Computed Tomography-Navigation™ Electromagnetic System Compared to Conventional Computed Tomography Guidance for Percutaneous Lung Biopsy: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Computed Tomography Procedures Selection
2.2.1. Conventional CT-Guided Procedures: CT-Group
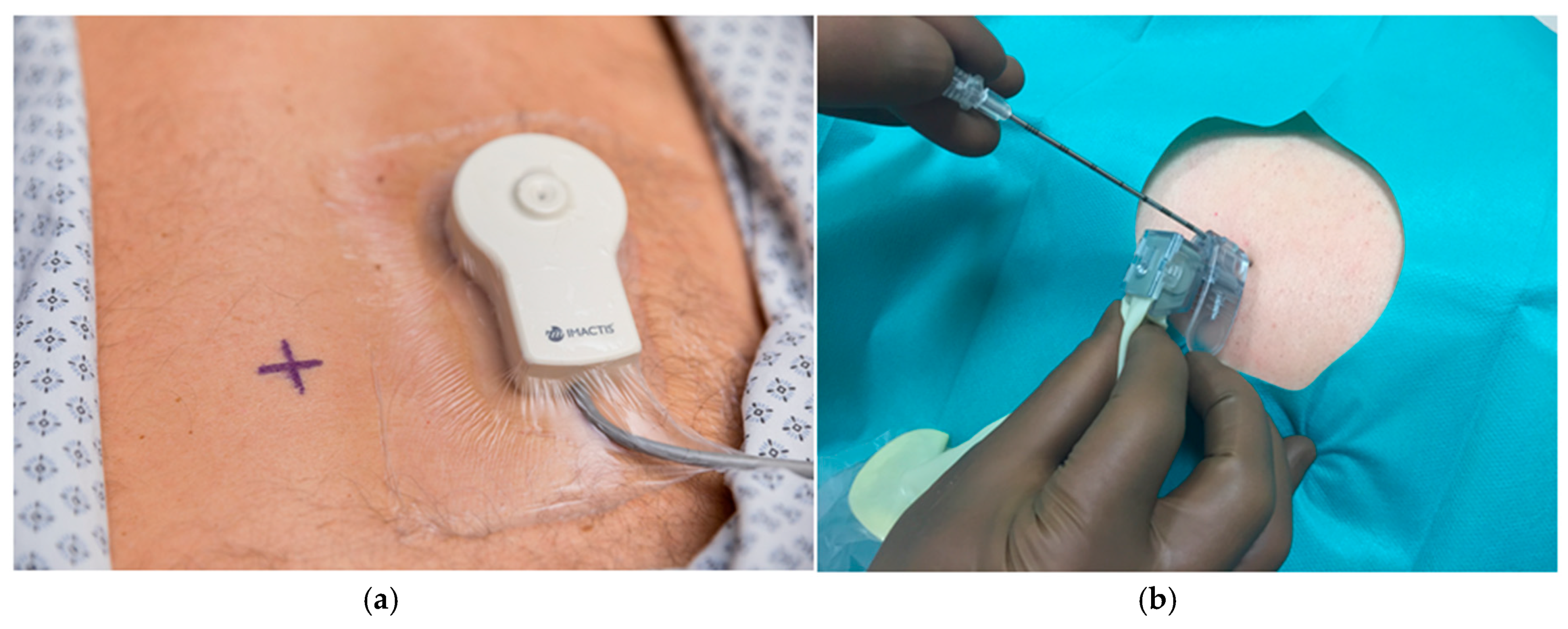
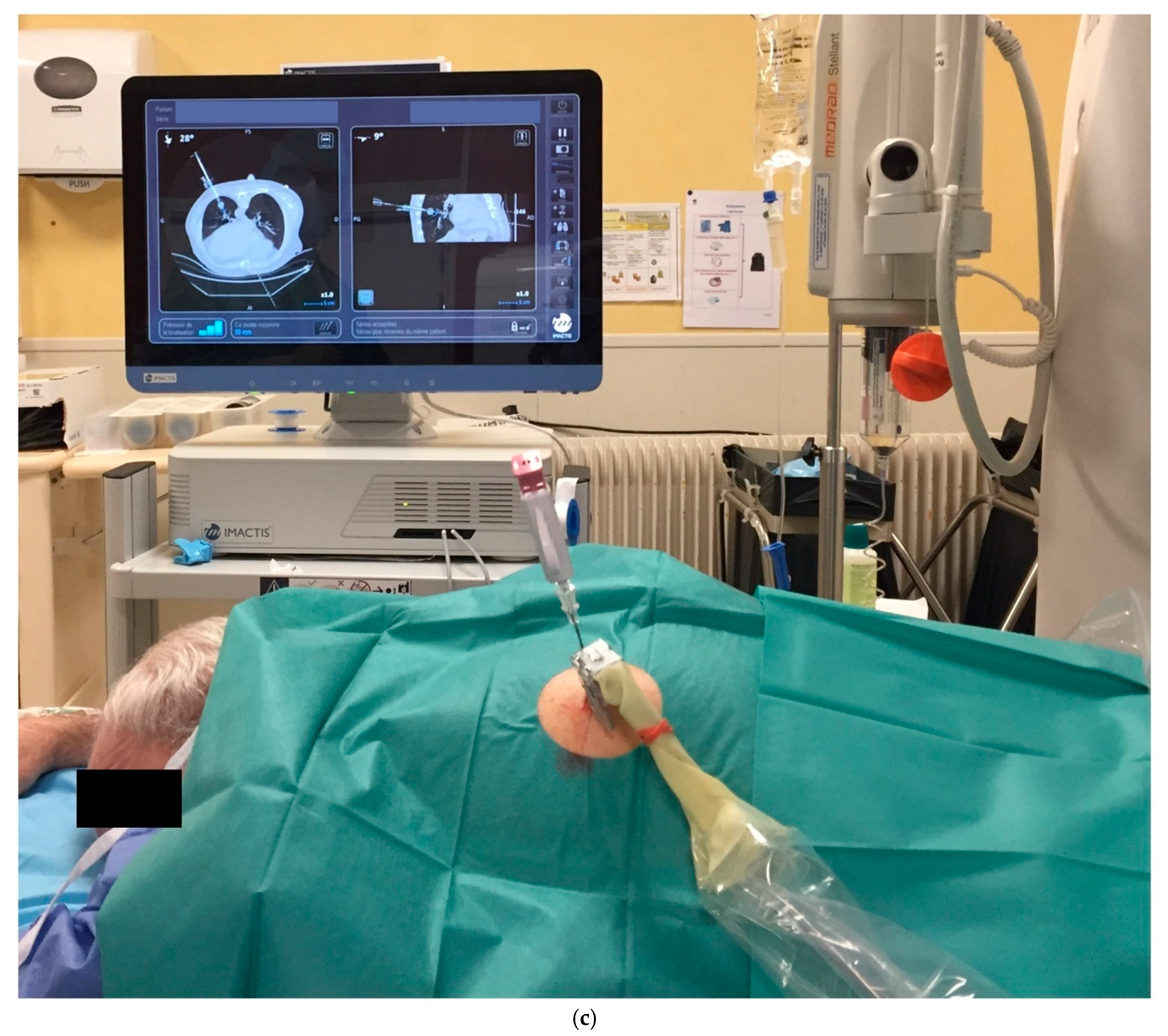
2.2.2. CT-Navigation™ Guided Procedures: NAV-Group
2.3. Outcomes Definition
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Operators Experience
3.3. Lesions Characteristics
3.4. Technical and Diagnostic Success Rates
3.5. Procedure Duration and Radiation Dose Exposure
3.5.1. Step ∆1
3.5.2. Step ∆2
3.5.3. Whole Procedure
3.6. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez, Y.; Anvari, A.; Samir, A.E.; Arellano, R.S.; Prabhakar, A.M.; Uppot, R.N. Navigational Guidance and Ablation Planning Tools for Interventional Radiology. Curr. Probl. Diagn. Radiol. 2017, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Gupta, S.; Hicks, M.E. Out-of-Plane Computed-Tomography-Guided Biopsy Using a Magnetic-Field-Based Navigation System. Cardiovasc. Intervent. Radiol. 2006, 29, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Hur, J.; Lee, S.M.; Lee, H.J.; Hong, Y.J.; Nam, J.E.; Kim, H.S.; Kim, Y.J.; Choi, B.W.; Kim, T.H.; et al. CT Fluoroscopy-Guided Lung Biopsy versus Conventional CT-Guided Lung Biopsy: A Prospective Controlled Study to Assess Radiation Doses and Diagnostic Performance. Eur. Radiol. 2011, 21, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Prosch, H.; Stadler, A.; Schilling, M.; Bürklin, S.; Eisenhuber, E.; Schober, E.; Mostbeck, G. CT Fluoroscopy-Guided vs. Multislice CT Biopsy Mode-Guided Lung Biopsies: Accuracy, Complications and Radiation Dose. Eur. J. Radiol. 2012, 81, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.C.; Li, T.F.; Han, X.W.; Wu, G.; Ma, J.; Fu, M.T.; Sun, Q.; Beilner, J. Clinical Applications of the C-Arm Cone-Beam CT-Based 3D Needle Guidance System in Performing Percutaneous Transthoracic Needle Biopsy of Pulmonary Lesions. Diagn. Interv. Radiol. 2014, 20, 470–474. [Google Scholar] [CrossRef]
