Laser Doppler Imaging for Treating Vascular Complications from Procedures Involving Dermal Fillers: Case Series and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Design
2.2. Pre-Intervention Treatment Evaluation
2.3. LDI Procedure
2.4. Needling Procedure
2.5. Ancillary Therapies
2.6. Post-Treatment Evaluations
3. Results
3.1. Patients’ Characteristics
3.2. LDI Provides an Accurate Delineation of the Hypoperfused Region
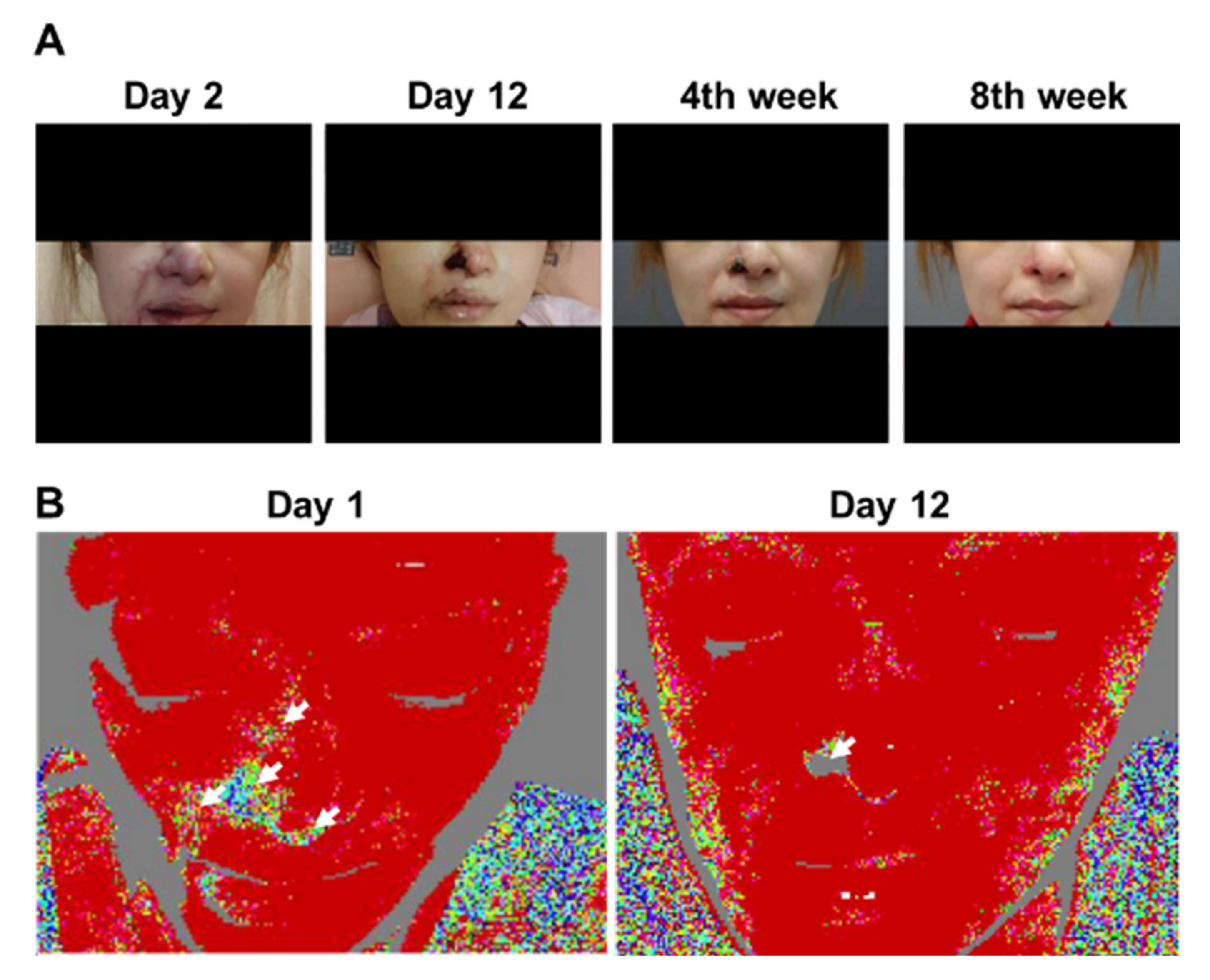
3.3. Patient 3
3.4. Patient 6
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dermal Fillers (Soft Tissue Fillers). Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/ucm619837.html (accessed on 3 April 2017).
