Brain and Muscle Oxygen Saturation Combined with Kidney Injury Biomarkers Predict Cardiac Surgery Related Acute Kidney Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. NIRS Monitoring
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasta, J.F.; Kane-Gill, S.L.; Durtschi, A.J.; Pathak, D.S.; Kellum, J.A. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol. Dial. Transplant. 2008, 23, 1970–1974. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.R.; Cochran, R.P.; Dacey, L.J.; Ross, C.S.; Kunzelman, K.S.; Dunton, R.F.; Braxton, J.H.; Charlesworth, D.C.; Clough, R.A.; Helm, R.E.; et al. Perioperative Increases in Serum Creatinine Are Predictive of Increased 90-Day Mortality after Coronary Artery Bypass Graft Surgery. Circulation 2006, 114 (Suppl. I), I409. [Google Scholar] [CrossRef] [Green Version]
- Karkouti, K.; Wijeysundera, D.N.; Yau, T.M.; Callum, J.L.; Cheng, D.C.; Crowther, M.; Dupuis, J.; Fremes, S.E.; Kent, B.; Laflamme, C.; et al. Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 2009, 119, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Haase, M.; Devarajan, P.; Haase-Fielitz, A.; Bellomo, R.; Cruz, D.N.; Wagener, G.; Krawczeski, C.D.; Koyner, J.L.; Murray, P.; Zappitelli, M.; et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclini-cal acute kidney injury: A multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 2011, 57, 1752–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagener, G.; Jan, M.; Kim, M.; Mori, K.; Barasch, J.M.; Sladen, R.N.; Lee, H.T. Association between Increases in Urinary Neutrophil Gelatinase–associated Lipocalin and Acute Renal Dysfunction after Adult Cardiac Surgery. Anesthesiology 2006, 105, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kalisnik, J.M.; Hrovat, E.; Hrastovec, A.; Žibert, J.; Jerin, A.; Skitek, M.; Santarpino, G.; Klokocovnik, T. Creatinine, neutrophil gelatinase-associated lipocalin, and cystatin C in determining acute kidney injury after heart operations using cardiopulmonary bypass. Artif. Organs 2017, 41, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Maiti, R.; Chakraborty, B.; Candilio, L.; Clayton, T.; Evans, R.; Jenkins, D.P.; Kolvekar, S.; Kunst, G.; Laing, C.; et al. Neutrophil gelatinase-associated lipocalin prior to cardiac surgery predicts