- Lustig, J.P.; Aubry, S.; Vidal, C.; Pazart, L.; Moreau-Gaudry, A.; Bricault, I. Body Interventional Procedures: Which Is the Best Method for CT Guidance? Eur. Radiol. 2020, 30, 1593–1600. [Google Scholar] [CrossRef]
- Gruber-Rouh, T.; Schulz, B.; Eichler, K.; Naguib, N.N.N.; Vogl, T.J.; Zangos, S. Radiation Dose and Quickness of Needle CT-Interventions Using a Laser Navigation System (LNS) Compared with Conventional Method. Eur. J. Radiol. 2015, 84, 1976–1980. [Google Scholar] [CrossRef]
- Moser, C.; Becker, J.; Deli, M.; Busch, M.; Boehme, M.; Groenemeyer, D.H.W. A Novel Laser Navigation System Reduces Radiation Exposure and Improves Accuracy and Workflow of CT-Guided Spinal Interventions: A Prospective, Randomized, Controlled, Clinical Trial in Comparison to Conventional Freehand Puncture. Eur. J. Radiol. 2013, 82, 627–632. [Google Scholar] [CrossRef]
- Chehab, M.A.; Brinjikji, W.; Copelan, A.; Venkatesan, A.M. Navigational Tools for Interventional Radiology and Interventional Oncology Applications. Semin. Interv. Radiol. 2015, 32, 416–427. [Google Scholar] [CrossRef]
- Uruc, V.; Ozden, R.; Dogramacı, Y.; Kalacı, A.; Dikmen, B.; Yıldız, O.S.; Yengil, E. The Comparison of Freehand Fluoroscopic Guidance and Electromagnetic Navigation for Distal Locking of Intramedullary Implants. Injury 2013, 44, 863–866. [Google Scholar] [CrossRef]
- Mahan, M.; Spetzler, R.F.; Nakaji, P. Electromagnetic Stereotactic Navigation for External Ventricular Drain Placement in the Intensive Care Unit. J. Clin. Neurosci. 2013, 20, 1718–1722. [Google Scholar] [CrossRef]
