Radiolabeling Strategies of Nanobodies for Imaging Applications
Abstract
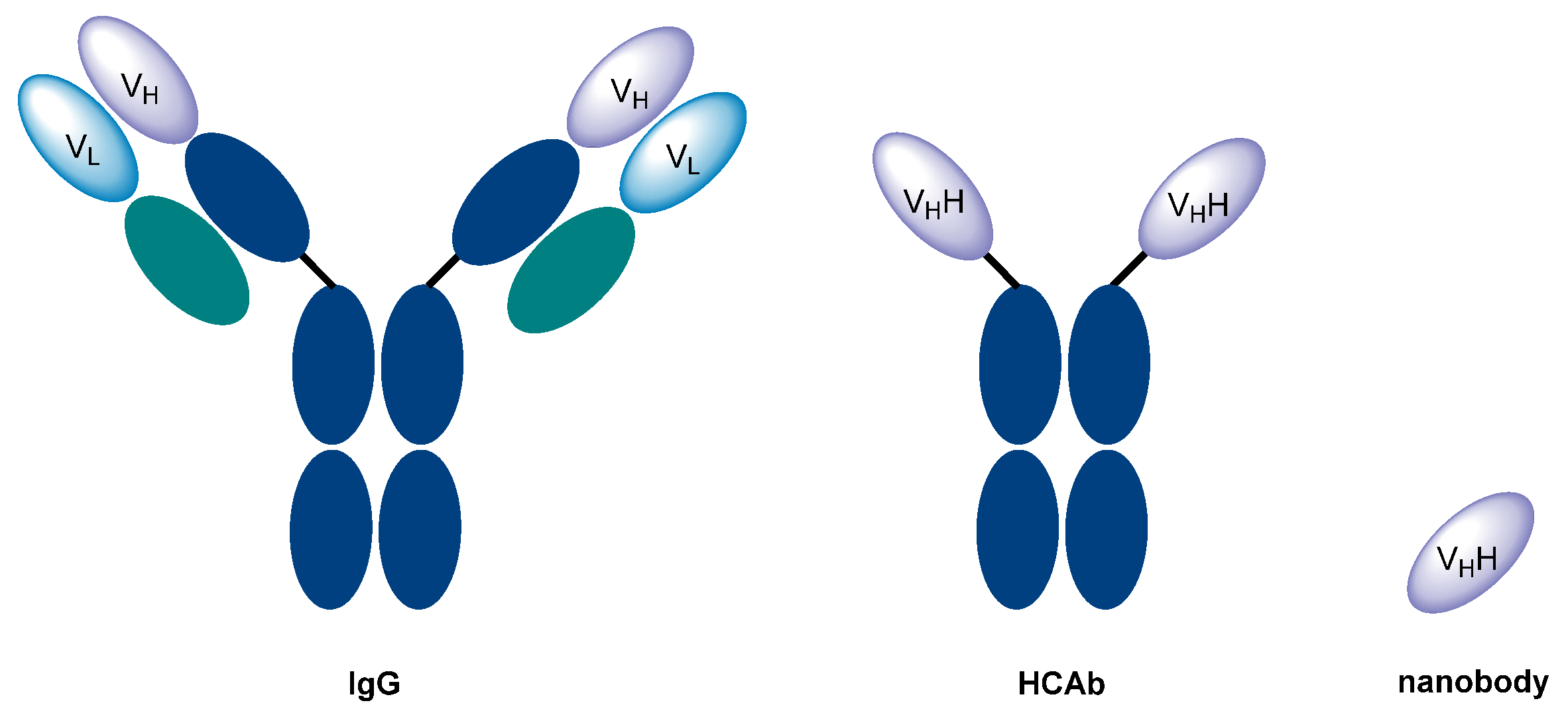
1. Introduction
2. Radiolabeling Strategies of Nanobodies
2.1. Radiohalogens
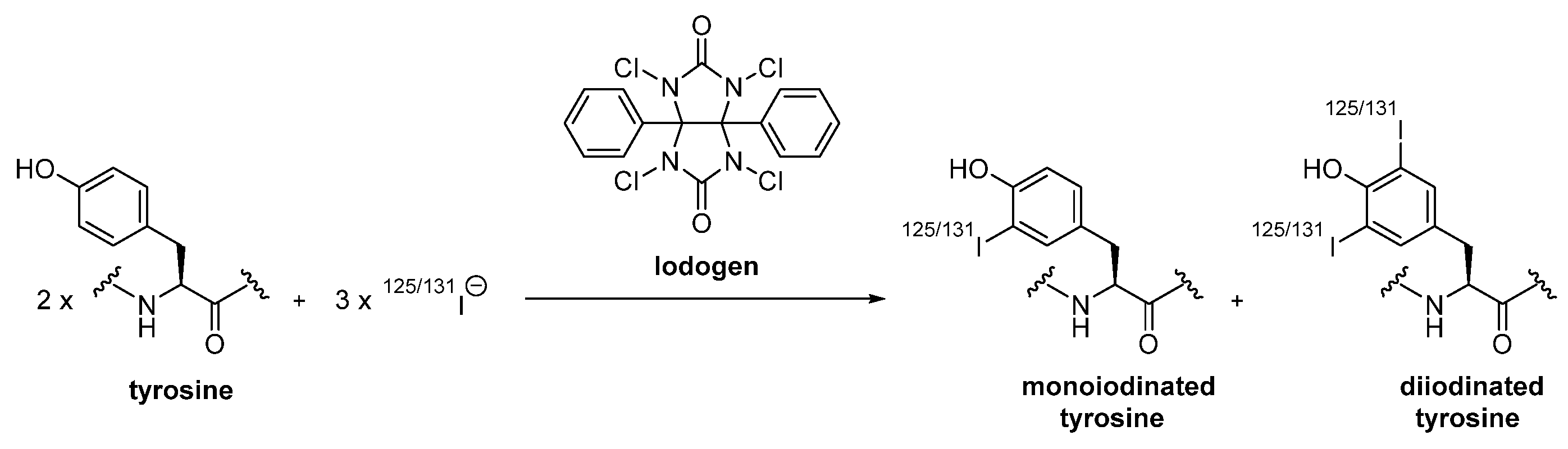
2.1.1. Direct Radiohalogenation
2.1.2. Indirect Radiohalogenation
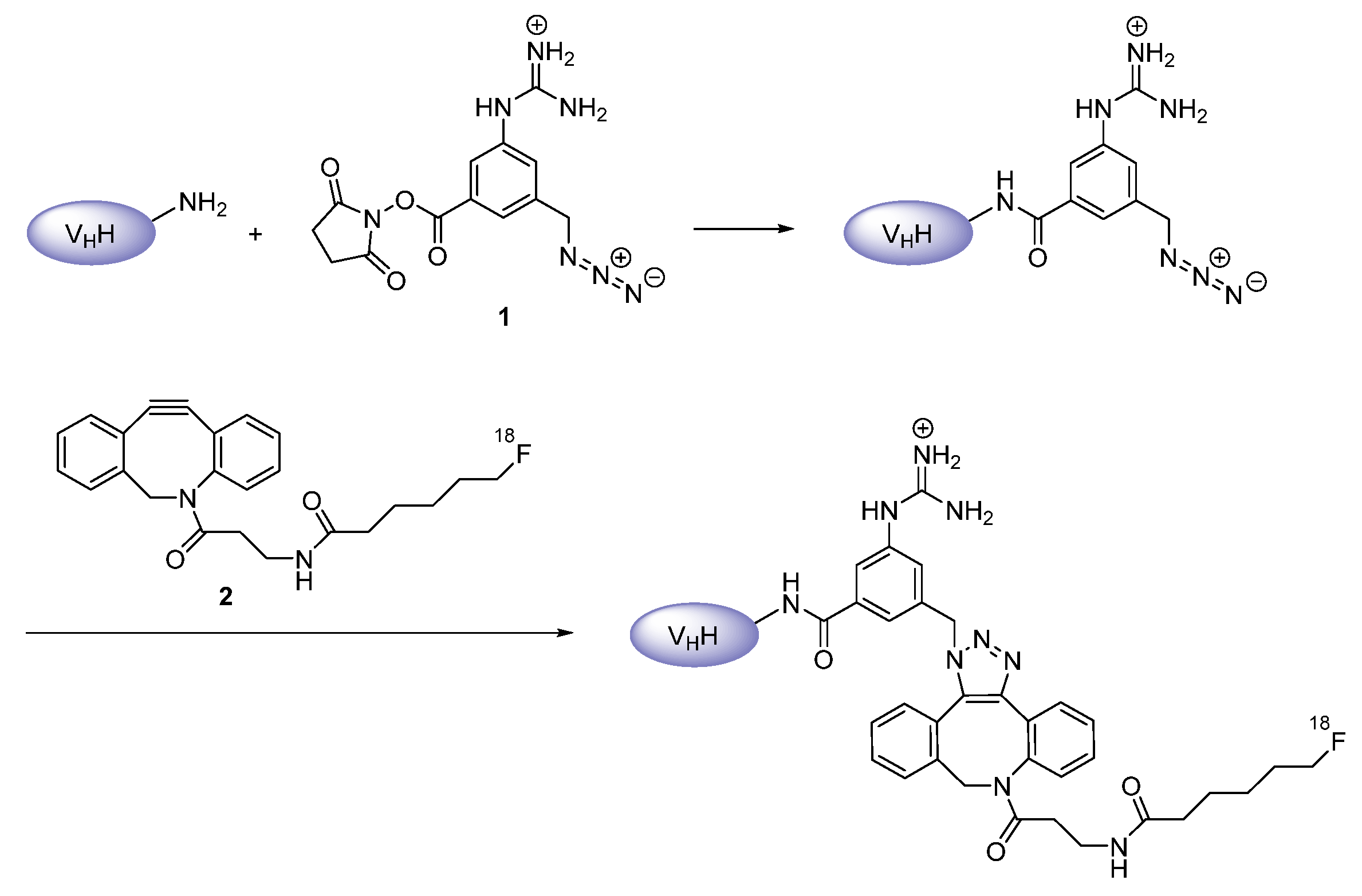
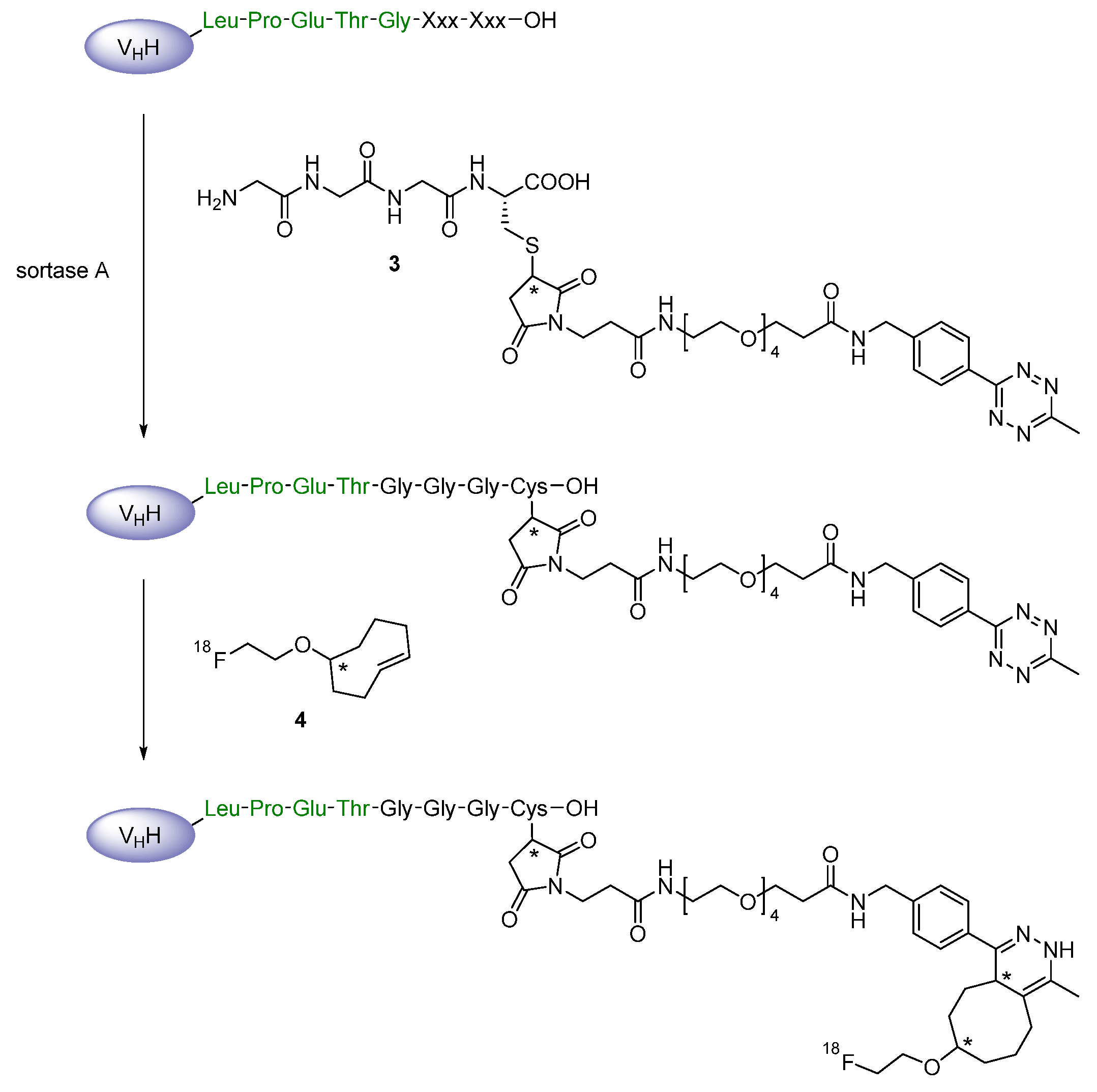
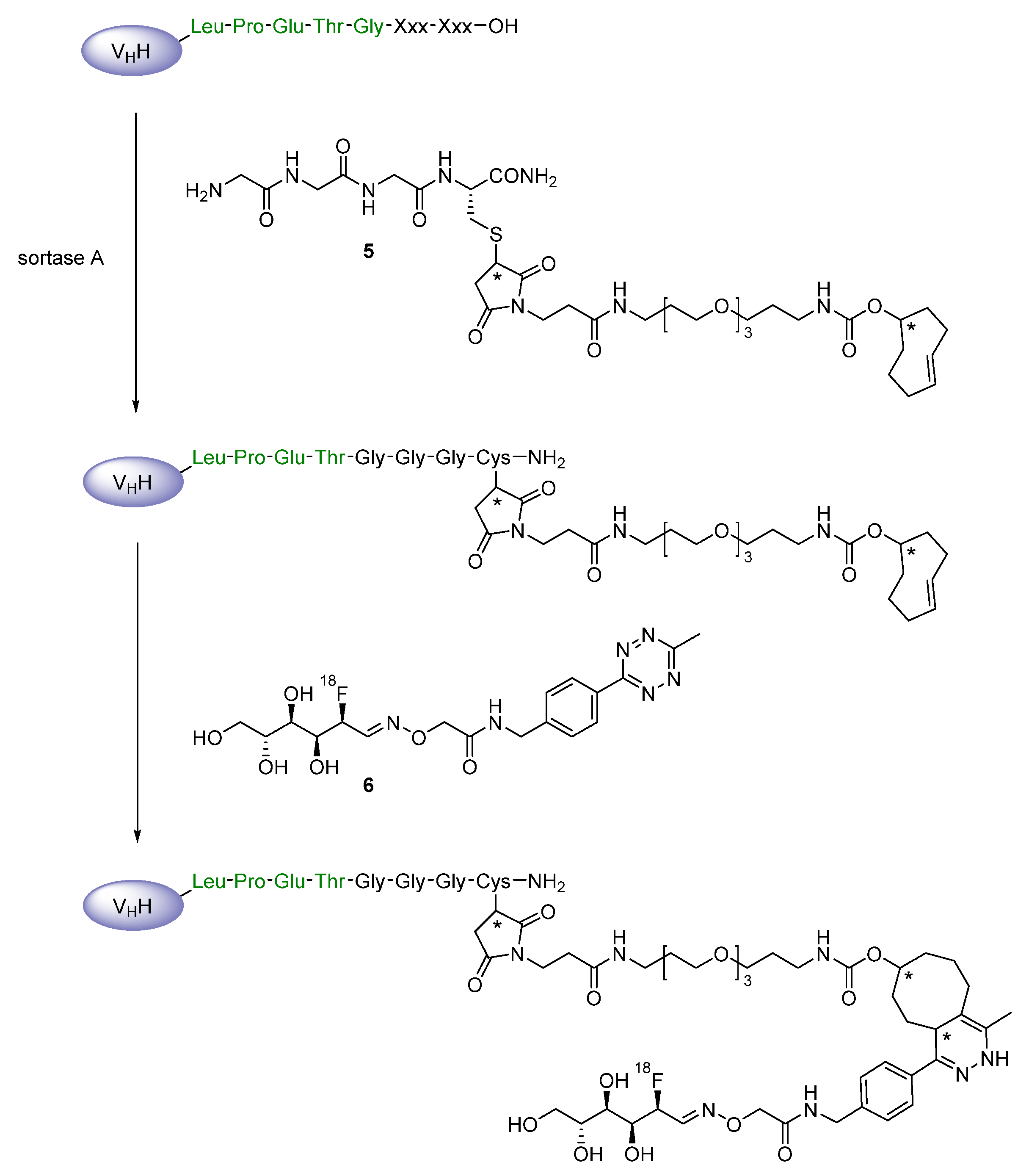
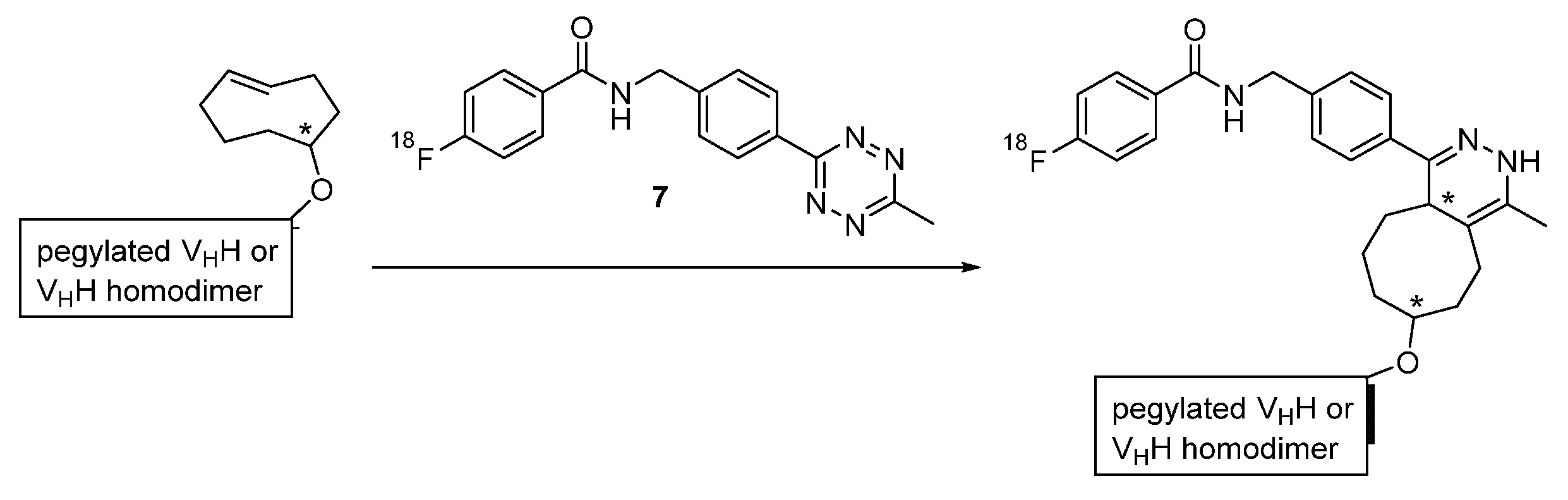
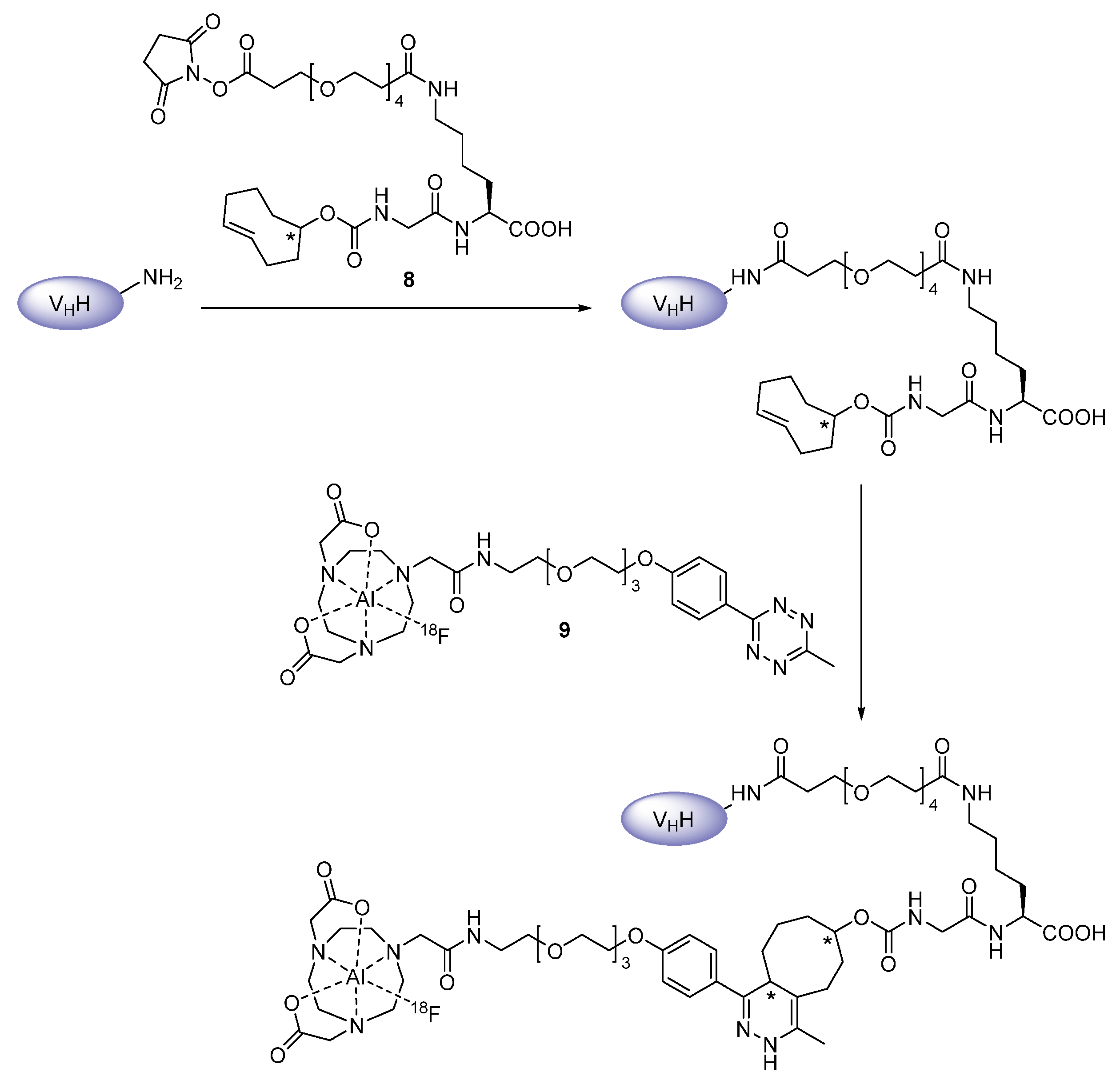
Prosthetic Groups
Chelation
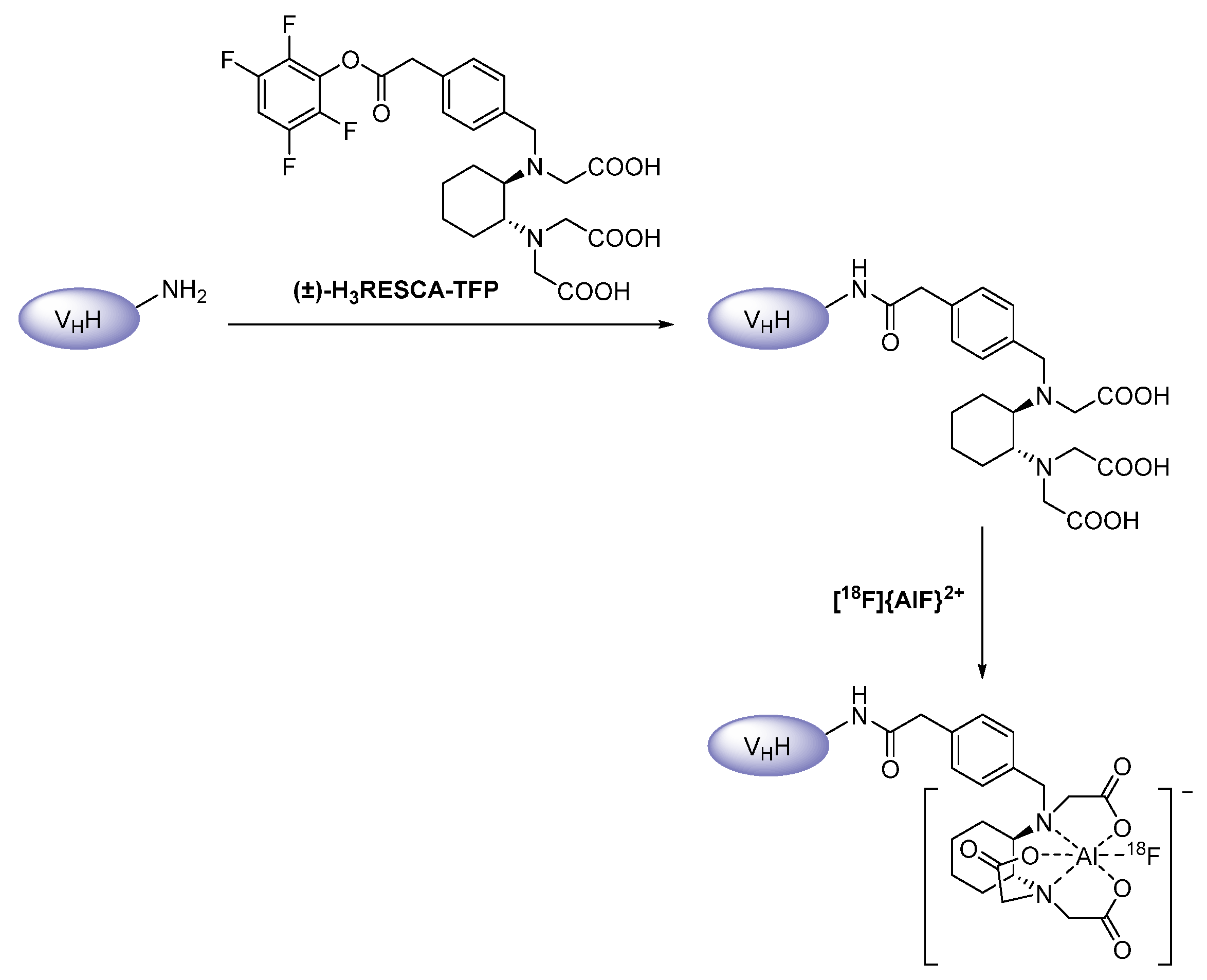
2.2. Radiometals
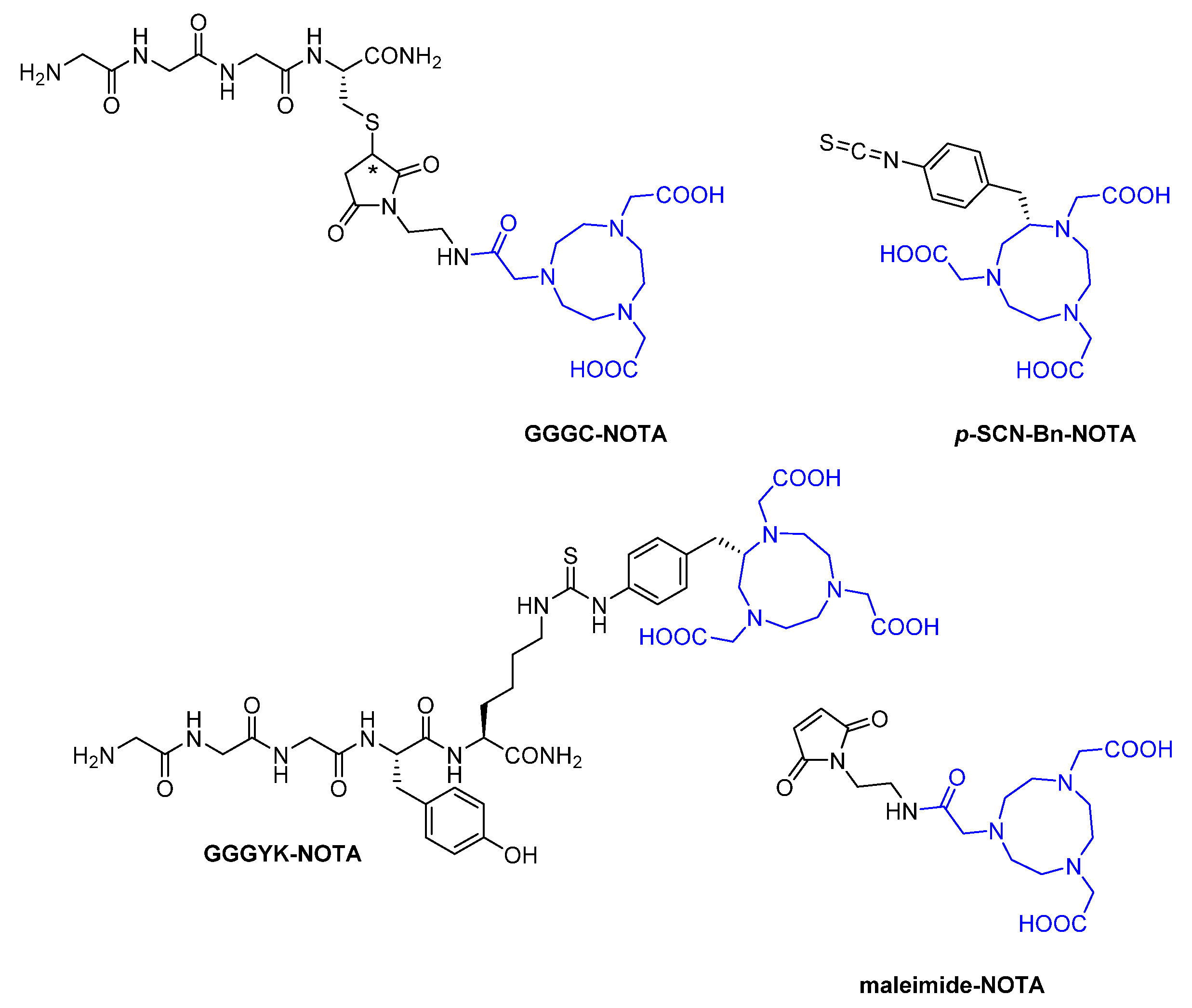
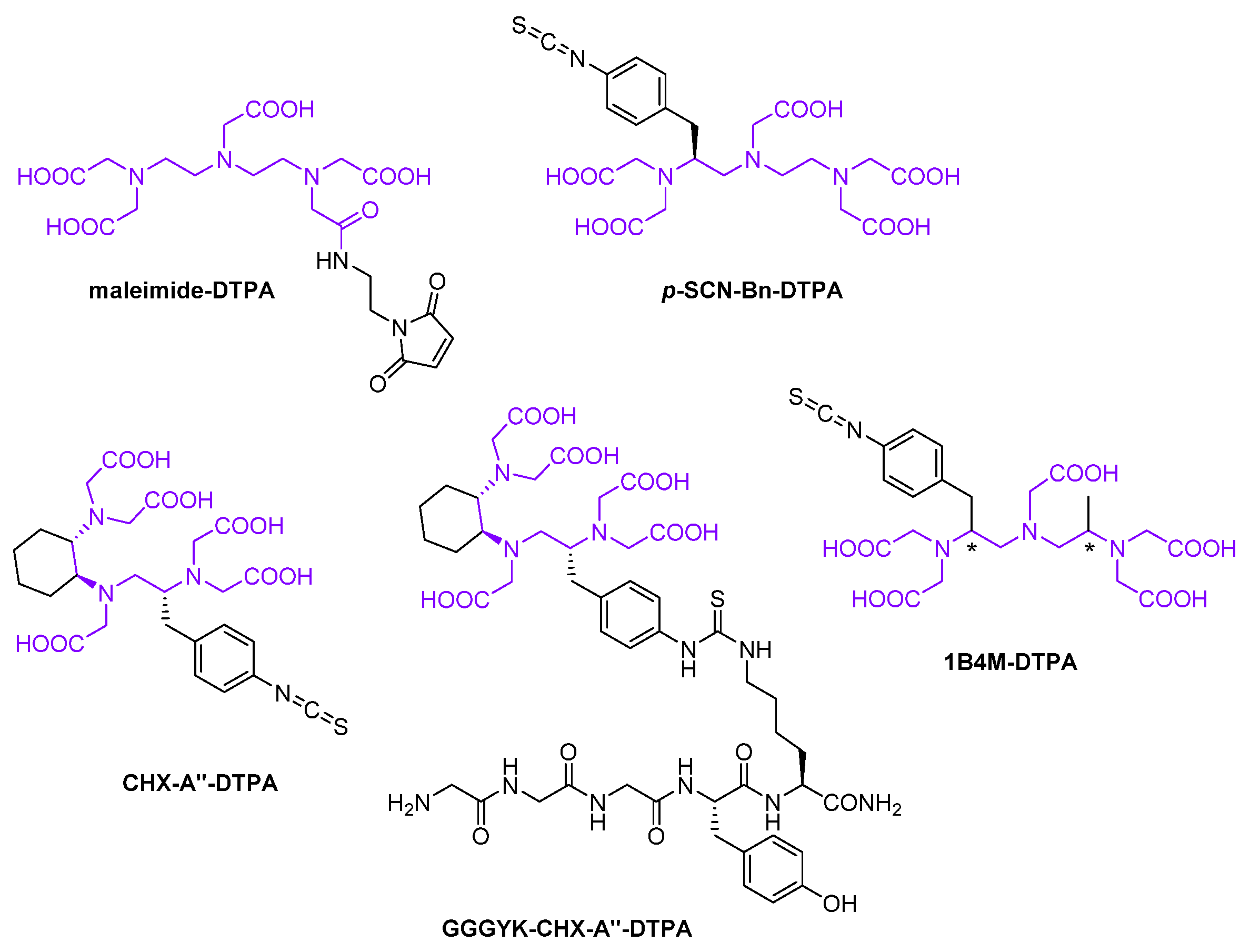
2.2.1. Synthetic Chelators
Macrocyclic
Acyclic
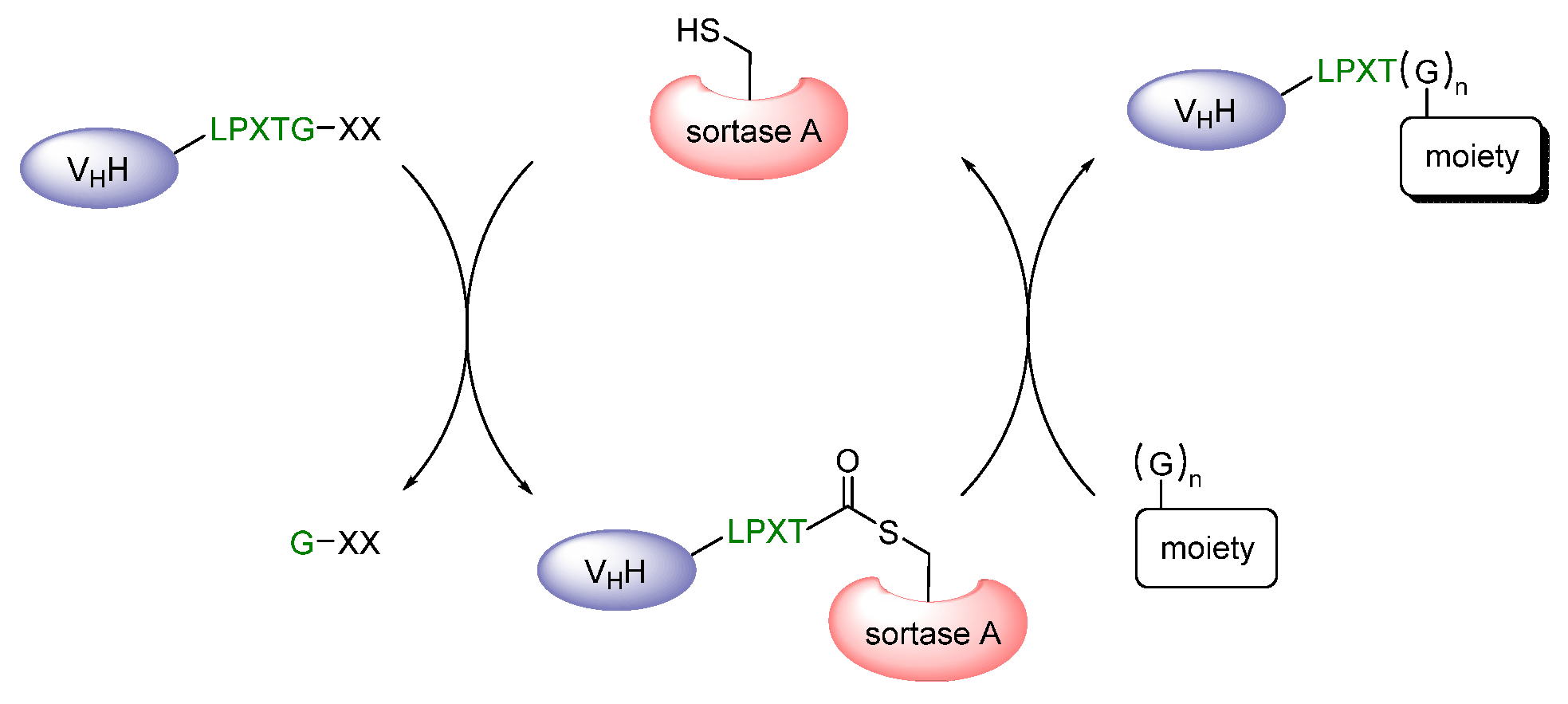
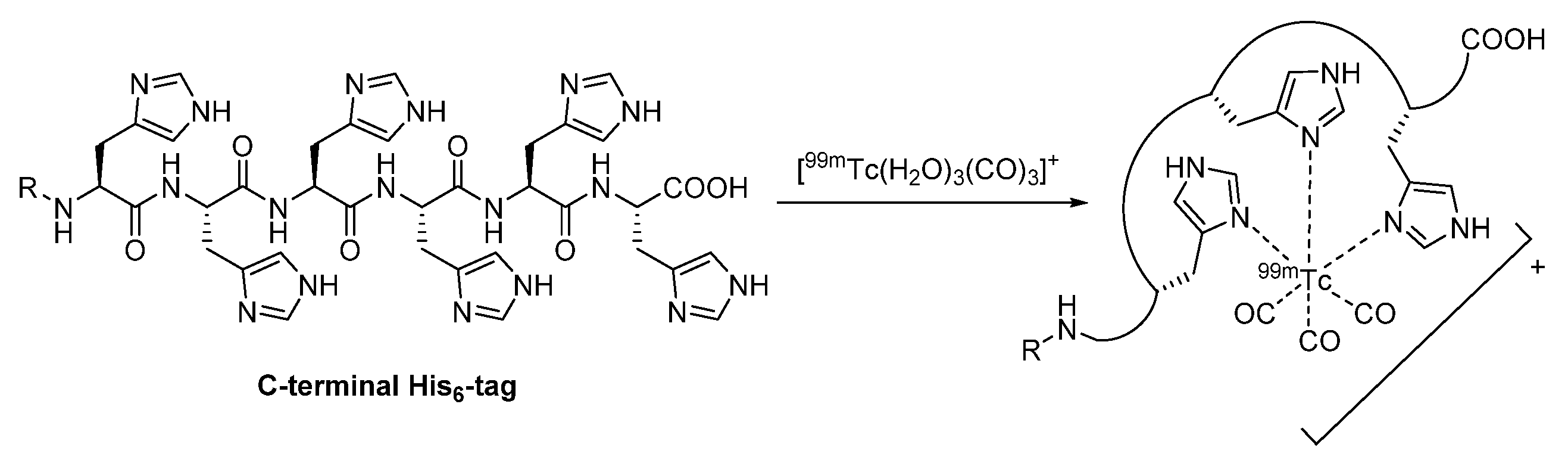
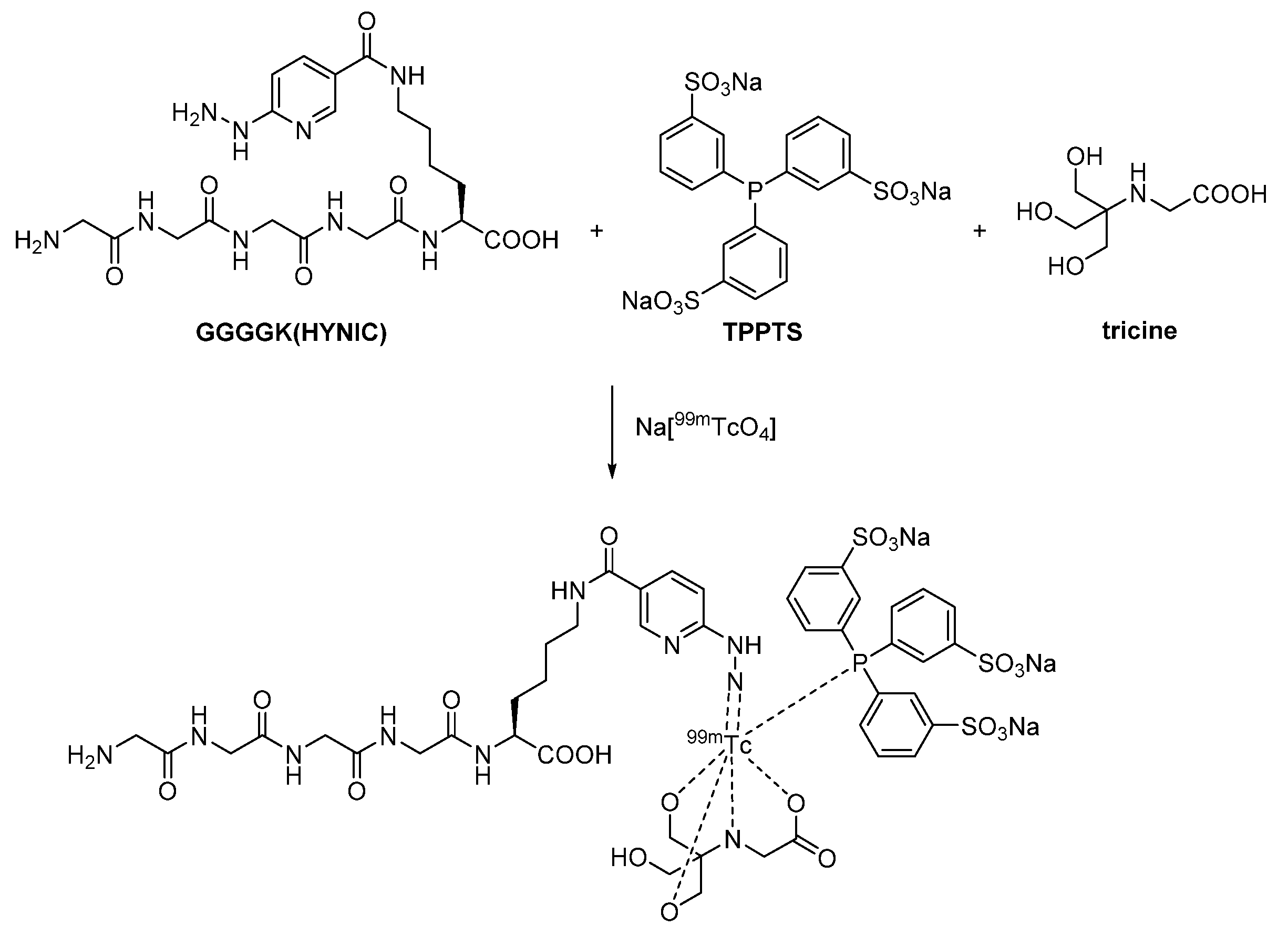
2.2.2. Proteinogenic Chelator
2.2.3. Heteroleptic Complex
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lecocq, Q.; De Vlaeminck, Y.; Hanssens, H.; D’Huyvetter, M.; Raes, G.; Goyvaerts, C.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. Theranostics in immuno-oncology using nanobody derivatives. Theranostics 2019, 9, 7772–7791. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, S.; Ying, T. Single–domain antibodies as therapeutics against human viral diseases. Front. Immunol. 2017, 8, 1802. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. (18)F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Rashidian, M.; Ploegh, H. Nanobodies as non-invasive imaging tools. Immuno-Oncol. Technol. 2020, 7, 2–14. [Google Scholar] [CrossRef]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; Van Willigen, D.M.; Van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef]