acute kidney injury and mortality. Heart 2018, 104, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Luo, Q.; Wang, L.; Han, L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: A meta-analysis. Eur. J. Cardio-Thorac. Surg. 2016, 49, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Heise, D.; Rentsh, K.; Brauer, A.; Friedrich, M.; Quintel, M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and α1- microglobulin for early detection of acute renal injury after cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2011, 39, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlroy, D.R.; Farkas, D.; Pan, K.; Pickering, J.W.; Lee, H.T. Combining Novel Renal Injury Markers with Delta Serum Creatinine Early after Cardiac Surgery and Risk-Stratification for Serious Adverse Outcomes: An Exploratory Analysis. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2190–2200. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Xie, B.; Huang, R.; Yan, Y.; Liu, S.; Zhu, M.; Lu, R.; Qian, J.; Ni, Z.; et al. Early serum cystatin C-enhanced risk prediction for acute kidney injury post cardiac surgery: A prospective, observational, cohort study. Biomarkers 2020, 25, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Liebetrau, C.; Dörr, O.; Baumgarten, H.; Gaede, L.; Szardien, S.; Blumenstein, J.; Rolf, A.; Möllmann, H.; Hamm, C.; Walther, T.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand. J. Clin. Lab. Investig. 2013, 73, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.R.; Kandler, K.; Nielsen, R.V.; Jakobsen, P.C.; Knudsen, N.N.; Ranucci, M.; Nilsson, J.C.; Ravn, H.B. Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol. Scand. 2019, 63, 1290–1297. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Fernández-Molina, M.; Fierro, I.; Jorge-Monjas, P.; Carrascal, Y.; Gómez-Herreras, J.I.; Tamayo, E. Postoperative kidney oxygen saturation as a novel marker for acute kidney injury after adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2019, 157, 2340–2351. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W., Jr. Real time continuous monitoring of cerebral blood flow autoregulation using bear-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M.; Adams, S.J.; Novick, R.J.; Quantz, M.; Bainbridge, D.; Iglesias, I.; Andrew, C.; Betsy, S.; Beverly, I.; Stephanie, F. Monitoring brain oxygen saturation during coronary bypass surgery: A randomized, prospective study. Anesth. Analg. 2007, 104, 51–58. [Google Scholar] [CrossRef]