- Grand, D.J.; Atalay, M.A.; Cronan, J.J.; Mayo-Smith, W.W.; Dupuy, D.E. CT-Guided Percutaneous Lung Biopsy: Comparison of Conventional CT Fluoroscopy to CT Fluoroscopy with Electromagnetic Navigation System in 60 Consecutive Patients. Eur. J. Radiol. 2011, 79, e133–e136. [Google Scholar] [CrossRef] [PubMed]
- Durand, P.; Moreau-Gaudry, A.; Silvent, A.S.; Frandon, J.; Chipon, E.; Médici, M.; Bricault, I. Computer Assisted Electromagnetic Navigation Improves Accuracy in Computed Tomography Guided Interventions: A Prospective Randomized Clinical Trial. PLoS ONE 2017, 12, e0173751. [Google Scholar] [CrossRef] [PubMed]
- Volpi, S.; Tsoumakidou, G.; Loriaud, A.; Hocquelet, A.; Duran, R.; Denys, A. Electromagnetic Navigation System Combined with High-Frequency-Jet-Ventilation for CT-Guided Hepatic Ablation of Small US-Undetectable and Difficult to Access Lesions. Int. J. Hyperth. 2019, 36, 1051–1057. [Google Scholar] [CrossRef]
- Sánchez, Y.; Trifanov, D.S.; Kattapuram, T.M.; Tao, H.; Prabhakar, A.M.; Arellano, R.S.; Uppot, R.N. Use of an Electromagnetic Navigation System on a Phantom as a Training Simulator for CT-Guided Procedures. J. Am. Coll. Radiol. 2017, 14, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Moncharmont, L.; Moreau-Gaudry, A.; Medici, M.; Bricault, I. Phantom Evaluation of a Navigation System for Out-of-Plane CT-Guided Puncture. Diagn. Interv. Imaging 2015, 96, 531–536. [Google Scholar] [CrossRef][Green Version]
- Rouchy, R.C.; Moreau-Gaudry, A.; Chipon, E.; Aubry, S.; Pazart, L.; Lapuyade, B.; Durand, M.; Hajjam, M.; Pottier, S.; Renard, B.; et al. Evaluation of the Clinical Benefit of an Electromagnetic Navigation System for CT-Guided Interventional Radiology Procedures in the Thoraco-Abdominal Region Compared with Conventional CT Guidance (CTNAV II): Study Protocol for a Randomised Controlled Trial. Trials 2017, 18, 306. [Google Scholar] [CrossRef]