- De Vos, J.; Devoogdt, N.; Lahoutte, T.; Muyldermans, S. Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: Adjusting the bullet to its target. Expert Opin. Biol. Ther. 2013, 13, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted nanobody-based molecular tracers for nuclear imaging and image-guided surgery. Antibodies (Basel) 2019, 8, 12. [Google Scholar] [CrossRef]
- Weber, J.; Haberkorn, U.; Mier, W. Cancer stratification by molecular imaging. Int. J. Mol. Sci. 2015, 16, 4918–4946. [Google Scholar] [CrossRef] [PubMed]
- Shoghi, K.I. Quantitative small animal PET. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 365–373. [Google Scholar]
- Franc, B.L.; Seo, Y.; Flavell, R.; Aparici, C.M. Preclinical SPECT and SPECT-CT in Oncology. Recent Results Cancer Res. 2020, 216, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W.D. PET/CT: A new imaging technology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody fragment and affibody immunopet imaging agents: Radiolabelling strategies and applications. ChemMedChem 2018, 13, 2466–2478. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Jodal, A.; Pape, F.; Becker-Pauly, C.; Maas, O.; Schibli, R.; Béhé, M. Evaluation of ¹¹¹In-labelled exendin-4 derivatives containing different meprin β-specific cleavable linkers. PLoS ONE 2015, 10, e0123443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. An efficient method for labeling single domain antibody fragments with 18f using tetrazine- trans-cyclooctene ligation and a renal brush border enzyme-cleavable linker. Bioconjug. Chem. 2018, 29, 4090–4103. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; De Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; Muyldermans, S.; Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin. Drug Deliv. 2016, 13, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; Van Leeuwen, F.W.B.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016, 11, 328–339. [Google Scholar] [CrossRef]