- Denault, A.; Deschamps, A.; Murkin, J.M. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 274–281. [Google Scholar] [CrossRef]
- Rogers, C.A.; Stoica, S.; Ellis, L.; Stokes, E.A.; Wordsworth, S.; Dabner, L.; Clayton, G.; Downes, R.; Nicholson, E.; Bennett, S.; et al. Randomized trial of near-infrared spectroscopy for personalized optimization of cere-bral tissue oxygenation during cardiac surgery. Br. J. Anaesth. 2017, 119, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Moerman, A.; Bove, T.; Francois, K.; Jacobs, S.; Deblaere, I.; Wouters, P.; De Hert, S. The effect of blood pressure regulation during aortic coarctation repair on brain, kidney, and muscle oxygen saturation measured by near-infrared spectroscopy: A randomised, clinical study. Anesth. Analg. 2013, 116, 760–766. [Google Scholar] [CrossRef]
- Brady, K.M.; Mytar, J.O.; Lee, J.K.; Cameron, D.E.; Vricella, L.A.; Thompson, W.R.; Hogue, C.W.; Easley, R.B. Monitoring Cerebral Blood Flow Pressure Autoregulation in Pediatric Patients during Cardiac Surgery. Stroke 2010, 41, 1957–1962. [Google Scholar] [CrossRef] [Green Version]
- Hazle, M.A.; Gajarski, R.J.; Aiyagari, R.; Yu, S.; Abraham, A.; Donohue, J.; Blatt, N.B. Urinary biomarkers and renal near-infrared spectroscopy predict ICU outcomes following cardiac surgery in infants under 6 months of age. J. Thorac. Cardiovasc. Surg. 2013, 146, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Ruf, B.; Bonelli, V.; Balling, G.; Hörer, J.; Nagdyman, N.; Braun, S.L.; Ewert, P.; Reiter, K. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: A case–control study. Crit. Care 2015, 19, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, D.; Hogue, C.; Adachi, H.; Max, L.; Price, J.; Sciortino, C.; Zehr, K.; Conte, J.; Cameron, D.; Mandal, K. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interactive Cardiovasc. Thorac. Surg. 2016, 22, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Serraino, G.F.; Murphy, G.J. Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: A systematic review of randomised trials. BMJ Open 2017, 7, e016613. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Lameire, N. KDIGO AKI Guideline Work group. Diagnosis, evaluation and management of acute kidney injury: KDIGO clinical practice guideline for acute kidney injury (Section 2). Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar]