- Moulin, B.; Tselikas, L.; De Baere, T.; Varin, F.; Abed, A.; Debays, L.; Bardoulat, C.; Hakime, A.; Teriitehau, C.; Deschamps, F.; et al. CT Guidance Assisted by Electromagnetic Navigation System for Percutaneous Fixation by Internal Cemented Screws (FICS). Eur. Radiol. 2020, 30, 943–949. [Google Scholar] [CrossRef]
- Iannelli, G.; Caivano, R.; Villonio, A.; Semeraro, V.; Lucarelli, N.M.; Ganimede, M.P.; Gisone, V.; Dinardo, G.; Bruno, S.; Macarini, L.; et al. Percutaneous Computed Tomography-Guided Lung Biopsies Using a Virtual Navigation Guidance: Our Experience. Cancer Investig. 2018, 36, 349–355. [Google Scholar] [CrossRef]
- Grasso, R.F.; Luppi, G.; Cazzato, R.L.; Faiella, E.; D’Agostino, F.; Beomonte Zobel, D.; De Lena, M. Percutaneous Computed Tomography-Guided Lung Biopsies: Preliminary Results Using an Augmented Reality Navigation System. Tumori 2012, 98, 775–782. [Google Scholar] [CrossRef]
- Grasso, R.F.; Faiella, E.; Luppi, G.; Schena, E.; Giurazza, F.; Del Vescovo, R.; D’Agostino, F.; Cazzato, R.L.; Beomonte Zobel, B. Percutaneous Lung Biopsy: Comparison between an Augmented Reality CT Navigation System and Standard CT-Guided Technique. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Frauenfelder, G.; Santucci, D.; Luppi, G.; Schena, E.; Beomonte Zobel, B.; Grasso, R.F. Percutaneous Low-Dose CT-Guided Lung Biopsy with an Augmented Reality Navigation System: Validation of the Technique on 496 Suspected Lesions. Clin. Imaging 2018, 49, 101–105. [Google Scholar] [CrossRef]
- Grasso, R.F.; Cazzato, R.L.; Luppi, G.; D’Agostino, F.; Schena, E.; Del Vescovo, R.; Giurazza, F.; Faiella, E.; Beomonte Zobel, B. Percutaneous Lung Biopsies: Performance of an Optical CT-Based Navigation System with a Low-Dose Protocol. Eur. Radiol. 2013, 23, 3071–3076. [Google Scholar] [CrossRef]
- Radiologie Interventionnelle Assistée par Ordinateur. Available online: http://www.imactis.com/ (accessed on 3 March 2021).