- Cascini, G.L.; Niccoli Asabella, A.; Notaristefano, A.; Restuccia, A.; Ferrari, C.; Rubini, D.; Altini, C.; Rubini, G. 124Iodine: A longer-life positron emitter isotope-new opportunities in molecular imaging. Biomed. Res. Int. 2014, 2014, 672094. [Google Scholar] [CrossRef]
- Josefsson, A.; Forssell-Aronsson, E. Dosimetric analysis of (123) I, (125) I and (131) I in thyroid follicle models. EJNMMI Res. 2014, 4, 23. [Google Scholar] [CrossRef]
- Wang, P.; Ma, N.; Zhang, S.; Ning, X.; Guo, C.; Zhang, Q.; Cheng, Q.; Li, Y. Iodine-125 interstitial brachytherapy for malignant lacrimal sac tumours: An innovative technique. Eye Lond. 2020, 35, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, Y.; Chen, Y.; Liu, Z.; Han, B.; Li, Z.; Wang, J. Imidazolate ionic liquids for high-capacity capture and reliable storage of iodine. Commun. Chem. 2018, 1, s42004–s42018. [Google Scholar] [CrossRef]
- Cavaliere, A.; Probst, K.C.; Paisey, S.J.; Marshall, C.; Dheere, A.K.H.; Aigbirhio, F.; McGuigan, C.; Westwell, A.D. Radiosynthesis of 18F-Labelled Pro-Nucleotides (ProTides). Molecules 2020, 25, 704. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; O’Hagan, D. Strategies for radiolabelling antibody, antibody fragments and affibodies with fluorine-18 as tracers for positron emission tomography (PET). J. Fluor. Chem. 2017, 203, 31–46. [Google Scholar] [CrossRef][Green Version]
- Bailey, G.S. The iodogen method for radiolabeling protein. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1996; pp. 673–674. ISBN 978-0-89603-338-2. [Google Scholar]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Zalutsky, M.R. Targeting breast carcinoma with radioiodinated anti-HER2 Nanobody. Nucl. Med. Biol. 2013, 40, 52–59. [Google Scholar] [CrossRef]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Farah, K.; Farouk, N. Electrophilic radioiodination of tyrosine derivatives. J. Label. Compd. Radiopharm. 1998, 41, 255–259. [Google Scholar] [CrossRef]
