A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules
Abstract
:1. Introduction
2. Materials and Methods
2.1. The bioMILD Trial
2.1.1. Imaging Acquisition and Interpretation
- Negative LDCT (LDCT-): no PN detected, nodule with fat or benign pattern of calcifications, SN < 113 mm3 or NSN < 5 mm;
- Indeterminate LDCT (LDCT Ind): SN 113–260 mm3, PSN with solid component < 5 mm or NSN > 5 mm;
- Positive LDCT (LDCT+): SN > 260 mm3, PSN with solid component > 5 mm.
2.1.2. Quantitative Analysis and Radiomic Feature Measurement
2.1.3. Dataset Composition
- 544 PNs (dataset I), classified into SNs (324, 59.6%) and SSNs (220, 40.4%), of which 55 (25%) were PSNs and 165 (75%) NSNs—based on LDCT density measured by the readers as previously described (see Section 2.1.1) and used as reference standard I [36];
- 326 PNs (dataset II), sent to biopsy and then classified into malignant (32, 9.8%) and benign (294, 90.2%), based on histopathological features (reference standard II).
- 159 PNs (dataset III), classified into SNs (81, 50.9%) and SSNs (78, 49.1%)—further classified into PSNs (29, 37.2%) and NSNs (49, 62.8%);
- 104 PNs (dataset IV), sent to biopsy and then classified as malignant (13, 12.5%) and benign (91, 87.5%).
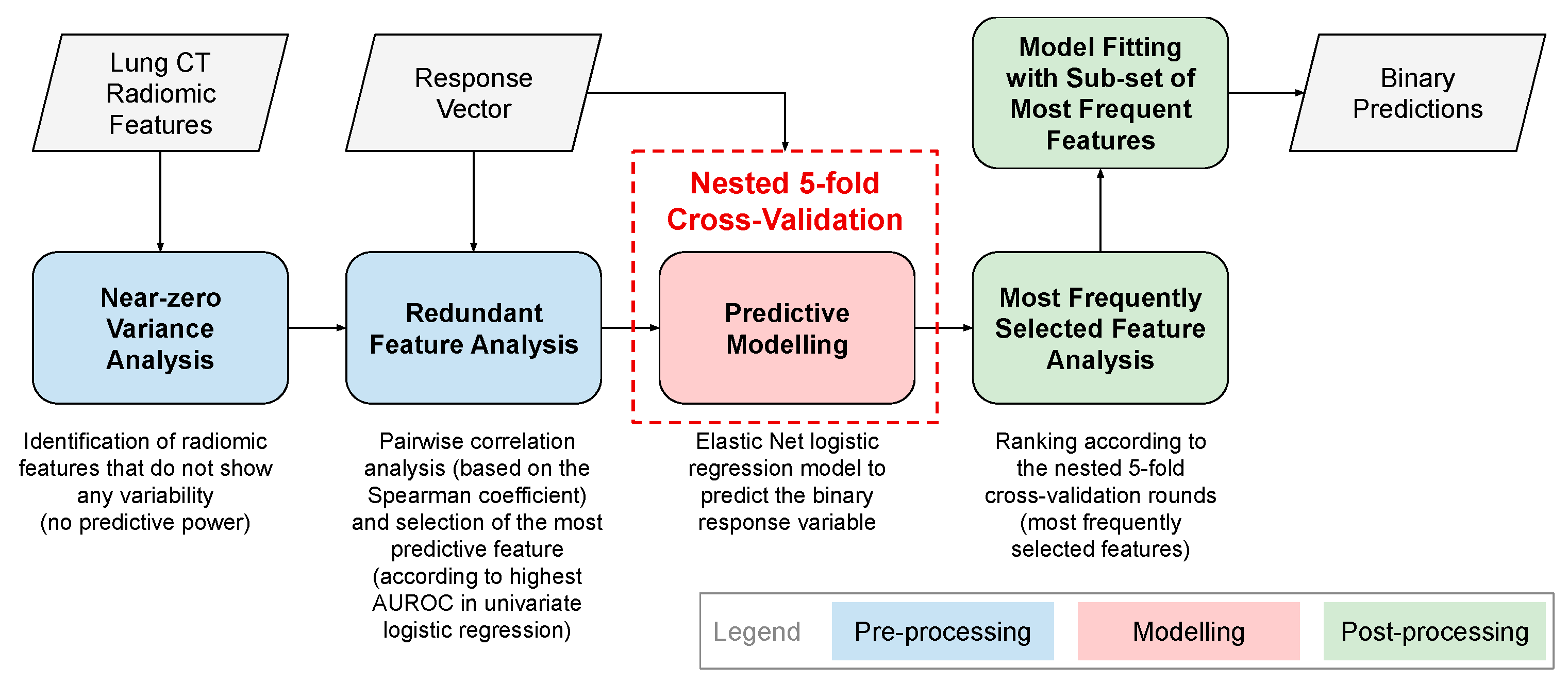
2.2. Radiomic Analyses
- Aim 1 (PN characterization): developing a SN vs. SSN radiomic automatic classifier. For this purpose, datasets I and III were used. Moreover, a sub-classification of SSN into NSN vs. PSN was performed by a second-level radiomic classifier.