- Cosmetic Plastic Surgery Statistics. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-%20full-report-2017.pdf (accessed on 3 April 2017).
- Pierre, S.; Liew, S.; Bernardin, A. Basics of dermal filler rheology. Dermatol. Surg. 2015, 41 (Suppl. 1), S120–S126. [Google Scholar] [CrossRef]
- Chiang, Y.Z.; Pierone, G.; Al-Niaimi, F. Dermal fillers: Pathophysiology, prevention and treatment of complications. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 405–413. [Google Scholar] [CrossRef]
- Hanke, C.W.; Higley, H.R.; Jolivette, D.M.; Swanson, N.A.; Stegman, S.J. Abscess formation and local necrosis after treatment with Zyderm or Zyplast collagen implant. J. Am. Acad. Dermatol. 1991, 25, 319–326. [Google Scholar] [CrossRef]
- Beleznay, K.; Humphrey, S.; Carruthers, J.D.; Carruthers, A. Vascular compromise from soft tissue augmentation: Experience with 12 cases and recommendations for optimal outcomes. J. Clin. Aesthetic Dermatol. 2014, 7, 37–43. [Google Scholar]
- DeLorenzi, C. Complications of injectable fillers, part 2: Vascular complications. Aesthetic Surg. J. 2014, 34, 584–600. [Google Scholar] [CrossRef] [Green Version]
- Rzany, B.; DeLorenzi, C. Understanding, Avoiding, and Managing Severe Filler Complications. Plast. Reconstr. Surg. 2015, 136, 196S–203S. [Google Scholar] [CrossRef]
- Liu, Y.C.; Tsai, M.F.; Chen, Y.F. Near Complete Recovery of Visual Acuity After Calcium Hydroxylapatite Injection-Related Vision Loss: A Case Report and Literature Review. Ann. Plast. Surg. 2020, 84, S123–S127. [Google Scholar] [CrossRef]
- Woodward, J.; Khan, T.; Martin, J. Facial Filler Complications. Facial Plast. Surg. Clin. North Am. 2015, 23, 447–458. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, B.; Hsiai, T.K.; Shung, K.K. A Review of Intravascular Ultrasound-based Multimodal Intravascular Imaging: The Synergistic Approach to Characterizing Vulnerable Plaques. Ultrason. Imaging 2016, 38, 314–331. [Google Scholar] [CrossRef] [Green Version]
- Dyson, M.; Moodley, S.; Verjee, L.; Verling, W.; Weinman, J.; Wilson, P. Wound healing assessment using 20 MHz ultrasound and photography. Skin Res. Technol. 2003, 9, 116–121. [Google Scholar] [CrossRef]
- Porter-Armstrong, A.P.; Adams, C.; Moorhead, A.S.; Donnelly, J.; Nixon, J.; Bader, D.L.; Lyder, C.; Stinson, M.D. Do high frequency ultrasound images support clinical skin assessment? ISRN Nurs. 2013, 2013, 314248. [Google Scholar] [CrossRef]