- Kothari, P.; De, B.P.; He, B.; Chen, A.; Chiuchiolo, M.J.; Kim, D.; Nikolopoulou, A.; Amor-Coarasa, A.; Dyke, J.P.; Voss, H.U.; et al. Radioiodinated capsids facilitate in vivo non-invasive tracking of adeno-associated gene transfer vectors. Sci. Rep. 2017, 7, 39594. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Pruszynski, M.; Koumarianou, E.; Zhou, Z.; Zalutsky, M.R. N-Succinimidyl 3-((4-(4-(18) Ffluorobutyl)-1H-1,2,3-triazol-1-yl) methyl)-5-(guanidinomethyl) benzoate ((18) FSFBTMGMB): A residualizing label for (18) F-labeling of internalizing biomolecules. Org. Biomol. Chem. 2016, 14, 1261–1271. [Google Scholar] [CrossRef]
- Tran, L.; Baars, J.; Damen, C.; Beijnen, J.; Huitema, A. Three spectroscopic techniques evaluated as a tool to study the effects of iodination of monoclonal antibodies, exemplified by rituximab. J. Pharm. Biomed. Anal. 2011, 56, 609–614. [Google Scholar] [CrossRef]
- Buell, A.K.; White, D.A.; Meier, C.; Welland, M.E.; Knowles, T.P.J.; Dobson, C.M. Surface attachment of protein fibrils via covalent modification strategies. J. Phys. Chem. B 2010, 114, 10925–10938. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; McDougald, D.; Pruszynski, M.; Osada, T.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. N-Succinimidyl guanidinomethyl iodobenzoate protein radiohalogenation agents: Influence of isomeric substitution on radiolabeling and target cell residualization. Nucl. Med. Biol. 2014, 41, 802–812. [Google Scholar] [CrossRef]
- Zhou, Z.; Vaidyanathan, G.; McDougald, D.; Kang, C.M.; Balyasnikova, I.; Devoogdt, N.; Ta, A.N.; McNaughton, B.R.; Zalutsky, M.R. Fluorine-18 labeling of the HER2-targeting single-domain antibody 2Rs15d using a residualizing label and preclinical evaluation. Mol. Imaging Biol. 2017, 19, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; McDougald, D.; Meshaw, R.; Balyasnikova, I.; Zalutsky, M.R.; Vaidyanathan, G. Labeling single domain antibody fragments with 18F using a novel residualizing prosthetic agent–N–succinimidyl 3-(1-(2-(2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)-5-(guanidinomethyl)benzoate. Nucl. Med. Biol. 2021, 100-101, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chitneni, S.K.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Fluorine–18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain–promoted click reaction: Chemistry and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; McDougald, D.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Labeling single domain antibody fragments with fluorine-18 Using 2,3,5,6-tetrafluorophenyl 6-[18F] fluoronicotinate resulting in high tumor-to-kidney ratios. Mol. Pharm. 2019, 16, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Blykers, A.; Schoonooghe, S.; Xavier, C.; D’hoe, K.; Laoui, D.; D’Huyvetter, M.; Vaneycken, I.; Cleeren, F.; Bormans, G.; Heemskerk, J.; et al. pet imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18f-radiolabeled camelid single-domain antibody fragments. J. Nucl. Med. 2015, 56, 1265–1271. [Google Scholar] [CrossRef]
- Bala, G.; Blykers, A.; Xavier, C.; Descamps, B.; Broisat, A.; Ghezzi, C.; Fagret, D.; Van Camp, G.; Caveliers, V.; Vanhove, C.; et al. Targeting of vascular cell adhesion molecule-1 by 18F-labelled nanobodies for PET/CT imaging of inflamed atherosclerotic plaques. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1001–1008. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.; Dougan, M.; Juras, P.K.; Cavallari, M.; Wojtkiewicz, G.R.; Jacobsen, J.; Edens, J.G.; Tas, J.M.G.; Victora, G.; et al. The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS Cent. Sci. 2015, 1, 142–147. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.J.; Bilate, A.M.; Duarte, J.N.; Wojtkiewicz, G.R.; Jacobsen, J.T.; Cragnolini, J.; Swee, L.K.; Victora, G.D.; Weissleder, R.; et al. Noninvasive imaging of immune responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6146–6151. [Google Scholar] [CrossRef]