- Aim 2 (PN risk): developing a malignant vs. benign nodule radiomic automatic classifier. For this purpose, datasets II and IV were used.
2.2.1. Pre-Processing of Radiomic Features
Near-Zero Variance Analysis
Redundant Feature Analysis
2.2.2. Cross-Validation of Radiomic Classifiers on Discovery Datasets
2.2.3. Post-Processing of Radiomic Features: Radiomic Predictors
2.2.4. Integration and Comparisons of Radiomic Predictors with Clinical Features and Semantic LDCT Features
- Best solidity radiomic features: best radiomic predictors of Aim 1, as an objective measure of solidity of each PN;
- Clinical features: body mass index (BMI), forced expiratory volume in 1 second (FEV1), and C-reactive protein (CRP);
- Semantic LDCT features: emphysema extent, type (centrilobular, paraseptal, or panlobular) and location (lobes involved); anterior descending, circumflex and right coronary artery calcifications (categorical values); and bronchial wall thickening (dichotomous variable).
2.2.5. Testing Radiomic Classifiers on Blinded Test Dataset
2.2.6. Re-Training Classifier with Feature Class Imbalance Correction
Minority Class Over-Sampling
Majority Class Under-Sampling
2.2.7. Impact of Radiomic Classifiers on PN Characterization and Screening Recall Intervals
3. Results
3.1. Pre-Processing of Radiomic Features on Discovery Datasets
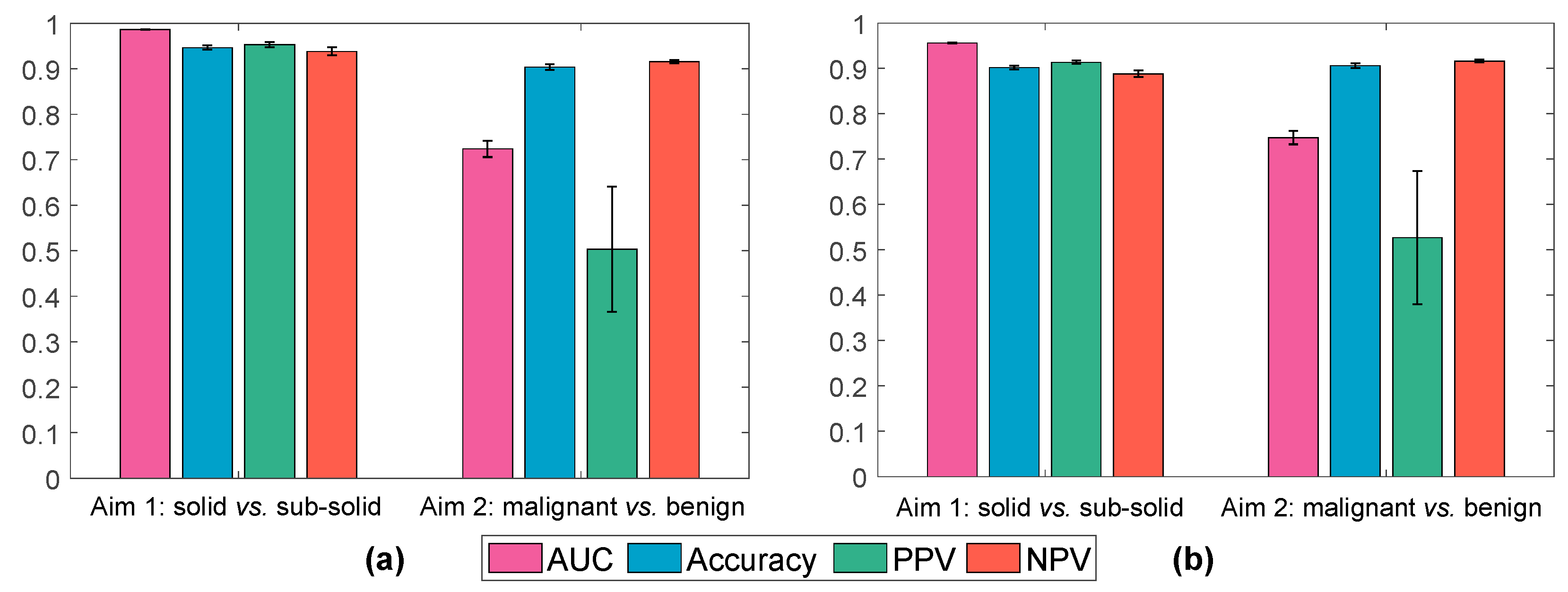
3.2. Cross-Validation of Radiomic Classifiers on Discovery Datasets
3.3. Post-Processing of Radiomic Features: Radiomic Predictors
- Shape: Sphericity;
- NGTDM: Contrast;
- GLSZM: Small Area High Gray Level Emphasis;
- GLRLM: Gray Level Variance.