- Ueta, M.; Sugama, J.; Konya, C.; Matsuo, J.; Matsumoto, M.; Yabunaka, K.; Nakatani, T.; Tabata, K. Use of ultrasound in assessment of necrotic tissue in pressure ulcers with adjacent undermining. J. Wound Care 2011, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Draijer, M.; Hondebrink, E.; van Leeuwen, T.; Steenbergen, W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med. Sci. 2009, 24, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Hecht, N.; Woitzik, J.; Dreier, J.P.; Vajkoczy, P. Intraoperative monitoring of cerebral blood flow by laser speckle contrast analysis. Neurosurg. Focus 2009, 27, E11. [Google Scholar] [CrossRef]
- Sandker, S.C.; Hondebrink, E.; Grandjean, J.G.; Steenbergen, W. Laser speckle contrast analysis for quantifying the Allen test: A feasibility study. Lasers Surg. Med. 2014, 46, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Clough, G.F. Scanning laser Doppler imaging and dermal microdialysis in the investigation of skin inflammation. Allergy Clin. Immunol. Int. 1997, 113, 41–46. [Google Scholar]
- Murray, A.K.; Herrick, A.L.; King, T.A. Laser Doppler imaging: A developing technique for application in the rheumatic diseases. Rheumatology 2004, 43, 1210–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opazo Saez, A.M.; Mosel, F.; Nurnberger, J.; Rushentsova, U.; Gossl, M.; Mitchell, A.; Schafers, R.F.; Philipp, T.; Wenzel, R.R. Laser Doppler imager (LDI) scanner and intradermal injection for in vivo pharmacology in human skin microcirculation: Responses to acetylcholine, endothelin-1 and their repeatability. Br. J. Clin. Pharmacol. 2005, 59, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Droog, E.J.; Steenbergen, W.; Sjoberg, F. Measurement of depth of burns by laser Doppler perfusion imaging. Burns 2001, 27, 561–568. [Google Scholar] [CrossRef]
- Niazi, Z.B.; Essex, T.J.; Papini, R.; Scott, D.; McLean, N.R.; Black, M.J. New laser Doppler scanner, a valuable adjunct in burn depth assessment. Burns 1993, 19, 485–489. [Google Scholar] [CrossRef]
- Pape, S.A.; Skouras, C.A.; Byrne, P.O. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 2001, 27, 233–239. [Google Scholar] [CrossRef]
- Urdiales-Galvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Moreno, A.; Ortiz-Marti, F.; Del Rio-Reyes, R.; Romero-Alvarez, N.; Del Cueto, S.R.; et al. Treatment of Soft Tissue Filler Complications: Expert Consensus Recommendations. Aesthetic Plast. Surg. 2018, 42, 498–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLorenzi, C. Complications of injectable fillers, part I. Aesthetic Surg. J. 2013, 33, 561–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C. Avoiding the “danger zones” when injecting dermal fillers and volume enhancers. Plast. Surg. Nurs. 2014, 34, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Tansatit, T.; Apinuntrum, P.; Phetudom, T. A Dark Side of the Cannula Injections: How Arterial Wall Perforations and Emboli Occur. Aesthetic Plast. Surg. 2017, 41, 221–227. [Google Scholar] [CrossRef]