- Rashidian, M.; Wang, L.; Edens, J.G.; Jacobsen, J.T.; Hossain, I.; Wang, Q.; Victora, G.D.; Vasdev, N.; Ploegh, H.; Liang, S.H. Enzyme-mediated modification of single-domain antibodies for imaging modalities with different characteristics. Angew. Chem. Int. Ed. Engl. 2016, 55, 528–533. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, T.; Hong, H.; Zhou, Z.; Wu, Z. Sortase a-mediated on-resin peptide cleavage and in situ ligation: An efficient one-pot strategy for the synthesis of functional peptides and proteins. Org. Chem. Front. 2017, 4, 2058–2062. [Google Scholar] [CrossRef]
- Guimaraes, C.P.; Witte, M.D.; Theile, C.S.; Bozkurt, G.; Kundrat, L.; Blom, A.E.M.; Ploegh, H.L. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 2013, 8, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, M.; Massa, S.; Martin, C.; Betti, C.; Ballet, S.; Devoogdt, N.; Xavier, C.; Muyldermans, S. Site-Specific radioactive labeling of nanobodies. Methods Mol. Biol. 2018, 1827, 505–540. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Dougan, M.; Rashidian, M.; Knoll, M.; Keliher, E.J.; Garrett, S.; Garforth, S.; Blomberg, O.S.; Espinosa, C.; Bhan, A.; et al. PD–L1 is an activation-independent marker of brown adipocytes. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Blomberg, O.S.; Rashidian, M.; Ali, L.; Garforth, S.; Fedorov, E.; Fedorov, A.A.; Bonanno, J.B.; Le Gall, C.; Crowley, S.; et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc. Natl. Acad. Sci. USA 2018, 115, 3912–3917. [Google Scholar] [CrossRef]