- Shape: Maximum 2D Diameter Slice, Least Axis Length;
- GLCM: Correlation, Cluster Shade;
- GLSZM: Size Zone Non-Uniformity Normalized, Size Zone Non-Uniformity.
3.4. Integration of Radiomic Predictors with Semantic LDCT Features and Clinical Features and Comparison
3.5. Testing Radiomic Classifiers on Blinded Test Dataset
3.6. Re-Training Classifier with Feature Class Imbalance Correction
3.6.1. Minority Class Over-Sampling
3.6.2. Majority Class under-Sampling
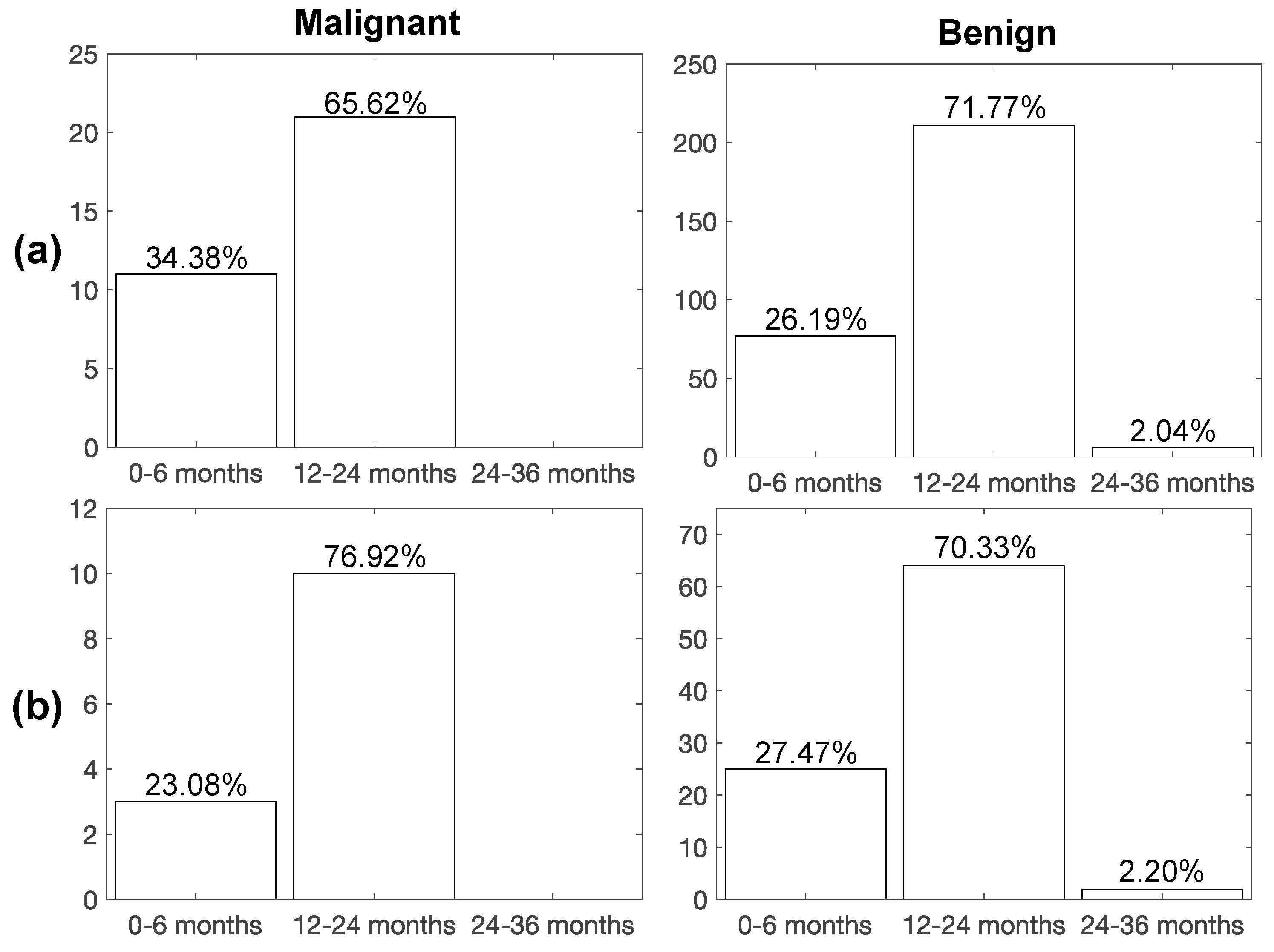
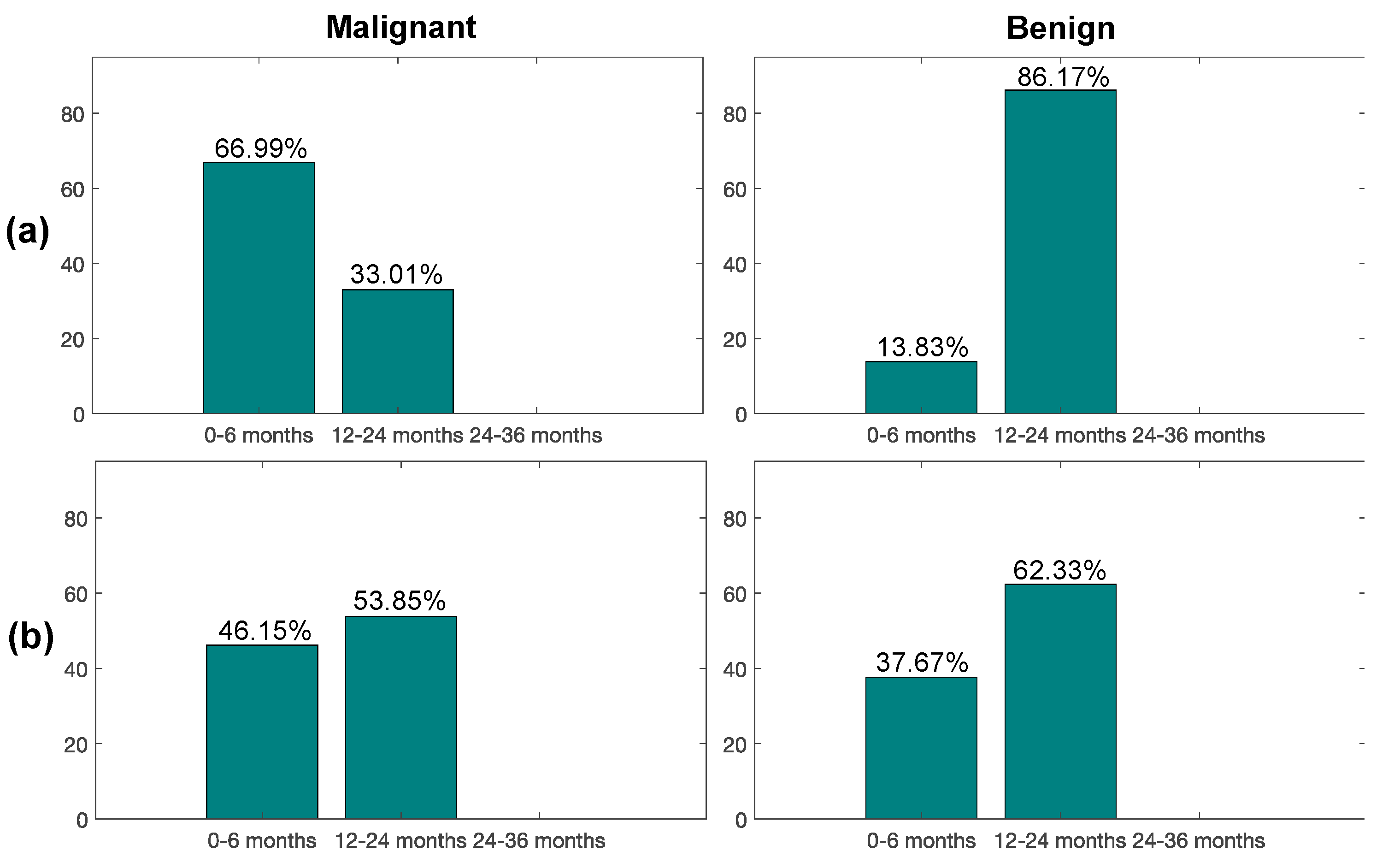
3.7. Impact of Radiomic Classifiers on PN Characterization and Screening Recall Intervals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Berg, C.D.; Black, W.C.; Church, T.R.; Fagerstrom, R.M.; Galen, B.; Gareen, I.F.; Gatsonis, C.; Goldin, J.; et al. The National Lung Screening Trial: Overview and Study Design. Radiology 2011, 258, 243–253. [Google Scholar] [PubMed] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Saul, E.E.; Guerra, R.B.; Saul, M.E.; da Silva, L.L.; Aleixo, G.F.P.; Matuda, R.M.K.; Lopes, G. The Challenges of Implementing Low-Dose Computed Tomography for Lung Cancer Screening in Low- and Middle-Income Countries. Nat. Cancer 2020, 1, 1140–1152. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchianò, A. Prolonged Lung Cancer Screening Reduced 10-Year Mortality in the MILD Trial: New Confirmation of Lung Cancer Screening Efficacy. Ann. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef]
- Hunger, T.; Wanka-Pail, E.; Brix, G.; Griebel, J. Lung Cancer Screening with Low-Dose CT in Smokers: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1040. [Google Scholar] [CrossRef]
- Tammemagi, M.C.; Schmidt, H.; Martel, S.; McWilliams, A.; Goffin, J.R.; Johnston, M.R.; Nicholas, G.; Tremblay, A.; Bhatia, R.; Liu, G.; et al. Participant Selection for Lung Cancer Screening by Risk Modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] Study): A Single-Arm, Prospective Study. Lancet Oncol. 2017, 18, 1523–1531. [Google Scholar] [CrossRef]
- Swensen, S.J.; Jett, J.R.; Sloan, J.A.; Midthun, D.E.; Hartman, T.E.; Sykes, A.-M.; Aughenbaugh, G.L.; Zink, F.E.; Hillman, S.L.; Noetzel, G.R.; et al. Screening for Lung Cancer with Low-Dose Spiral Computed Tomography. Am. J. Respir. Crit. Care Med. 2002, 165, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Lung–RADS® Version 1.1, Assessment Categories (Release date: 2019). Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf?la=en (accessed on 16 August 2021).