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Krücker, J.; Xu, S.; Venkatesan, A.; Locklin, J.K.; Amalou, H.; Glossop, N.; Wood, B.J. Clinical Utility of Real-Time Fusion Guidance for Biopsy and Ablation. J. Vasc. Interv. Radiol. 2011, 22, 515–524. [Google Scholar] [CrossRef]
- Tomiyama, N.; Yasuhara, Y.; Nakajima, Y.; Adachi, S.; Arai, Y.; Kusumoto, M.; Eguchi, K.; Kuriyama, K.; Sakai, F.; Noguchi, M.; et al. CT-Guided Needle Biopsy of Lung Lesions: A Survey of Severe Complication Based on 9783 Biopsies in Japan. Eur. J. Radiol. 2006, 59, 60–64. [Google Scholar] [CrossRef]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.M.; Vliegenthart, R.; Oudkerk, M. Complication Rates of CT-Guided Transthoracic Lung Biopsy: Meta-Analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef]
- Teriitehau, C.; Rabeh, H.; Pessis, E.; Sénéchal, Q.; Besse, F.; Bravetti, M. Reduction of Patient Radiation Dose during Percutaneous CT Vertebroplasty: Impact of a New Computer-Assisted Navigation (CAN) System. Radioprotection 2020, 55, 11–16. [Google Scholar] [CrossRef]
- Glossop, N.D. Advantages of Optical Compared with Electromagnetic Tracking. J. Bone Jt. Surg. Am. 2009, 91, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mavrovi, E.; Pialat, J.B.; Beji, H.; Kalenderian, A.C.; Vaz, G.; Richioud, B. Percutaneous osteosynthesis and cementoplasty for stabilization of malignant pathologic fractures of the proximal femur. Diagn. Interv. Imaging 2017, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ringe, K.I.; Pöhler, G.H.; Rabeh, H.; Wacker, F. Electromagnetic Navigation System-Guided Microwave Ablation of Hepatic Tumors: A Matched Cohort Study. Cardiovasc. Intervent. Radiol. 2021, 44, 500–506. [Google Scholar] [CrossRef] [PubMed]
| NAV Group | CT Group | p | |
|---|---|---|---|
| No. of patients | 60 | 60 | |
| Sex ratio M/F | 28/32 | 33/27 | 0.465 |
| Mean age (range) (years) | 66 (58; 72) | 68 (60; 73) | 0.576 |
| BMI (range) (kg.m−2) | 26 (23; 28) | 24 (21; 29) | 0.187 |
| Position (P/SU/SI) | 25/33/2 | 33/26/1 | 0.187 |
| Operator | |||
| Senior (%) | 57 (95.0) | 54 (90.0) | 0.328 |
| Resident (%) | 3 (5.0) | 6 (10.0) | |
| Lesion size (mm) | 20.0 [12.8; 37.0] | 29.5 [20.0; 49.5] | 0.007 |
| No. of lesions < 2 cm (%) | 29 (48.3) | 14 (23.3) | 0.007 |