- Bailey, S.H.; Cohen, J.L.; Kenkel, J.M. Etiology, prevention, and treatment of dermal filler complications. Aesthetic Surg. J. 2011, 31, 110–121. [Google Scholar] [CrossRef]
- Maruyama, S. A Histopathologic Diagnosis of Vascular Occlusion After Injection of Hyaluronic Acid Filler: Findings of Intravascular Foreign Body and Skin Necrosis. Aesthetic Surg. J. 2017, 37, NP102–NP108. [Google Scholar] [CrossRef]
- De Boulle, K.; Heydenrych, I. Patient factors influencing dermal filler complications: Prevention, assessment, and treatment. Clin. Cosmet. Investig. Dermatol. 2015, 8, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Cotofana, S.; Pretterklieber, B.; Lucius, R.; Frank, K.; Haas, M.; Schenck, T.L.; Gleiser, C.; Weyers, I.; Wedel, T.; Pretterklieber, M. Distribution Pattern of the Superior and Inferior Labial Arteries: Impact for Safe Upper and Lower Lip Augmentation Procedures. Plast. Reconstr. Surg. 2017, 139, 1075–1082. [Google Scholar] [CrossRef]
- Goodman, G.J.; Roberts, S.; Callan, P. Experience and Management of Intravascular Injection with Facial Fillers: Results of a Multinational Survey of Experienced Injectors. Aesthetic Plast. Surg. 2016, 40, 549–555. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Li, H.; Li, P.; Wang, J. Vascular Complications After Chin Augmentation Using Hyaluronic Acid. Aesthetic Plast. Surg. 2018, 42, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.E.H.; van Haasterecht, L.; van Zuijlen, P.P.M.; Mokkink, L.B. A systematic review on the quality of measurement techniques for the assessment of burn wound depth or healing potential. Burns 2019, 45, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Identification and Complications of Cosmetic Fillers: Sonography First. J. Ultrasound Med. 2015, 34, 1163–1172. [Google Scholar] [CrossRef]
- Lin, C.-P.; Honye, J.; Chang, C.-J.; Kuo, C.-T. Clinical Application of Intravascular Ultrasound in Coronary Artery Disease: An Update. Acta Cardiol. Sin. 2011, 27, 1–13. [Google Scholar]
- DeLorenzi, C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events. Aesthetic Surg. J. 2017, 37, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.S.; Zhu, G.Z.; Wang, H.B.; Xu, X.; Cai, B.; Zeng, L.; Yang, J.Q.; Luo, S.K. Clinical Outcomes of Impending Nasal Skin Necrosis Related to Nose and Nasolabial Fold Augmentation with Hyaluronic Acid Fillers. Plast. Reconstr. Surg. 2015, 136, 434e–441e. [Google Scholar] [CrossRef]
- Han, J.; He, Y.; Liu, K.; Yang, Q. Necrosis of the Glabella After Injection With Hyaluronic Acid Into the Forehead. J. Craniofacial Surg. 2018, 29, e726–e727. [Google Scholar] [CrossRef]