- Gierada, D.S.; Rydzak, C.E.; Zei, M.; Rhea, L. Improved Interobserver Agreement on Lung-RADS Classification of Solid Nodules Using Semiautomated CT Volumetry. Radiology 2020, 297, 675–684. [Google Scholar] [CrossRef] [PubMed]
- De Margerie-Mellon, C.; Gill, R.R.; Monteiro Filho, A.C.; Heidinger, B.H.; Onken, A.; VanderLaan, P.A.; Bankier, A.A. Growth Assessment of Pulmonary Adenocarcinomas Manifesting as Subsolid Nodules on CT: Comparison of Diameter-Based and Volume Measurements. Acad. Radiol. 2020, 27, 1385–1393. [Google Scholar] [CrossRef]
- Borghesi, A.; Michelini, S.; Scrimieri, A.; Golemi, S.; Maroldi, R. Solid indeterminate pulmonary nodules of less than 300 mm3: Application of different volume doubling time cut-offs in clinical practice. Diagnostics 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy, M.C.B.; Naidich, D.P. Subsolid Pulmonary Nodules and the Spectrum of Peripheral Adenocarcinomas of the Lung: Recommended Interim Guidelines for Assessment and Management. Radiology 2009, 253, 606–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghesi, A.; Farina, D.; Michelini, S.; Ferrari, M.; Benetti, D.; Fisogni, S.; Tironi, A.; Maroldi, R. Pulmonary adenocarcinomas presenting as ground-glass opacities on multidetector CT: Three-dimensional computer-assisted analysis of growth pattern and doubling time. Diagn. Interv. Radiol. 2016, 22, 525–533. [Google Scholar] [CrossRef]
- Esserman, L.J.; Thompson, I.M., Jr.; Reid, B. Overdiagnosis and Overtreatment in Cancer: An Opportunity for Improvement. JAMA 2013, 310, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.L.; Coletti, R.J.; Harris, R.P. Quantifying and Monitoring Overdiagnosis in Cancer Screening: A Systematic Review of Methods. BMJ 2015, 350, g7773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, P.B. Overdiagnosis in Lung Cancer: Different Perspectives, Definitions, Implications. Thorax 2008, 63, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.X.; Raz, D.J.; Brown, L.; Sun, V. Psychological Burden Associated With Lung Cancer Screening: A Systematic Review. Clin. Lung Cancer 2016, 17, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI Applications to Medical Images: From Machine Learning to Deep Learning. Phys. Med. 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Ninatti, G.; Kirienko, M.; Neri, E.; Sollini, M.; Chiti, A. Imaging-Based Prediction of Molecular Therapy Targets in NSCLC by Radiogenomics and AI Approaches: A Systematic Review. Diagnostics 2020, 10, 359. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Milanese, G.; Pagano, P.; Madeddu, D.; Gnetti, L.; Trentini, F.; Falco, A.; Frati, C.; Lorusso, B.; Lagrasta, C.; et al. Integrated CT Imaging and Tissue Immune Features Disclose a Radio-Immune Signature with High Prognostic Impact on Surgically Resected NSCLC. Lung Cancer 2020, 144, 30–39. [Google Scholar] [CrossRef]
- Antunovic, L.; Gallivanone, F.; Sollini, M.; Sagoma, A.; Invento, A.; Manfrinato, G.; Kirienko, M.; Tinterri, C.; Chiti, A. [18F]FDG PET/CT features for the molecular characterization of primary breast tumors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1945–1954. [Google Scholar] [CrossRef]
- Huang, P.; Park, S.; Yan, R.; Lee, J.; Chu, L.C.; Lin, C.T.; Hussien, A.; Rathmell, J.; Thomas, B.; Chen, C.; et al. Added Value of Computer-Aided CT Image Features for Early Lung Cancer Diagnosis with Small Pulmonary Nodules: A Matched Case-Control Study. Radiology 2018, 286, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Rundo, L.; Tangherloni, A.; Galimberti, S.; Cazzaniga, P.; Woitek, R.; Sala, E. HaraliCU: GPU-powered Haralick feature extraction on medical images exploiting the full dynamics of gray-scale levels. In Proceedings of the International Conference on Parallel Computing Technologies (PaCT) 2019, Almaty, Kazakhstan, 19–23 August 2019; LNCS. Springer: Cham, Switzerland, 2019; Volume 11657, pp. 304–318. [Google Scholar]
