangeon, M.; Tomasini, P.; Chaleat, S.; Jeanson, A.; Souquet-Bressand, M.; Khobta, N.; Bermudez, J.; Trigui, Y.; Greillier, L.; Blanchon, M.; et al. Association Between Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18 F-FDG PET/CT Longitudinal Studies in Patients with Advanced Metastatic Melanoma for Response Evaluation of Combination Treatment with Vemurafenib and Ipilimumab. Melanoma Res. 2019, 29, 178–186. [Google Scholar] [CrossRef]
- Ferrari, C.; Maggialetti, N.; Masi, T.; Nappi, A.G.; Santo, G.; Asabella, A.N.; Rubini, G. Early Evaluation of Immunotherapy Response in Lymphoma Patients by 18F-FDG PET/CT: A Literature Overview. J. Pers. Med. 2021, 11, 217. [Google Scholar] [CrossRef]
- Jin, P.; Li, J.; Meng, Y.; Wu, L.; Bai, M.; Yu, J. PET/CT Metabolic Patterns in Systemic Immune Activation: A New Perspective on the Assessment of Immunotherapy Response and Efficacy. Cancer Lett. 2021, 520, 91–99. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number |
|---|---|
| Total Number of Patients | 28 |
| Median age at diagnosis (years) | 65 (range 48–87) |
| Sex | |
| Male | 22 (79%) |
| Female | 6 (21%) |
| Histological variant | |
| Adenocarcinoma | 22 (79%) |
| Squamous Cell Carcinoma | 4 (14%) |
| Others | 2 (7%) |
| Previous lung surgery | |
| No | 21 (75%) |
| Yes | 7 (25%) |
| Immunotherapy | |
| First line | 8 (29%) |
| ≥Second line | 20 (71%) |
| Drugs | |
| Nivolumab | 15 (54%) |
| Pembrolizumab | 13 (46%) |
| Patients (n = 5) | Age, Sex | Disease | Therapy | irAEs | Final Outcome |
|---|---|---|---|---|---|
| 1 | 58, F | NSCLC | Nivolumab | Thyroiditis | PD |
| 2 | 61, F | NSCLC | Nivolumab | Thyroiditis | SD |
| 3 | 64, M | NSCLC | Pembrolizumab | Colitis | PR |
| 4 | 64, M | NSCLC | Nivolumab | Arthritis | PD |
| 5 | 60, M | NSCLC | Nivolumab | Pneumonitis and sarcoid reaction | PR |
| Overall (n = 28) | |||
|---|---|---|---|
| CD | PET Parameters | Median ± SD | p |
| preSUVmaxTL | 13.0 ± 5.4 | 0.751 | |
| preSUVpeakTL | 10.0 ± 4.2 | 0.525 | |
| preTLGWB | 425,737 ± 586.6 | 0.130 | |
| preMTVWB | 203.0 ± 302.9 | 0.387 | |
| ΔSUVmax TL | −0.5 ± 6.7 | 0.003 | |
| ΔSUVpeak TL | −0.04 ± 7.2 | <0.001 | |
| ΔTLGWB | 242.8 ± 1375.6 | <0.001 | |
| ΔMTVWB | 34.8 ± 443.9 | 0.022 | |
| CB | Lymphoid Cell-Rich Organs | Median ± SD | p |
| postSUVmaxSp | 2.3 ± 0.6 | 0.586 | |
| postSUVmaxBM | 2.0 ± 0.4 | 0.464 | |
| Patients (n = 28) | Controlled Disease | Clinical Benefit | ||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Sex (male, female) | 0.113 (−0.216, 0.441) | 0.487 | 0.289 (−0.036, 0.614) | 0.079 |
| Histological variant (adenocarcinoma, squamous) | 0.246 (−0.219, 0.712) | 0.287 | 0.022 (−0.473, 0.518) | 0.927 |
| Previous lung surgery (yes, no) | 0.036 (−0.314, 0.386) | 0.835 | −0.233 (−0.585, 0.119) | 0.185 |
| Line immunotherapy (first, ≥second) | −0.390 (−0.719, −0.060) | 0.022 | 0.289 (−0.073, 0.651) | 0.113 |
| Drugs (pembrolizumab, nivolumab) | 0.292 (−0.093, 0.678) | 0.131 | −0.100 (−0.518, 0.318) | 0.627 |
| SUVmaxTL (<11.4 vs. >11.4) | −0.072 (−0.475, 0.331) | 0.717 | - | - |
| TLGWB (<194.1 vs. >194.1) | 0.215 (−0.179, 0.610) | 0.272 | - | - |
| MTVWB (<54 vs. >54) | 0.215 (−0.179, 0.610) | 0.272 | - | - |
| SUVpeakTL (<9 vs. >9) | 0.005 (−0.398, 0.408) | 0.979 | 0.056 (−0.363, 0.475) | 0.787 |
| ΔSUVmaxTL (<0.3 vs. >0.3) | −0.359 (−0.736, 0.018) | 0.061 | - | - |
| ΔTLGWB (<4.35 vs. >4.35) | −0.790 (−1.039, −0.541) | <0.001 | 0.622 (0.285, 0.960) | <0.001 |
| ΔMTVWB (<−2.55 vs. >−2.55) | −0.426 (−0.790, −0.061) | 0.024 | 0.678 (0.359, 0.996) | <0.001 |
| ΔSUVpeakTL (<−0.21 vs. >−0.21 | −0.503 (−0.852, −0.153) | 0.007 | 0.156 (−0.260, 0.572) | 0.449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Santo, G.; Merenda, N.; Branca, A.; Mammucci, P.; Pizzutilo, P.; Gadaleta, C.D.; Rubini, G. Immune Checkpoint Inhibitors in Advanced NSCLC: [18F]FDG PET/CT as a Troubleshooter in Treatment Response. Diagnostics 2021, 11, 1681. https://doi.org/10.3390/diagnostics11091681
Ferrari C, Santo G, Merenda N, Branca A, Mammucci P, Pizzutilo P, Gadaleta CD, Rubini G. Immune Checkpoint Inhibitors in Advanced NSCLC: [18F]FDG PET/CT as a Troubleshooter in Treatment Response. Diagnostics. 2021; 11(9):1681. https://doi.org/10.3390/diagnostics11091681
Chicago/Turabian StyleFerrari, Cristina, Giulia Santo, Nunzio Merenda, Alessia Branca, Paolo Mammucci, Pamela Pizzutilo, Cosmo Damiano Gadaleta, and Giuseppe Rubini. 2021. "Immune Checkpoint Inhibitors in Advanced NSCLC: [18F]FDG PET/CT as a Troubleshooter in Treatment Response" Diagnostics 11, no. 9: 1681. https://doi.org/10.3390/diagnostics11091681
APA StyleFerrari, C., Santo, G., Merenda, N., Branca, A., Mammucci, P., Pizzutilo, P., Gadaleta, C. D., & Rubini, G. (2021). Immune Checkpoint Inhibitors in Advanced NSCLC: [18F]FDG PET/CT as a Troubleshooter in Treatment Response. Diagnostics, 11(9), 1681. https://doi.org/10.3390/diagnostics11091681






