Is It Possible to Establish a Reliable Correlation between Maximum Standardized Uptake Value of 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Histological Types of Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Database
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. PET/CT Image Acquisition
2.3. Endpoints of the Study
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Italian VATS Group Collaborator List
Conflicts of Interest
References
- Khuri, F.R.; Herbst, R.S.; Fossella, F.V. Emerging therapies in non-small-cell lung cancer. Ann. Oncol. 2001, 12, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Lackey, A.; Donnington, J.S. Surgical management of Lung Cancer. Semin. Int. Radiol. 2013, 30, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.-Y.; Chen, J.; Zhou, F.-M.; Hu, Y.; Li, M.-X.; Wu, Q.-W.; Han, D.-M. CT-pathologic correlation in lung adenocarcinoma and squamous cell carcinoma. Medicine (B0altimore) 2018, 97, e13362. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Grgic, A.; Yuksel, Y.; Groschel, A.; Schafers, H.J.; Sybrecht, G.W.; Kirsch, C.M.; Hellwig, D. Risk Stratification of Solitary Pulmonary nodules by means of PET using (18) F-fluorodeoxyglucose and SUV qualification. Eur. J. Nucl. Med. Mol. Imag. 2010, 37, 1087–1094. [Google Scholar] [CrossRef]
- Divisi, D.; Barone, M.; Crisci, R. Current role of standardized uptake valuemax-derived ratios in N2 fluorine-18 fluorodeoxyglucose positron-emission tomography non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 503–507. [Google Scholar] [CrossRef]
- Divisi, D.; Di Tommaso, S.; Di Leonardo, G.; Brianzoni, E.; De Vico, A.; Crisci, R. 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography with Computerized Tomography Versus Computerized Tomography Alone for the Management of Solitary Lung Nodules with Diameters Inferior to 1.5 cm. Thorac. Cardiovasc. Surg. 2010, 58, 422–426. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.; Mao, Y.; Mu, J.; Xue, Q.; Feng, X.; He, J. Surgical Outcomes of Synchronous Multiple Primary Non-Small Cell Lung Cancers. Sci. Rep. 2016, 6, 23252. [Google Scholar] [CrossRef]