- Galloway, M.M. Texture Analysis Using Gray Level Run Lengths. Comput. Graph. Image Process. 1975, 4, 172–179. [Google Scholar] [CrossRef]
- Thibault, G.; Angulo, J.; Meyer, F. Advanced Statistical Matrices for Texture Characterization: Application to Cell Classification. IEEE Trans. Biomed. Eng. 2014, 61, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wee, W.G. Neighboring Gray Level Dependence Matrix for Texture Classification. Comput. Graph. Image Process. 1982, 20, 297. [Google Scholar] [CrossRef]
- Amadasun, M.; King, R. Textural Features Corresponding to Textural Properties. IEEE Trans. Syst. Man Cybern. 1989, 19, 1264–1274. [Google Scholar] [CrossRef]
- Revel, M.-P.; Mannes, I.; Benzakoun, J.; Guinet, C.; Léger, T.; Grenier, P.; Lupo, A.; Fournel, L.; Chassagnon, G.; Bommart, S. Subsolid Lung Nodule Classification: A CT Criterion for Improving Interobserver Agreement. Radiology 2018, 286, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, N.; Matos, C.; Koh, D.M. How to Develop a Meaningful Radiomic Signature for Clinical Use in Oncologic Patients. Cancer Imaging 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso: A Retrospective. R. Stat. Soc. B 2011, 73, 273–282. [Google Scholar] [CrossRef]
- Gill, A.B.; Rundo, L.; Wan, J.C.M.; Lau, D.; Zawaideh, J.P.; Woitek, R.; Zaccagna, F.; Beer, L.; Gale, D.; Sala, E.; et al. Correlating Radiomic Features of Heterogeneity on CT with Circulating Tumor DNA in Metastatic Melanoma. Cancers 2020, 12, 3493. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 2000, 42, 80–86. [Google Scholar] [CrossRef]
- Parvandeh, S.; Yeh, H.-W.; Paulus, M.P.; McKinney, B.A. Consensus Features Nested Cross-Validation. Bioinformatics 2020, 36, 3093–3098. [Google Scholar] [CrossRef]
- Cawley, G.C. Over-Fitting in Model Selection and Its Avoidance. In Advances in Intelligent Data Analysis XI (IDA 2012); LNCS; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7619, p. 1. [Google Scholar]
- Briggs, W.M.; Zaretzki, R. The Skill Plot: A Graphical Technique for Evaluating Continuous Diagnostic Tests. Biometrics 2008, 64, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Han, H.; Wang, W.-Y.; Mao, B.-H. Borderline-SMOTE: A New Over-Sampling Method in Imbalanced Data Sets Learning. In Advances in Intelligent Computing (ICIC 2005); LNCS; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3644, pp. 878–887. [Google Scholar]
- Bunkhumpornpat, C.; Sinapiromsaran, K.; Lursinsap, C. Safe-Level-SMOTE: Safe-Level-Synthetic Minority Over-Sampling TEchnique for Handling the Class Imbalanced Problem. In Advances in Knowledge Discovery and Data Mining; LNCS; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5476, pp. 475–482. [Google Scholar]
- He, H.; Bai, Y.; Garcia, E.A.; Li, S. ADASYN: Adaptive Synthetic Sampling Approach for Imbalanced Learning. In Proceedings of the 2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence) 2008, Hong Kong, China, 1–6 June 2008; pp. 1322–1328. [Google Scholar]
- Hotelling, H. Analysis of a Complex of Statistical Variables into Principal Components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Van der Maaten, L.; Hinton, G. Visualizing Data Using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Ramentol, E.; Vluymans, S.; Verbiest, N.; Caballero, Y.; Bello, R.; Cornelis, C.; Herrera, F. IFROWANN: Imbalanced Fuzzy-Rough Ordered Weighted Average Nearest Neighbor Classification. IEEE Trans. Fuzzy Syst. 2015, 23, 1622–1637. [Google Scholar] [CrossRef]