- Barkhordari, K.; Yasseri, A.M.F.; Yousefshahi, F.; Shafiee, A. Risk Factors for Acute Kidney Injury in Coronary Artery Bypass Graft Surgery Patients Based on the Acute Kidney Injury Network Critera. J. Tehran Univ. Heart Cent. 2018, 13, 52–57. [Google Scholar]
- Kang, W.; Wu, X. Pre-, Intra-, and Post-Operative Factors for Kidney Injury of Patients Underwent Cardiac Surgery: A Retrospective Cohort Study. Med. Sci. Monit. 2019, 25, 5841–5849. [Google Scholar] [CrossRef]
- Brown, J.R.; Cochran, R.P.; Leavitt, B.J.; Dacey, L.J.; Ross, C.S.; MacKenzie, T.A.; Kunzelman, K.S.; Kramer, R.S.; Hernandez, F., Jr.; Helm, R.E.; et al. Multivariable Prediction of Renal Insufficiency Developing after Cardiac Surgery. Circulation 2007, 116 (Suppl. I), I139–I143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.R.; Cochran, R.P.; MacKenzie, T.A.; Furnary, A.P.; Kunzelman, K.S.; Ross, C.S.; Langner, C.W.; Charlesworth, D.C.; Leavitt, B.J.; Dacey, L.J.; et al. Long-Term Survival after Cardiac Surgery is Predicted by Estimated Glomerular Filtration Rate. Ann. Thorac. Surg. 2008, 86, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Wijeysundera, D.N.; Beattie, W.S. RBC Investigators. Risk associated with preoperative anemia in cardiac surgery: A multicenter cohort study. Circulation 2008, 117, 478–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, M.; Yerebakan, H.; Sorabella, R.A.; Donovan, D.J.; Kossar, A.P.; Sreekanth, S.; Kurlansky, P.; Borger, M.A.; Argenziano, M.; Smith, C.R.; et al. Acute kidney injury following surgical aortic valve replacement. J. Card. Surg. 2015, 30, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; LeClerc, J.-L.; Vincent, J.