- Divisi, D.; Barone, M.; Zaccagna, G.; Crisci, R. Fluorine-18 fluorodeoxyglucose positron emission tomography in the management of solitary pulmonary nodule: A review. Ann. Med. 2017, 49, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.; Ohja, B.; Bartolucci, A.A. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J. Thorac. Cardiovasc. Surg. 2005, 130, 151–159. [Google Scholar] [CrossRef]
- Divisi, D.; Italian VATS Group; Barone, M.; Bertolaccini, L.; Rocco, G.; Solli, P.; Crisci, R. Standardized uptake value and radiological density attenuation as predictive and prognostic factors in patients with solitary pulmonary nodules: Our experience on 1592 patients. J. Thorac. Dis. 2017, 9, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imag. 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Hochhegger, B.; Alves, G.R.T.; Irion, K.; Fritscher, C.C.; Fritscher, L.G.; Concatto, N.H.; Marchiori, E. PET/CT imaging in lung cancer: Indications and findings. J. Bras. Pneumol. 2015, 41, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Divisi, D.; Barone, M.; Bertolaccini, L.; Zaccagna, G.; Gabriele, F.; Crisci, R. Diagnostic performance of fluorine-18 fluorodeoxy-glucose positron emission tomography in the management of solitary pulmonary nodule: A meta-analysis. J. Thorac. Dis. 2018, 10 (Suppl. 7), S779–S789. [Google Scholar] [CrossRef]
- Schreyogg, J.; Weller, J.; Stargardt, T.; Herrmann, K.; Bluemel, C.; Dechow, T.; Glatting, G.; Krause, B.J.; Mottaghy, F.; Reske, S.N.; et al. Cost-Effectiveness of Hybrid PET/CT for Staging of Non-Small Cell Lung Cancer. J. Nucl. Med. 2010, 51, 1668–1675. [Google Scholar] [CrossRef]
- Kapucu, L.; Meltzer, C.C.; Townsend, D.W.; Keenan, R.J.; Luketich, J.D. Fluorine-18-fluorodeoxyglucose uptake in pneumonia. J. Nucl. Med. 1998, 39, 1267–1269. [Google Scholar] [PubMed]
- Ollemberger, G.P.; Knight, S.; Tauro, A. False-positive FDG positron emission tomography in Pulmonary Amyloidosis. Clin. Nucl. Med. 2004, 29, 657–658. [Google Scholar] [CrossRef]
- Thie, J.A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004, 45, 1431–1434. [Google Scholar]