- Salval, A.; Ciancio, F.; Margara, A.; Bonomi, S. Impending Facial Skin Necrosis and Ocular Involvement After Dermal Filler Injection: A Case Report. Aesthetic Plast. Surg. 2017, 41, 1198–1201. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, D.K.; Jeong, H.S.; Suh, I.S. Treatment algorithm of complications after filler injection: Based on wound healing process. J. Korean Med. Sci. 2014, 29 (Suppl. 3), S176–S182. [Google Scholar] [CrossRef] [Green Version]
- De Boulle, K. Management of complications after implantation of fillers. J. Cosmet. Dermatol. 2004, 3, 2–15. [Google Scholar] [CrossRef]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.P.; Fagien, S. Treatment of injectable soft tissue filler complications. Dermatol. Surg. 2009, 35 (Suppl. 2), 1672–1680. [Google Scholar] [CrossRef]
- Kleydman, K.; Cohen, J.L.; Marmur, E. Nitroglycerin: A review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol. Surg. 2012, 38, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
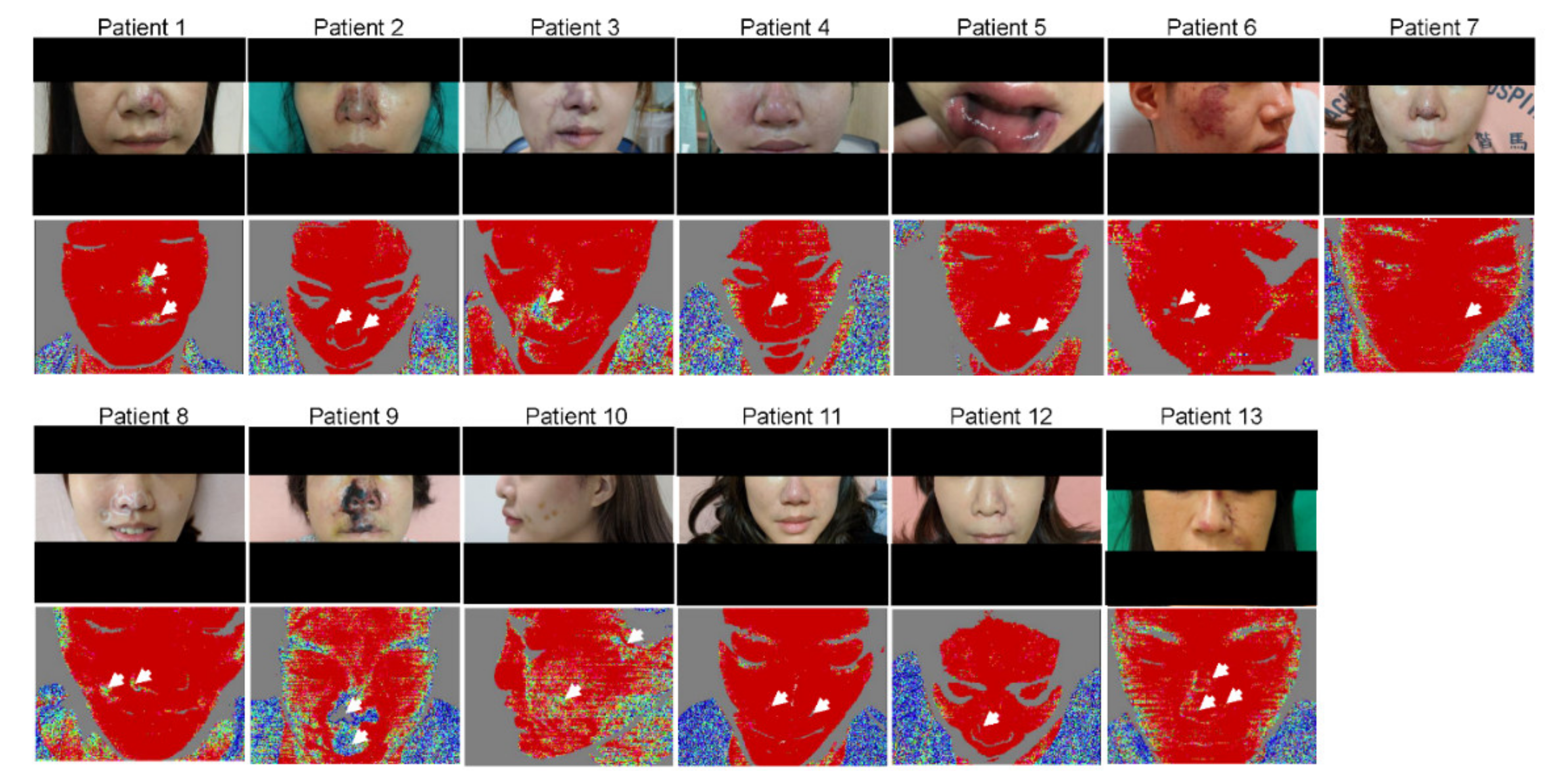

| Patients | Age (Years) | Sex | Past History | Location and Details of Dermal Filler Injections | Type of Dermal Filler | Overall Follow-Up (Days) | Day of Dermal Filler Injection before Admission |
|---|---|---|---|---|---|---|---|
| Patient 1 | 40 | F | Two NLF rejuvenations with PLLA in the past 3 years | NLF R/L 1 mL/1 mL (with 23-G cannula) | HA | 14 | D2 |
| Patient 2 | 25 | F | Nil | Nasal tip 0.3 mL/dorsum 0.3 mL/radix 0.3 mL/chin 0.6 mL (with 27-G needle) | CaHA | 24 | D4 |
| Patient 3 | 31 | F | Open rhinoplasty and septal reconstruction 1 year ago | NLF R/L 1 mL/1 mL (with 25-G cannula) | HA | 53 | D0 (6 h) |
| Patient 4 | 42 | F | Nil | Nasal tip 0.2 mL/dorsum 0.1 mL (with 27-G needle) | HA | 11 | D4 |
| Patient 5 | 45 | F | Nil | Upper and lower lips 1 mL (with 27-G needle) | HA | 13 | D1 |
| Patient 6 | 25 | M | Nil | Acne scar treatment (multiple sites involving bilateral cheeks): Subcision followed-by HA injection (with 27-G needle): 1 mL totally | HA | 20 | D6 |
| Patient 7 | 43 | F | Close rhinoplasty with prosthesis | Nose augmentation 2 c.c. (with 27-G needle) | HA | 13 | D3 |
| Patient 8 | 33 | F | Nil | Bilateral NLF R/L: 1.6 c.c./0.4 c.c. (with 27-G needle) | HA | 12 | D0 (6 h) |
| Patient 9 | 61 | F | Nil | Nasal tip 0.8 c.c. (with 27-G cannula) | HA | 100 | D6 |
| Patient 10 | 23 | F | Nil | Bilateral forehead and temporal fossa R/L: 3 c.c./3 c.c. (with 21-G cannula) | HA | 10 | D2 |
| Patient 11 | 43 | F | Nil | Bilateral NLF R/L: 0.4 c.c./0.4 c.c. Left tear trough: 0.3 c.c. (with 21-G cannula) | HA | 28 | D1 |