-L. Inflamatory response to Cardiopulmonary Bypass, mechanism involved and possible therapeutic strategies. Chest 1997, 112, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Uusaro, A.; Ruokoen, E.; Takala, J. Splanchnic oxygen transport after cardiac surgery: Evidence for inadequate tissue perfusion after stabilization of hemodynamics. Intensive Care Med. 1996, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Hasibeder, W.R.; Hengl, C.; Pajk, W.; Schwarz, B.; Margreiter, J.; Ulmer, H.; Engl, J.; Knotzer, H. Effects of phenylephrine on the sublingual microcirculation during cardiopulmonary bypass. Br. J. Anaesth. 2009, 102, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbers, P.W.G.; Ozdemir, A.; van Iterson, M.; van Dongen, E.P.; Ince, C. Microcirculatory Imaging in Cardiac Anesthesia: Ketanserin Reduces Blood Pressure but Not Perfused Capillary Density. J. Cardiothorac. Vasc. Anesth. 2009, 23, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.B.; Suneja, M.; Riou, B. Cardiopulmonary Bypass–associated Acute Kidney Injury. Anesthesiology 2011, 114, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Arnaoutakis, G.J.; Fine, D.M.; Brady, K.; Easley, R.B.; Zheng, Y.; Brown, C.; Katz, N.M.; Grams, M.; Hogue, C.W. Blood Pressure Excursions Below the Cerebral Autoregulation Threshold during Cardiac Surgery are Associated with Acute Kidney Injury*. Crit. Care Med. 2013, 41, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Balci, C.; Haftaci, E.; Kunt, A.T. Use of cerebral oxygen saturation and hemoglobin concentration to predict acute kidney injury after cardiac surgery. J. Int. Med. Res. 2018, 46, 1130–1137. [Google Scholar] [CrossRef]
- Duret, J.; Pottecher, J.; Bouzat, P.; Brun, J.; Harrois, A.; Payen, J.-F.; Duranteau, J. Skeletal muscle oxygenation in severe trauma patients during haemorrhagic shock resuscitation. Crit. Care 2015, 19, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Biedrzycka, A.; Kowalik, M.; Pawlaczyk, R.; Jagielak, D.; Świetlik, D.; Szymanowicz, W.; Lango, R. Aortic cross-clamping phase of cardiopulmonary bypass is related to decreased microvascular reactivity after short-term ischaemia of the thenar muscle both under intravenous and volatile anaesthesia: A randomized trial. Interact Cardiovasc. Thorac. Surg. 2016, 23, 770–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgaard, F.; Vedel, A.G.; Lange, T.; Nilsson, J.C.; Ravn, H.B. Impact of 2 distinct levels of mean arterial pressure on near-infrared spectroscopy during cardiac surgery: Secondary outcome from a randomized clinical trial. Anaesth. Analg. 2019, 128, 1081–1088. [Google Scholar] [CrossRef] [PubMed]