- Aquino, S.L.; Halpern, E.F.; Kuester, L.B.; Fischman, A.J. FDG-PET and CT features of non-small cell lung cancer based on tumor type. Int. J. Mol. Med. 2007, 19, 495–499. [Google Scholar] [CrossRef][Green Version]
- de Geus-Oei, L.F.; van Krieken, J.H.; Aliredjo, R.P.; Krabbe, P.F.M.; Frielink, C.; Verhagen, A.F.T.; Boerman, O.C.; Oyen, W.J.G. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 2007, 55, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, N.; Ito, M.; Qiao, S.; Uchida, K.; Takao, M.; Yamada, T.; Takeda, K.; Murashima, S. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 2011, 72, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Usuda, K.; Sagawa, M.; Aikawa, H.; Ueno, M.; Tanaka, M.; Machida, Y.; Zhao, X.-T.; Ueda, Y.; Higashi, K.; Sakuma, T. Correlation between glucose transporter-1 expression and 18F-fluoro-2-deoxyglucose uptake on positron emission tomography in lung cancer. Gen. Thorac. Cardiovasc. Surg. 2010, 58, 405–410. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lee, S.J.; Park, S.Y. Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters. Lung Cancer 2017, 104, 31–37. [Google Scholar] [CrossRef]
- Nakamura, H.; Saji, H.; Shinmyo, T.; Tagaya, R.; Kurimoto, N.; Koizumi, H.; Takagi, M. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung Cancer 2015, 87, 28–33. [Google Scholar] [CrossRef]
- Kobayashy, Y.; Mitsudomi, T. Management of ground-glasse opacities: Should all pulmonary lesions with ground-glass opacity be surgically recected? Transl. Lung Cancer Res. 2013, 2, 354–363. [Google Scholar]
- Chiu, C.-H.; Yeh, Y.-C.; Lin, K.-H.; Wu, Y.-C.; Lee, Y.-C.; Chou, T.-Y.; Tsai, C.-M. Histological Subtypes of Lung Adenocarcinoma Have Differential 18F-Fluorodeoxyglucose Uptakes on the Positron Emission Tomography/Computed Tomography Scan. J. Thorac. Oncol. 2011, 6, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xiao, J.; Xiu, Y.; Fu, Z.; Shi, H.; Cheng, D. Correlation of PD-L1 expression on tumor cell and tumor infiltrating immune cell with 18F-flurodeoxyglucose uptake on PET/computed tomography in surgically resected pulmonary adenocarcinoma. Nucl. Med. Commun. 2020, 41, 252–259. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Du, B.; Diao, Y.; Li, Y. PD-L1 in Lung Adenocarcinoma: Insights into the Role of 18F-FDG PET/CT. Cancer Manag. Res. 2020, 12, 6385–6395. [Google Scholar] [CrossRef]