| Patient 12 | 29 | F | Nil | Bilateral NLF R/L: 0.4 c.c./0.4 c.c. (with 27-G needle) | HA | 12 | D1 |
| Patient 13 | 47 | F | Nil | Bilateral NLF R/L: HA 1 c.c./1 c.c. (with 22-G cannula) | HA | 12 | D2 |
| Patients | Day of or 1 Day after Admission | Three Days after Admission | Approximately 2 Weeks after Admission | |||
|---|---|---|---|---|---|---|
| Clinical/Photographic Findings | LDI Findings | Clinical/Photographic Findings | LDI Findings | Clinical/Photographic Findings | LDI Findings | |
| Patient 1 | Cyanotic change of the L nasal ala and the L upper lip | L nasal alar area partial perfusion injury | Few gangrenous changes of the L nasal ala | No perfusion defect | Few scars on and PIH of the L nasal ala | NA |
| Patient 2 | Cyanotic change of bilateral nasal ala and nasal tip | R nasal alar perfusion defect | Gangrenous change of the R nasal ala and few cyanotic changes of L nasal ala and nasal tip | No perfusion defect | Few scars on and PIH of the R cheek | NA |
| Patient 3 | Cyanotic change of the nasal tip, R nasal ala, R NLF, R mouth angle/R upper and lower lips, R glabellar region | R nasal ala, R NLF, R lower lip partial perfusion defect | Cyanotic change of the R nasal ala, R mouth angle/right upper and lower lips | R nasal alar perfusion defect | R alar eschar | No perfusion over R nasal ala |
| Patient 4 | Ischemic change of the nasal tip/bilateral nasal ala/dorsum and R glabellar region | R and L nasal alar area perfusion defects | Ischemic change of the nasal tip/dorsum, gangrenous change of bilateral nasal ala | NA | NA | NA |
| Patient 5 | Cyanotic changes and swelling of the upper and lower lips | NA | R oral ulcer (MBD) | No perfusion defect | Healing R oral ulcer | NA |
| Patient 6 | R cheek skin necrosis | R cheek spot perfusion deformity | Healing skin necrosis of the R cheek | R cheek spot perfusion deformity disappeared compared with 2 days prior | Few scars on and PIH of the R cheek | NA |
| Patient 7 | Cyanotic changes of the nasal tip and columella | NA | Few cyanotic changes and swelling of nasal tip | No perfusion defect | Minimal scar and PIH on nasal tip | NA |
| Patient 8 | Cyanotic changes of the right nasal ala and right NLF | Right nasal ala and right NLF perfusion defect | Few cyanotic changes of the right nasal alar and right NLF | No perfusion defect | Few PIH on right nasal ala | NA |
| Patient 9 | Ischemic changes of the bilateral nasal ala, nasal tip, right NLF, and upper lip | Bilateral nasal ala, nasal tip, right NLF, and upper lip perfusion defect | Gangrene changes of the bilateral nasal ala, nasal tip, right NLF, and upper lip | NA | Gangrene changes of the bilateral nasal ala, nasal tip, right NLF, and upper lip | NA |