- McBride, W.J.; D’Souza, C.A.; Karacay, H.; Sharkey, R.M.; Goldenberg, D.M. New lyophilized kit for rapid radiofluorination of peptides. Bioconjug. Chem. 2012, 23, 538–547. [Google Scholar] [CrossRef]
- Cleeren, F.; Lecina, J.; Ahamed, M.; Raes, G.; Devoogdt, N.; Caveliers, V.; McQuade, P.; Rubins, D.J.; Li, W.; Verbruggen, A.; et al. Al18F-labeling of heat-sensitive biomolecules for positron emission tomography imaging. Theranostics 2017, 7, 2924–2939. [Google Scholar] [CrossRef]
- Cleeren, F.; Lecina, J.; Bridoux, J.; Devoogdt, N.; Tshibangu, T.; Xavier, C.; Bormans, G. Direct fluorine-18 labeling of heat-sensitive biomolecules for positron emission tomography imaging using the Al18F-RESCA method. Nat. Protoc. 2018, 13, 2330–2347. [Google Scholar] [CrossRef]
- Bridoux, J.; Neyt, S.; Debie, P.; Descamps, B.; Devoogdt, N.; Cleeren, F.; Bormans, G.; Broisat, A.; Caveliers, V.; Xavier, C.; et al. Improved detection of molecular markers of atherosclerotic plaques using sub-millimeter PET imaging. Molecules 2020, 25, 1838. [Google Scholar] [CrossRef]
- Zhou, Z.; Zalutsky, M.R.; Vaidyanathan, G. Labeling a TCO-functionalized single domain antibody fragment with 18F via inverse electron demand Diels Alder cycloaddition using a fluoronicotinyl moiety-bearing tetrazine derivative. Bioorg. Med. Chem. 2020, 28, 115634. [Google Scholar] [CrossRef] [PubMed]
- Bala, G.; Baudhuin, H.; Remory, I.; Gillis, K.; Debie, P.; Krasniqi, A.; Lahoutte, T.; Raes, G.; Devoogdt, N.; Cosyns, B.; et al. Evaluation of [99mTc] radiolabeled macrophage mannose receptor-specific nanobodies for targeting of atherosclerotic lesions in mice. Mol. Imaging Biol. 2018, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga–NOTA–anti–HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tu, Y.; Hu, X.; Bao, A.; Chen, H.; Ma, X.; Doyle, T.; Shi, H.; Cheng, Z. Pilot study of 64Cu(I) for PET imaging of melanoma. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, M.E. Guest edited collection: Radioisotopes and radiochemistry in health science. Sci. Rep. 2020, 10, 340. [Google Scholar] [CrossRef]