- Van Baardwijk, A.; Dooms, C.; Van Suylen, R.J. The maximum uptake of (18)F-deoxyglucose on Positron Emission Tomography scan correlates with survival hypoxia inducible factor-1alpha and GLUT-1 in Non-Small Cell Lung Cancer. Eur. J. Cancer 2007, 43, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Lee, W.W.; Chung, J.-H.; Park, S.Y.; Kim, Y.K.; Kim, S.E. Correlation between FDG uptake and glucose transporter type 1 expression in neuroendocrine tumors of the lung. Lung Cancer 2008, 61, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A Comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in Pulmonary Neuroendocrine Tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef] [PubMed]


| Clinical Data | N | % | Mean | Interval |
|---|---|---|---|---|
| Gender | ||||
| Male | 4879 | 59.9% | ||
| Female | 3260 | 40.1% | ||
| Age | 67.9 | 18–90 | ||
| Nodule Size (CT) | ||||
| Group 0 (T < 2 cm) | 3869 | 47.6% | ||
| Group 1 (2.1 < T > 3 cm) | 2198 | 27% | ||
| Group 2 (3.1 < T > 5 cm) | 1669 | 20.5% | ||
| Group 3 (5.1 < T > 7 cm) | 345 | 4.2% | ||
| Group 4 (T > 7 cm) | 58 | 0.7% | ||
| Nodule Density (CT) | ||||
| Solid | 6469 | 79.5% | ||
| Part-solid | 1452 | 17.8% | ||
| Pure GGO | 218 | 2.7% | ||
| SUVmax (PET) | 6.19 | 0–78 | ||
| Type of lesion | ||||
| Malignant (primary) | 7783 | 95.6% | ||
| Malignant (secondary) | 356 | 4.4% | ||
| Type of resection | ||||
| Upper Lobectomy | 4407 | 54.1% | ||
| Middle Lobectomy | 590 | 7.3% | ||
| Lower Lobectomy | 2742 | 33.7% | ||
| Upper Bilobectomy | 69 | 0.9% | ||
| Lower Bilobectomy | 49 | 0.6% | ||
| Basal Segmentectomy | 39 | 0.5% | ||
| Apical segmentectomy of upper lobe | 99 | 1.2% | ||
| Apical segmentectomy of lower lobe | 83 | 1.0% | ||
| Lingulectomy | 61 | 0.7% |
| Types | N | % | |
|---|---|---|---|
| Adenocarcinoma * | |||
| Path 0 | Preinvasive lesion | 187 | 2.3% |
| Path 1 | Minimally invasive | 767 | 9.4% |
| Path 2 | Invasive Adenocarcinoma | 4580 | 56.3% |
| Path 3 | Squamous Cell Carcinoma * | 1254 | 15.4% |