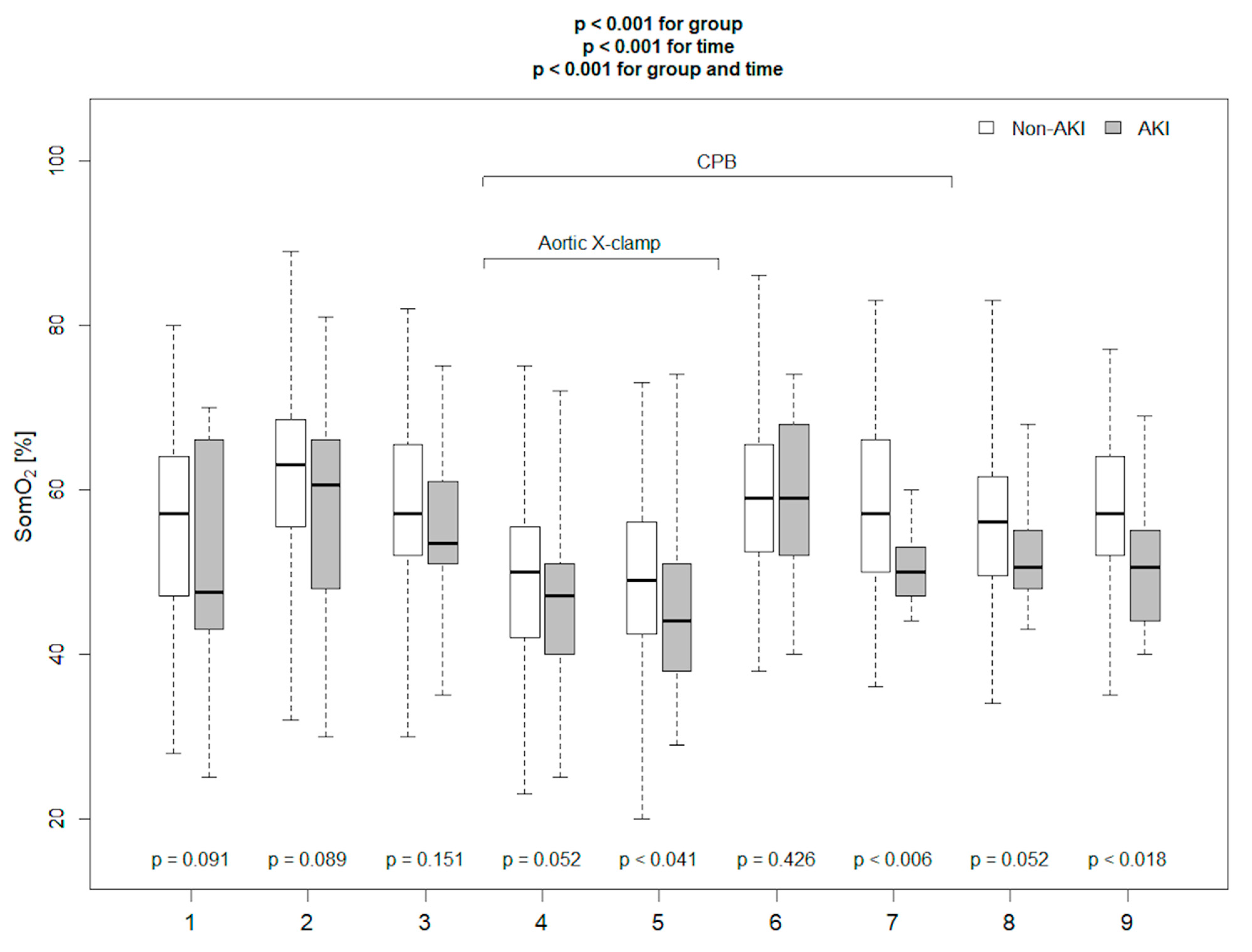
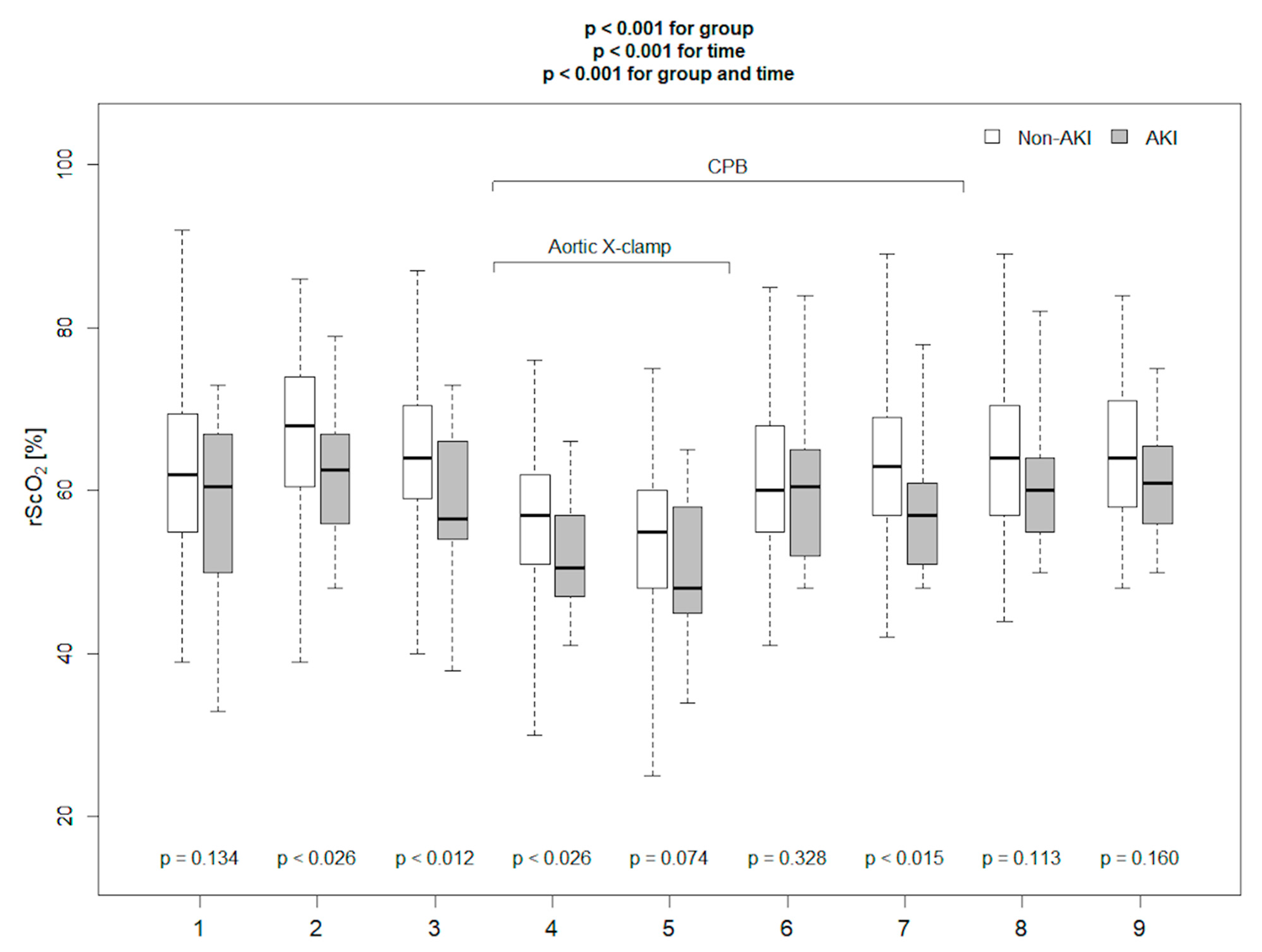
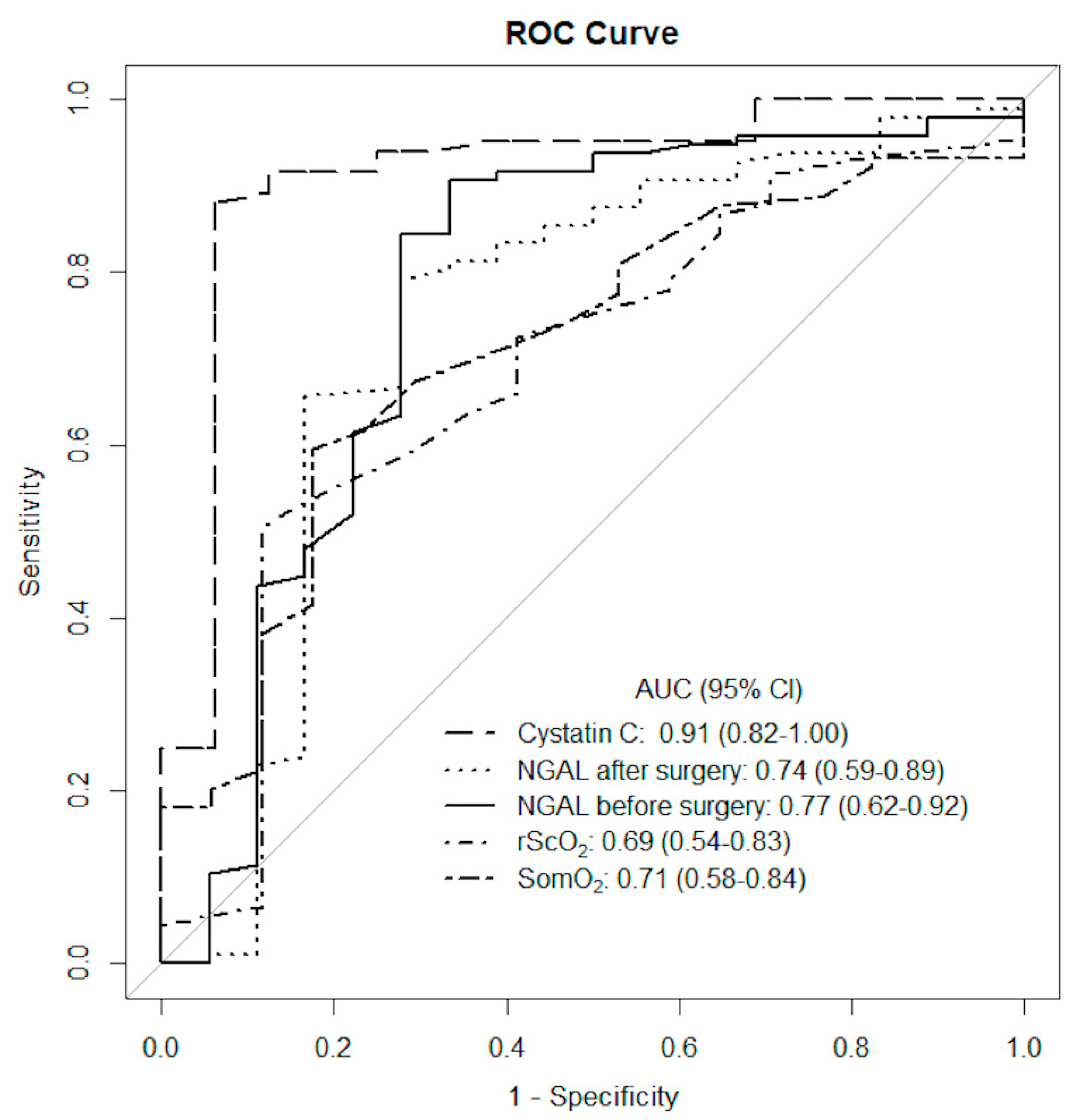
| AKI Patients n = 18 | Non-AKI Patients n = 96 | p | |
|---|---|---|---|
| PREOPERATIVE CHARACTERISTICS | |||
| Age (years) | 74 (66–78) | 67 (60–74) | 0.029 |
| Male, n (%) | 8 (44) | 49 (51) | 0.798 |
| Coronary artery disease, n (%) | 12 (67) | 62 (65) | 1.000 |
| Arterial hypertension, n (%) | 12 (67) | 74 (77) | 0.376 |
| Diabetes mellitus, n (%) | 7 (39) | 29 (30) | 0.725 |
| EUROScore (logistic) | 9.7 (5.1–13.5) | 5.5 (2.6–12.2) | 0.054 |
| LVEF% | 60 (45–64) | 60 (48–65) | 0.440 |
| Creatinine (mg/dL) | 1.02 (0.91–1.27) | 0.90 (0.79–1.05) | 0.018 |
| Haemoglobin (g/dL) | 12.8 (11.8–13.3) | 13.8 (12.6–14.6) | 0.019 |
| Preoperative anaemia *, n (%) | 7 (39) | 19 (20) | 0.076 |
| Leukocyte count (G/L) | 6.83 (6.43–8.64) | 7.34 (6.54–8.44) | 0.166 |
| Angiotensin-converting enzyme inhibitors/sartans before operation | 4 (22) | 28 (29) | 0.776 |
| Statins in premedication | 10 (56) | 63 (66) | 0.433 |
| INTRAOPERATIVE CHARACTERISTICS | |||
| Aortic valve surgery, n (%) | 6 (33) | 61 (64) | 0.021 |
| Mitral valve surgery, n (%) | 1 (5.6) | 12 (12.5) | 0.689 |
| Aortic and mitral valves surgery, n (%) | 3 (17) | 4 (4) | 0.077 |
| Ascending aorta surgery including Bentall operation n (%) | 3 (17) | 6 (6) | 0.150 |
| 3 valves surgery, n (%) | 4 (22) | 7 (7) | 0.071 |
| Other surgery, n (%) | 1 (5.6) | 6 (6) | 1.000 |
| CPB time (min) | 140 (116–168) | 119 (98–151) | 0.084 |
| Aortic cross-clamp time (min) | 95 (83–112) | 80 (67–103) | 0.048 |
| POSTOPERATIVE CHARACTERISTICS | |||
| Serum creatinine on the 1st day post-surgery (mg/dL) | 1.58 (1.31–1.95) | 0.90 (0.79–1.05) | 0.018 |
| Serum creatinine on the 2nd day post-surgery (mg/dL) | 1.52 (1.21–2.13) | 0.87 (0.74–1.03) | 0.001 |
| Serum creatinine on the 3rd day post-surgery (mg/dL) | 1.46 (0.99–1.67) | 0.79 (0.68–0.91) | 0.001 |
| Catecholamine infusion on the 1st day post-surgery n (%) | 10 (56) | 29 (30) | 0.059 |
| Catecholamine infusion on the 2nd day post-surgery n (%) | 7 (39) | 12 (13) | 0.014 |
| Catecholamine infusion on the 3rd day post-surgery n (%) | 4 (22) | 9 (9) | 0.223 |