| Patient 10 | Cyanotic changes of left forehead, left temporal fossa, and left preauricular region. Left upper eyelid ptosis | Left forehead, left temporal fossa, and left preauricular region perfusion defect | Cyanotic changes of left forehead, left temporal fossa, and left preauricular region. Left upper eyelid ptosis | NA | Few PIH on left forehead, left temporal fossa, and left preauricular region. | NA |
| Patient 11 | Cyanotic changes of the bilateral nasal ala, nasal tip, and left NLF | NA | Few cyanotic changes of the bilateral nasal ala and nasal tip | Bilateral nasal ala and nasal tip perfusion defect | Few PIH on nasal tip | NA |
| Patient 12 | Cyanotic changes of the left nasal ala and left NLF | No perfusion defect | Few cyanotic changes of the left nasal ala and left NLF | NA | Few PIH on left nasal ala and left NLF | NA |
| Patient 13 | Cyanotic changes of the nasal tip, left nasal ala, left nasal dorsum, and left NLF | Left nasal ala, nasal tip, and left nasal dorsum perfusion defect | Few cyanotic changes and swelling of the nasal tip, left nasal ala, left nasal dorsum, and left NLF | No perfusion defect | Few PIH on left nasal ala and nasal tip | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.-L.; Chen, Y.-F.; Yao, W.-T.; Liu, Y.-C.; Yu, C.-M.; Yu, C.-M.; Tu, C.-P.; Huang, W.-C.; Tung, K.-Y.; Tsai, M.-F. Laser Doppler Imaging for Treating Vascular Complications from Procedures Involving Dermal Fillers: Case Series and Literature Review. Diagnostics 2021, 11, 1640. https://doi.org/10.3390/diagnostics11091640
Lee A-L, Chen Y-F, Yao W-T, Liu Y-C, Yu C-M, Yu C-M, Tu C-P, Huang W-C, Tung K-Y, Tsai M-F. Laser Doppler Imaging for Treating Vascular Complications from Procedures Involving Dermal Fillers: Case Series and Literature Review. Diagnostics. 2021; 11(9):1640. https://doi.org/10.3390/diagnostics11091640
Chicago/Turabian StyleLee, An-Li, Yu-Fan Chen, Wen-Teng Yao, Ying-Chun Liu, Chia-Meng Yu, Chieh-Ming Yu, Chih-Peng Tu, Wen-Chen Huang, Kwang-Yi Tung, and Ming-Feng Tsai. 2021. "Laser Doppler Imaging for Treating Vascular Complications from Procedures Involving Dermal Fillers: Case Series and Literature Review" Diagnostics 11, no. 9: 1640. https://doi.org/10.3390/diagnostics11091640
APA StyleLee, A.-L., Chen, Y.-F., Yao, W.-T., Liu, Y.-C., Yu, C.-M., Yu, C.-M., Tu, C.-P., Huang, W.-C., Tung, K.-Y., & Tsai, M.-F. (2021). Laser Doppler Imaging for Treating Vascular Complications from Procedures Involving Dermal Fillers: Case Series and Literature Review. Diagnostics, 11(9), 1640. https://doi.org/10.3390/diagnostics11091640