| Path 4 | Adenosquamous Cell Carcinoma | 90 | 1.1% |
| Path 5 | Pleomorphic Carcinoma | 33 | 0.4% |
| Neuroendocrine Tumors * | |||
| Path 6 | Typical Carcinoid | 448 | 5.5% |
| Path 7 | Atypical Carcinoid | 107 | 1.3% |
| Path 8 | Small Cell Lung Cancer | 60 | 0.7% |
| Path 9 | Large Cell Neuroendocrine Carcinoma | 180 | 2.2% |
| Other Carcinomas * | |||
| Path 10 | NSCLC NAS | 23 | 0.3% |
| Path 11 | Mucoepidermoid Carcinoma | 7 | 0.1% |
| Path 12 | Carcinosarcoma | 26 | 0.3% |
| Path 13 | Adenoidocystic Carcinoma | 13 | 0.2% |
| Path 14 | Lymphoma * | 8 | 0.1% |
| Path 15 | Metastatic Cancer * | 356 | 4.4% |
| N | Mean | St. Dev | Median | Min | Max | |
|---|---|---|---|---|---|---|
| Path 0 | 187 | 4.88 | 3.82 | 4.00 | 0.00 | 24.00 |
| Path 1 | 767 | 5.49 | 4.10 | 4.10 | 0.00 | 31.00 |
| Path 2 | 4580 | 5.87 | 4.18 | 4.70 | 0.00 | 45.00 |
| Path 3 | 1254 | 8.85 | 6.70 | 8.00 | 0.00 | 51.00 |
| Path 4 | 90 | 6.55 | 5.39 | 6.00 | 0.00 | 21.00 |
| Path 5 | 33 | 8.15 | 6.47 | 8.50 | 0.00 | 24.00 |
| Path 6 | 448 | 3.83 | 2.76 | 3.00 | 0.00 | 78.00 |
| Path 7 | 107 | 6.41 | 3.48 | 4.00 | 0.00 | 77.00 |
| Path 8 | 60 | 7.13 | 5.05 | 6.65 | 0.00 | 21.46 |
| Path 9 | 180 | 8.64 | 6.07 | 7.27 | 0.00 | 39.00 |
| Path 10 | 23 | 7.11 | 5.51 | 7.00 | 0.00 | 18.39 |
| Path 11 | 7 | 3.86 | 3.13 | 4.00 | 0.00 | 8.00 |
| Path 12 | 26 | 9.36 | 7.30 | 8.00 | 0.00 | 28.00 |
| Path 13 | 13 | 4.00 | 3.39 | 4.80 | 0.00 | 10.00 |
| Path 14 | 8 | 3.41 | 2.56 | 3.00 | 0.00 | 8.00 |
| Path 15 | 356 | 5.57 | 3.57 | 4.60 | 0.00 | 31.00 |
| STAGE | N | % |
|---|---|---|
| IA1 | 2148 | 31.1% |
| IA2 | 1868 | 27% |
| IA3 | 715 | 10.3% |
| IB | 1061 | 15.3% |
| IIA | 190 | 2.8% |
| IIB | 580 | 8.4% |
| IIIA | 301 | 4.4% |
| IIIB | 45 | 0.7% |
| IIIC | 3 | 0% |
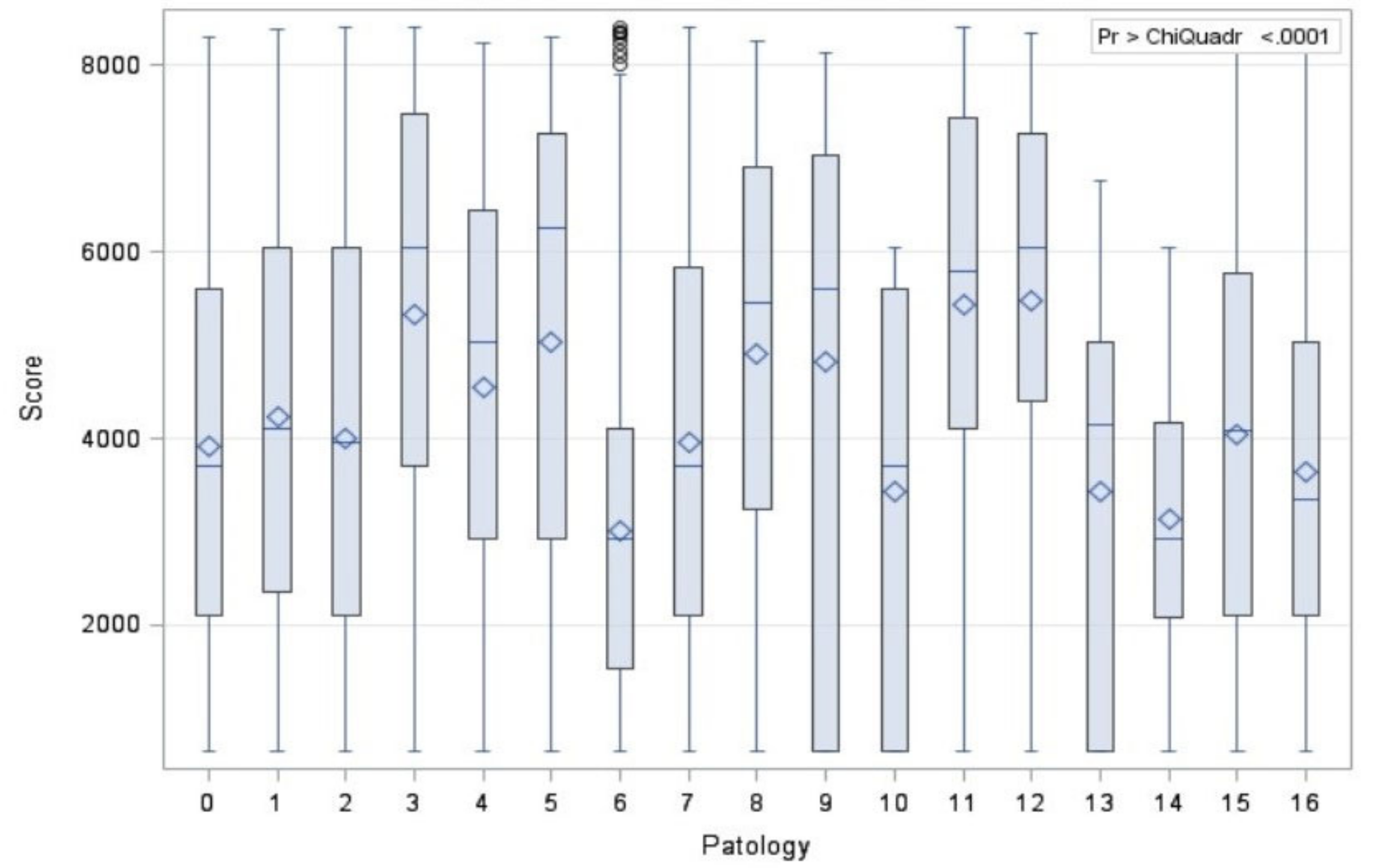
| Pathology | N | Average Rank | Different (p < 0.05) from Factor Nr. |
|---|---|---|---|
| Path 0 | 187 | 3094.74 | 3 |
| Path 1 | 767 | 3357.34 | 2–3 |
| Path 2 | 4580 | 3177.57 | 1–3 |
| Path 3 | 1254 | 4254.24 | 0–1–2 |
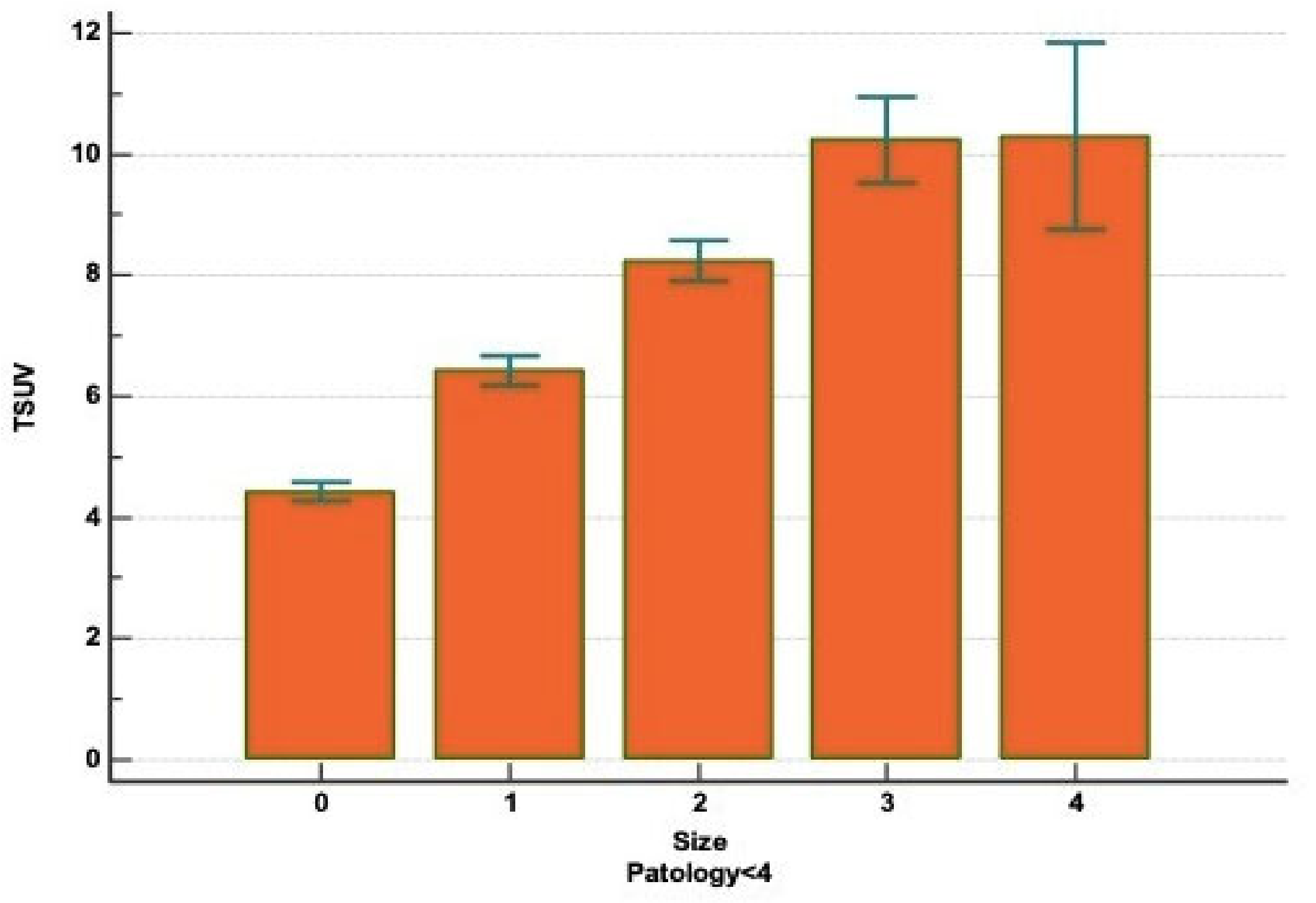
| Figure | N | Average Rank | Different (p < 0.05) from Factor Nr. |
|---|---|---|---|
| 0 (<2 cm) | 3052 | 2788.82 | 1–2–3–4 |