| Diuresis on the 1st day post-surgery (mL) | 1940 (1400–2470) | 2340 (2070–2690) | 0.019 |
| Fluid balance on the 1st day post-surgery (mL) | −20 (−769–918) | −650 (−923–−50) | 0.042 |
| Fluid balance on the 2nd day post-surgery (mL) | 297 (−435–860) | 0 (−950–400) | 0.093 |
| Fluid balance on the 3rd day post-surgery (mL) | −300 (−750–450) | −375 (−925–162.5) | 0.288 |
| Postoperative chest drainage on the 1st day post-surgery (mL) | 435 (273–950) | 320 (225–533) | 0.083 |
| Postoperative chest drainage on the 2nd day post-surgery (mL) | 130 (120–235) | 160 (98–255) | 0.497 |
| Furosemide on the 1st day post-surgery, n (%) | 10 (56) | 49 (51) | 0.007 |
| Time to extubation (hours) | 10.0 (8.5–11.5) | 8.0 (6.5–10.0) | 0.028 |
| CRP on the 1st day post-surgery | 38.2 (25.0–54.7) | 28.3 (17.5–42.7) | 0.043 |
| CRP on the 2nd day post-surgery | 79.3 (60.3–108.6) | 65.8 (44.0–93.82) | 0.086 |
| CRP on the 3rd day post-surgery | 113.3 (62.0–153.8) | 98.5 (62.3–142.6) | 0.286 |
| WBC on the 1st day post-surgery | 12.5 (10.8–15.2) | 12.9 (10.8–14.9) | 0.342 |
| WBC in the 2nd day post-surgery | 15.0 (12.7–19.4) | 14.0 (12.0–16.0) | 0.117 |
| WBC in the 3rd day post-surgery | 11.5 (10.7–14.7) | 10.3 (8.1–12.5) | 0.037 |
| Haemoglobin on the 1st day post-surgery | 10.5 (9.7–11.3) | 10.8 (10.1–11.4) | 0.239 |
| Haemoglobin on the 2nd day post-surgery | 9.6 (9.1–10.7) | 10.2 (9.6–10.7) | 0.074 |
| Haemoglobin on the 3rd day post-surgery | 10.0 (8.9–10.5) | 9.6 (8.9–10.4) | 0.315 |
| BIOMARKERS | |||
| Blood NGAL before surgery (ng/mL) | 123.5 (78.5–163.3) | 62.5 (50.8–86.5) | 0.001 |
| Blood NGAL 3 h after surgery (ng/mL) | 156.50 (94.00–181.00) | 74.00 (53.75–101.25) | 0.004 |
| Postoperative cystatin C (mg/L) | 1.56 (1.41–1.94) | 0.84 (0.72–1.07) | 0.001 |
| Parameters | Cut-off Value | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Cystatin C after surgery * (mg/L) | 1.23 | 91.4 (82.0–100.0) | 0.88 | 0.94 | 0.99 | 0.6 |
| Blood NGAL before surgery (ng/mL) | 91.5 | 73.9 (58.5–89.3) | 0.79 | 0.72 | 0.94 | 0.39 |
| Blood NGAL 3 h after surgery (ng/mL) | 140.5 | 77.1 (62.4–91.9) | 0.91 | 0.67 | 0.94 | 0.57 |
| The absolute SomO2 20 min after CPB (%) | 54.5 | 71.1 (58.1–84.0) | 0.6 | 0.82 | 0.95 | 0.28 |
| The absolute rScO2 20 min after CPB (%) | 62.5 | 68.6 (54.2–82.9) | 0.51 | 0.88 | 0.96 | 0.25 |