| Distance to the skin ± SD (mm) | 70.2 ± 22.1 | 62.2 ± 16.8 | 0.028 |
| Distance to pleura (mm) | 18 [10.0; 33.3] | 21.5 [7.0; 28.0] | 0.340 |
| Localization (%) | 0.754 | ||
| Right lower lobe | 12 (20.0) | 13 (21.7) | |
| Right upper lobe | 23 (38.3) | 19 (31.7) | |
| Middle lobe | 3 (5.0) | 4 (6.7) | |
| Left lower lobe | 10 (16.7) | 7 (11.7) | |
| Left upper lobe | 12 (20.0) | 17 (28.3) | |
| No. of easy lesions (%) | 33 (55.0) | 49 (81.7) | 0.002 |
| No. of difficult lesions (%) | 27 (45.0) | 11 (18.3) | |
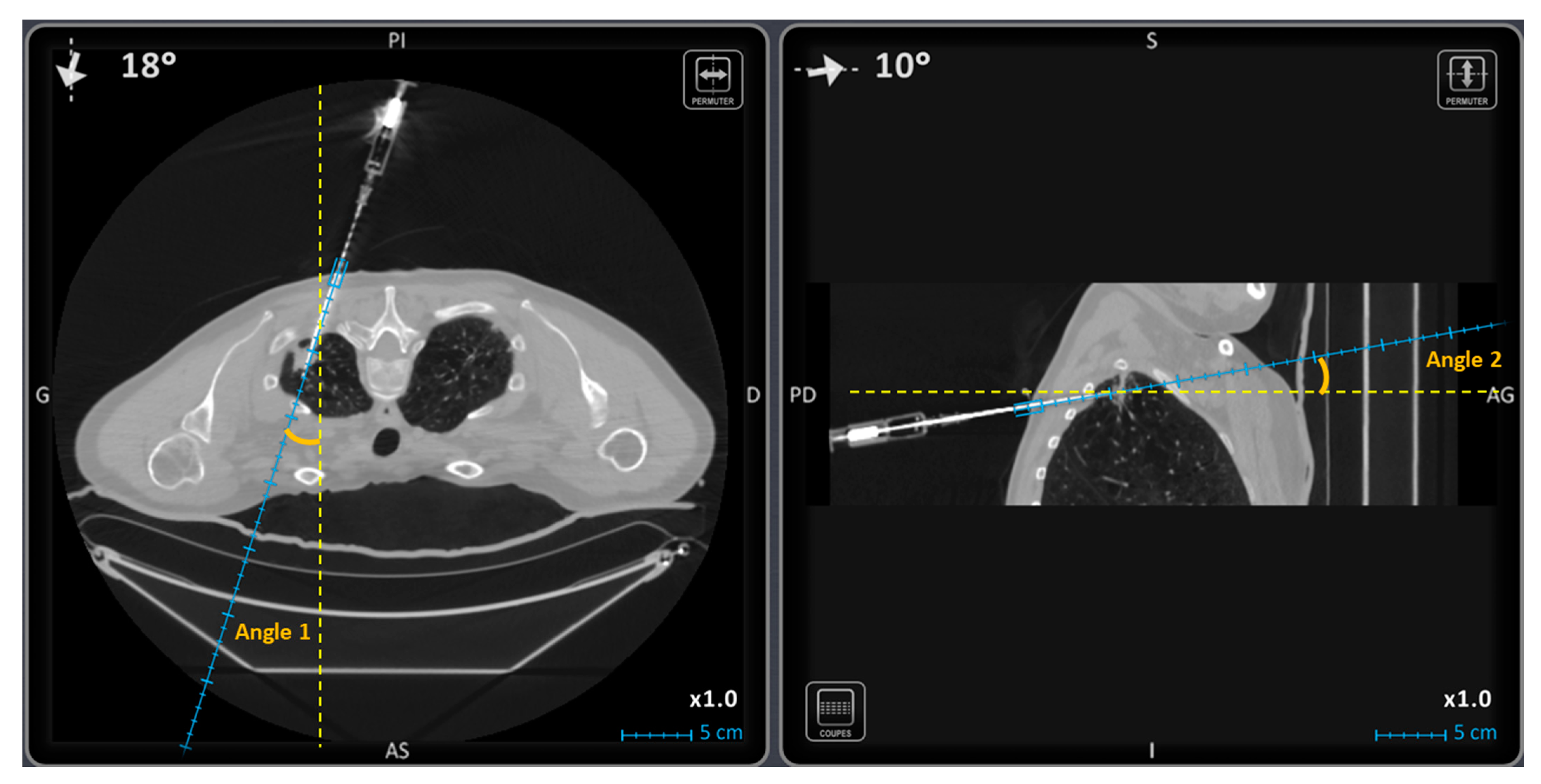
| Trajectory angle 1 (°) | 15.5 [3.0; 31.5] | 6.0 [2.0; 13.0] | 0.0008 |
| Trajectory angle 2 (°) | 10.0 [3.0; 16.3] | 1.0 [1.0; 5.0] | <0.0001 |
| NAV Group | CT Group | p | |
|---|---|---|---|
| Technical success (%) | 57 (95.0) | 56 (93.3) | 1.000 |
| Diagnostic success (%) | 56 (93.3) | 55 (91.6) | 1.000 |
| Step ∆1 | |||
| Duration (min) | 13 [11; 17] | 11 [8; 15] | 0.018 |
| No. of CT acquisitions | 1 [1; 1] | 1 [1; 1] | 1.000 |
| DLP (mGy.cm) | 142.9 [105.9; 219.5] | 188.2 [123.4; 360.7] | 0.013 |
| Step ∆2 | |||
| Duration (min) | 9 [7; 12] | 12 [9; 15] | 0.042 |
| No. of CT acquisitions | 6 [4; 8] | 7 [5; 9] | 0.053 |
| DLP (mGy.cm) | 611.6 [415.2; 888.7] | 849.5 [574.4; 1089.5] | 0.001 |
| Whole procedure: | |||
| Duration (min) | 28 [25; 35] | 29 [23; 33] | 0.497 |
| No. of CT acquisitions | 8 [7; 10] | 9 [8; 11] | 0.066 |
| DLP (mGy.cm) | 1059.3 [747.0; 1456.0] | 1481.3 [1255.5; 1872.9] | <0.0001 |
| NAV Group | CT Group | p | |
|---|---|---|---|
| No. of “easy” lesions (%) | 33 (55) | 49 (81.7) | |
| Diagnostic success (%) | 33 (100.0) | 47 (95.9) | 0.513 |
| Step ∆1 | |||
| Duration (min) | 12 [11; 15] | 11 [8; 13] | 0.079 |
| No. of CT acquisitions | 1 [1; 1] | 1 [1; 1] | 1.000 |
| DLP (mGy.cm) | 138.3 [96.5; 183.3] | 227.7 [123.4; 392.3] | 0.011 |
| Step ∆2 | |||
| Duration (min) | 9 [7; 10] | 11 [8; 15] | 0.015 |
| No. of CT acquisitions | 5 [4; 6] | 7 [5; 9] | <0.001 |
| DLP (mGy.cm) | 475.1 [322.4; 713.9] | 833.4 [548.9; 1058.2] | <0.001 |
| NAV Group | CT Group | p | |
|---|---|---|---|
| No. of difficult lesions (%) | 27 (45) | 11 (18.3) | |
| Diagnostic success (%) | 23 (85.2) | 8 (72.7) | 0.390 |
| Step ∆1 | |||
| Duration (min) | 14 [12; 19] | 14 [10; 19] | 0.701 |
| No. of CT acquisitions | 1 [1; 1] | 1 [1; 1] | 1.000 |