- Lokhandwala, T.; Bittoni, M.A.; Dann, R.A.; D’Souza, A.O.; Johnson, M.; Nagy, R.J.; Lanman, R.B.; Merritt, R.E.; Carbone, D.P. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin. Lung Cancer 2017, 18, e27–e34. [Google Scholar] [CrossRef]
- Freiman, M.R.; Clark, J.A.; Slatore, C.G.; Gould, M.K.; Woloshin, S.; Schwartz, L.M.; Wiener, R.S. Patients’ Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J. Thorac. Oncol. 2016, 11, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Oh, J.H.; Riyahi, S.; Liu, C.-J.; Jiang, F.; Chen, W.; White, C.; Rimner, A.; Mechalakos, J.G.; Deasy, J.O.; et al. Radiomics Analysis of Pulmonary Nodules in Low-Dose CT for Early Detection of Lung Cancer. Med. Phys. 2018, 45, 1537–1549. [Google Scholar] [CrossRef]
- Pérez-Morales, J.; Tunali, I.; Stringfield, O.; Eschrich, S.A.; Balagurunathan, Y.; Gillies, R.J.; Schabath, M.B. Peritumoral and Intratumoral Radiomic Features Predict Survival Outcomes among Patients Diagnosed in Lung Cancer Screening. Sci. Rep. 2020, 10, 10528. [Google Scholar] [CrossRef]
- Garau, N.; Paganelli, C.; Summers, P.; Choi, W.; Alam, S.; Lu, W.; Fanciullo, C.; Bellomi, M.; Baroni, G.; Rampinelli, C. External Validation of Radiomics-Based Predictive Models in Low-Dose CT Screening for Early Lung Cancer Diagnosis. Med. Phys. 2020, 47, 4125–4136. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Milanese, G.; Sestini, S.; Sabia, F.; Jacobs, C.; van Ginneken, B.; Prokop, M.; Schaefer-Prokop, C.M.; Marchianò, A.; Sverzellati, N.; et al. Lung Cancer Screening by Nodule Volume in Lung-RADS v1.1: Negative Baseline CT Yields Potential for Increased Screening Interval. Eur. Radiol. 2021, 31, 1956–1968. [Google Scholar] [CrossRef]
- Doran, S.J.; Kumar, S.; Orton, M.; d’Arcy, J.; Kwaks, F.; O’Flynn, E.; Ahmed, Z.; Downey, K.; Dowsett, M.; Turner, N.; et al. “Real-world” radiomics from multi-vendor MRI: An original retrospective study on the prediction of nodal status and disease survival in breast cancer, as an exemplar to promote discussion of the wider issues. Cancer Imaging 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA Signatures in Tissues and Plasma Predict Development and Prognosis of Computed Tomography Detected Lung Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianconi, F.; Palumbo, I.; Spanu, A.; Nuvoli, S.; Fravolini, M.L.; Palumbo, B. PET/CT Radiomics in Lung Cancer: An Overview. Appl. Sci. 2020, 10, 1718. [Google Scholar] [CrossRef] [Green Version]
- Fraioli, F.; Lyasheva, M.; Porter, J.C.; Bomanji, J.; Shortman, R.I.; Endozo, R.; Wan, S.; Bertoletti, L.; Machado, M.; Ganeshan, B.; et al. Synergistic Application of Pulmonary F-FDG PET/HRCT and Computer-Based CT Analysis with Conventional Severity Measures to Refine Current Risk Stratification in Idiopathic Pulmonary Fibrosis (IPF). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2023–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Features | AUC | Accuracy | PPV | NPV |
|---|---|---|---|---|
| Radiomic | 0.747 ± 0.015 | 0.906 ± 0.005 | 0.527 ± 0.147 | 0.916 ± 0.003 |
| Best radiomic predictors of Aim 2 + best radiomic predictors of Aim 1 (solidity) | 0.739 ± 0.014 | 0.905 ± 0.005 | 0.522 ± 0.137 | 0.915 ± 0.003 |
| Best radiomic predictors of Aim 2 + best radiomic predictors of Aim 1 (solidity) + clinical + semantic LDCT | 0.742 ± 0.021 | 0.898 ± 0.006 | 0.423 ± 0.120 | 0.913 ± 0.003 |
| Clinical + semantic LDCT | 0.521 ± 0.036 | 0.893 ± 0.006 | 0.039 ± 0.076 | 0.902 ± 0.002 |