| 1 (between 2.1 and 3 cm) | 1876 | 3651.76 | 0–2–3–4 |
| 2 (between 3.1 and 5 cm) | 1464 | 4083.42 | 0–1–3–4 |
| 3 (between 5.1 and 7 cm) | 323 | 4729.68 | 0–1–2 |
| 4 (>7 cm) | 73 | 4694.71 | 0–1–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divisi, D.; Rinaldi, M.; Necozione, S.; Curcio, C.; Rea, F.; Zaraca, F.; De Vico, A.; Zaccagna, G.; Di Leonardo, G.; Crisci, R.; et al. Is It Possible to Establish a Reliable Correlation between Maximum Standardized Uptake Value of 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Histological Types of Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Database. Diagnostics 2021, 11, 1901. https://doi.org/10.3390/diagnostics11101901
Divisi D, Rinaldi M, Necozione S, Curcio C, Rea F, Zaraca F, De Vico A, Zaccagna G, Di Leonardo G, Crisci R, et al. Is It Possible to Establish a Reliable Correlation between Maximum Standardized Uptake Value of 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Histological Types of Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Database. Diagnostics. 2021; 11(10):1901. https://doi.org/10.3390/diagnostics11101901
Chicago/Turabian StyleDivisi, Duilio, Marta Rinaldi, Stefano Necozione, Carlo Curcio, Federico Rea, Francesco Zaraca, Andrea De Vico, Gino Zaccagna, Gabriella Di Leonardo, Roberto Crisci, and et al. 2021. "Is It Possible to Establish a Reliable Correlation between Maximum Standardized Uptake Value of 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Histological Types of Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Database" Diagnostics 11, no. 10: 1901. https://doi.org/10.3390/diagnostics11101901
APA StyleDivisi, D., Rinaldi, M., Necozione, S., Curcio, C., Rea, F., Zaraca, F., De Vico, A., Zaccagna, G., Di Leonardo, G., Crisci, R., & on behalf of the Italian VATS Group. (2021). Is It Possible to Establish a Reliable Correlation between Maximum Standardized Uptake Value of 18-Fluorine Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Histological Types of Non-Small Cell Lung Cancer? Analysis of the Italian VATS Group Database. Diagnostics, 11(10), 1901. https://doi.org/10.3390/diagnostics11101901