| DLP (mGy.cm) | 148.6 [112.5; 230.6] | 165.3 [138.0; 173.5] | 1 |
| Step ∆2 | |||
| Duration (min) | 11 [9; 15] | 13 [12; 15] | 0.206 |
| No. of CT acquisitions | 8 [6; 9] | 9 [7; 10] | 0.459 |
| DLP (mGy.cm) | 821.7 [465.7; 1021.9] | 1085.9 [867.4; 1198.6] | 0.158 |
| NAV Group | CT Group | p | |
|---|---|---|---|
| No. of procedures | 60 | 60 | |
| No. of complications (%) | |||
| Pneumothorax | 15 (25.0) | 19 (31.6) | 0.418 |
| Pneumothorax requiring chest tube | 0 (0.0) | 3 (5.0) | 0.244 |
| Thoracic wall hematoma | 0 (0.0) | 0 (0.0) | - |
| Hemothorax | 0 (0.0) | 0 (0.0) | - |
| Intra-alveolar hemorrhage | 21 (35.0) | 16 (26.7) | 0.323 |
| Hemoptysis moderate or severe | 0 (0.0) | 0 (0.0) | - |
| Systemic air embolism | 0 (0.0) | 0 (0.0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanouzière, M.; Varbédian, O.; Chevallier, O.; Griviau, L.; Guillen, K.; Popoff, R.; Aho-Glélé, S.-L.; Loffroy, R. Computed Tomography-Navigation™ Electromagnetic System Compared to Conventional Computed Tomography Guidance for Percutaneous Lung Biopsy: A Single-Center Experience. Diagnostics 2021, 11, 1532. https://doi.org/10.3390/diagnostics11091532
Lanouzière M, Varbédian O, Chevallier O, Griviau L, Guillen K, Popoff R, Aho-Glélé S-L, Loffroy R. Computed Tomography-Navigation™ Electromagnetic System Compared to Conventional Computed Tomography Guidance for Percutaneous Lung Biopsy: A Single-Center Experience. Diagnostics. 2021; 11(9):1532. https://doi.org/10.3390/diagnostics11091532
Chicago/Turabian StyleLanouzière, Morgane, Olivier Varbédian, Olivier Chevallier, Loïc Griviau, Kévin Guillen, Romain Popoff, Serge-Ludwig Aho-Glélé, and Romaric Loffroy. 2021. "Computed Tomography-Navigation™ Electromagnetic System Compared to Conventional Computed Tomography Guidance for Percutaneous Lung Biopsy: A Single-Center Experience" Diagnostics 11, no. 9: 1532. https://doi.org/10.3390/diagnostics11091532
APA StyleLanouzière, M., Varbédian, O., Chevallier, O., Griviau, L., Guillen, K., Popoff, R., Aho-Glélé, S.-L., & Loffroy, R. (2021). Computed Tomography-Navigation™ Electromagnetic System Compared to Conventional Computed Tomography Guidance for Percutaneous Lung Biopsy: A Single-Center Experience. Diagnostics, 11(9), 1532. https://doi.org/10.3390/diagnostics11091532