| AUC | Accuracy | PPV | NPV | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Aim 1 first-level: SN vs. SSN | 0.887 ± 0.006 | 0.870 ± 0.010 | 0.830 ± 0.014 | 0.926 ± 0.002 | 0.938 ± 0.001 | 0.800 ± 0.020 |
| Aim 1 second-level: NSN vs. PSN | 0.800 ± 0.178 | 0.844 ± 0.005 | 0.988 ± 0.002 | 0.801 ± 0.005 | 0.580 ± 0.0134 | 0.991 ± 0.001 |
| Aim 2: benign vs. malignant | 0.564 ± 0.007 | 0.869 ± 0.005 | 0.367 ± 0.079 | 0.886 ± 0.004 | 0.102 ± 0.036 | 0.978 ± 0.001 |
| # Samples | AUC | Accuracy | PPV | NPV | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 32 | 0.768 ± 0.008 | 0.826 ± 0.007 | 0.568 ± 0.061 | 0.857 ± 0.007 | 0.292 ± 0.043 | 0.946 ± 0.009 |
| 64 | 0.788 ± 0.004 | 0.809 ± 0.008 | 0.669 ± 0.033 | 0.839 ± 0.008 | 0.432 ± 0.038 | 0.928 ± 0.012 |
| 128 | 0.826 ± 0.004 | 0.794 ± 0.007 | 0.741 ± 0.013 | 0.820 ± 0.008 | 0.644 ± 0.021 | 0.875 ± 0.009 |
| 192 | 0.795 ± 0.002 | 0.783 ± 0.006 | 0.806 ± 0.010 | 0.772 ± 0.005 | 0.657 ± 0.009 | 0.878 ± 0.009 |
| 256 | 0.817 ± 0.003 | 0.766 ± 0.006 | 0.830 ± 0.009 | 0.726 ± 0.004 | 0.670 ± 0.006 | 0.862 ± 0.009 |
| 295 | 0.802 ± 0.003 | 0.749 ± 0.005 | 0.840 ± 0.009 | 0.688 ± 0.004 | 0.648 ± 0.006 | 0.861 ± 0.010 |
| # Samples | AUC | Accuracy | PPV | NPV | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 32 | 0.602 ± 0.012 | 0.838 ± 0.019 | 0.317 ± 0.067 | 0.894 ± 0.002 | 0.229 ± 0.011 | 0.925 ± 0.021 |
| 64 | 0.591 ± 0.016 | 0.805 ± 0.018 | 0.229 ± 0.028 | 0.890 ± 0.002 | 0.231 ± 2.24 × 10−16 | 0.887 ± 0.021 |
| 128 | 0.571 ± 0.009 | 0.697 ± 0.018 | 0.163 ± 0.011 | 0.889 ± 0.004 | 0.346 ± 0.039 | 0.747 ± 0.024 |
| 192 | 0.574 ± 0.008 | 0.643 ± 0.008 | 0.165 ± 0.006 | 0.896 ± 0.003 | 0.457 ± 0.019 | 0.670 ± 0.010 |
| 256 | 0.556 ± 0.007 | 0.603 ± 0.010 | 0.149 ± 0.004 | 0.890 ± 0.002 | 0.462 ± 4.49 × 10−16 | 0.623 ± 0.011 |
| 295 | 0.548 ± 0.009 | 0.584 ± 0.009 | 0.142 ± 0.003 | 0.887 ± 0.002 | 0.462 ± 4.49 × 10−16 | 0.601 ± 0.011 |
| Dataset | Months of LC Diagnosis from Baseline LDCT in Subjects Recalled at 0–6 Months without the Use of the Radiomic Classifier for Aim 2 (PN Risk) | Months of LC Diagnosis from Baseline LDCT in Subjects to Be Recalled at 0–6 Months with the Use of the Radiomic Classifier for Aim 2 (PN Risk) |
|---|---|---|
| Discovery | 18 ± 23.00 | 44 ± 22.00; p = 0.0079 |
| Blinded test | 17 ± 23.25 | 27 ± 18.00; p = 0.5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rundo, L.; Ledda, R.E.; di Noia, C.; Sala, E.; Mauri, G.; Milanese, G.; Sverzellati, N.; Apolone, G.; Gilardi, M.C.; Messa, M.C.; et al. A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules. Diagnostics 2021, 11, 1610. https://doi.org/10.3390/diagnostics11091610
Rundo L, Ledda RE, di Noia C, Sala E, Mauri G, Milanese G, Sverzellati N, Apolone G, Gilardi MC, Messa MC, et al. A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules. Diagnostics. 2021; 11(9):1610. https://doi.org/10.3390/diagnostics11091610
Chicago/Turabian StyleRundo, Leonardo, Roberta Eufrasia Ledda, Christian di Noia, Evis Sala, Giancarlo Mauri, Gianluca Milanese, Nicola Sverzellati, Giovanni Apolone, Maria Carla Gilardi, Maria Cristina Messa, and et al. 2021. "A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules" Diagnostics 11, no. 9: 1610. https://doi.org/10.3390/diagnostics11091610
APA StyleRundo, L., Ledda, R. E., di Noia, C., Sala, E., Mauri, G., Milanese, G., Sverzellati, N., Apolone, G., Gilardi, M. C., Messa, M. C., Castiglioni, I., & Pastorino, U. (2021). A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules. Diagnostics, 11(9), 1610. https://doi.org/10.3390/diagnostics11091610