| Parameters | OR (95% CI) | p |
|---|---|---|
| NGAL before surgery ≥ 91.5 ng/mL | 9.88 (3.15–30.98) | 0.001 |
| NGAL 3 h after surgery ≥ 140.5 ng/mL | 19.33 (5.84–63.96) | 0.001 |
| Postoperative cystatin C ≥ 1.23 mg/L | 111 (13.2–933.33) | 0.001 |
| SomO2 20′ after CPB ≤ 54.5% | 6.87 (1.3–13.97) | 0.003 |
| rScO2 20′ after CPB ≤ 62.5% | 3.5 (1.14–10.78) | 0.003 |
| Blood NGAL before surgery ≥ 91.5 nl/mL and SomO2 20′ after CPB ≤ 54.5% | 12.7 (3.88–41.59) | 0.001 |
| Blood NGAL before surgery ≥ 91.5 nl/mL and rScO2 20′ after CPB ≤ 62.5% | 17.45 (5.16–59.06) | 0.001 |
| Blood NGAL 3 h after surgery ≥ 140.5 ng/mL and SomO2 20′ after CPB ≤ 54.5% | 30.36 (7.54–122.16) | 0.001 |
| Blood NGAL 3 h after surgery ≥ 140.5 ng/mL and rScO2 20′ after CPB ≤ 62.5% | 39.88 (9.71–163.67) | 0.001 |
| Postoperative cystatin C ≥ 1.23 mg/L and SomO2 20′ after CPB ≤ 54.5% | 58.5 (12.32–276.84) | 0.001 |
| Postoperative cystatin C ≥ 1.23 mg/L and rScO2 20′ after CPB ≤ 62.5% | 123.5 (20.49–744.46) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanowicz, W.; Daniłowicz-Szymanowicz, L.; Karolak, W.; Kowalik, M.M.; Lango, R. Brain and Muscle Oxygen Saturation Combined with Kidney Injury Biomarkers Predict Cardiac Surgery Related Acute Kidney Injury. Diagnostics 2021, 11, 1591. https://doi.org/10.3390/diagnostics11091591
Szymanowicz W, Daniłowicz-Szymanowicz L, Karolak W, Kowalik MM, Lango R. Brain and Muscle Oxygen Saturation Combined with Kidney Injury Biomarkers Predict Cardiac Surgery Related Acute Kidney Injury. Diagnostics. 2021; 11(9):1591. https://doi.org/10.3390/diagnostics11091591
Chicago/Turabian StyleSzymanowicz, Wiktor, Ludmiła Daniłowicz-Szymanowicz, Wojtek Karolak, Maciej Michał Kowalik, and Romuald Lango. 2021. "Brain and Muscle Oxygen Saturation Combined with Kidney Injury Biomarkers Predict Cardiac Surgery Related Acute Kidney Injury" Diagnostics 11, no. 9: 1591. https://doi.org/10.3390/diagnostics11091591
APA StyleSzymanowicz, W., Daniłowicz-Szymanowicz, L., Karolak, W., Kowalik, M. M., & Lango, R. (2021). Brain and Muscle Oxygen Saturation Combined with Kidney Injury Biomarkers Predict Cardiac Surgery Related Acute Kidney Injury. Diagnostics, 11(9), 1591. https://doi.org/10.3390/diagnostics11091591