- Varani, M.; Auletta, S.; Signore, A.; Galli, F. State of the art of natural killer cell imaging: A systematic review. Cancers (Basel) 2019, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of (177) Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Lemaire, M.; D’Huyvetter, M.; Lahoutte, T.; Van Valckenborgh, E.; Menu, E.; De Bruyne, E.; Kronenberger, P.; Wernery, U.; Muyldermans, S.; Devoogdt, N.; et al. Imaging and radioimmunotherapy of multiple myeloma with anti-idiotypic Nanobodies. Leukemia 2014, 28, 444–447. [Google Scholar] [CrossRef]
- Sadkin, V.; Sκuridin, V.; Nesterov, E.; Stasyuk, E.; Rogov, A.; Varlamova, N.; Zelchan, R. 99mTc-labeled nanocolloid drugs: Development methods. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Xavier, C.; Devoogdt, N.; Hernot, S.; Vaneycken, I.; D’Huyvetter, M.; De Vos, J.; Massa, S.; Lahoutte, T.; Caveliers, V. Site-specific labeling of his-tagged Nanobodies with ⁹⁹mTc: A practical guide. Methods Mol. Biol. 2012, 911, 485–490. [Google Scholar] [CrossRef]
- Van Elssen, C.H.M.J.; Rashidian, M.; Vrbanac, V.; Wucherpfennig, K.W.; Habre, Z.E.; Sticht, J.; Freund, C.; Jacobsen, J.T.; Cragnolini, J.; Ingram, J.; et al. Noninvasive imaging of human immune responses in a human xenograft model of graft-versus-host disease. J. Nucl. Med. 2017, 58, 1003–1008. [Google Scholar] [CrossRef]
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [Google Scholar] [CrossRef] [PubMed]
- Senders, M.L.; Hernot, S.; Carlucci, G.; Van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody–facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc. Imaging 2019, 12, 2015–2026. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Li, Y.; López Armbruster, N.; Braeuer, M.; Nekolla, S.G.; Habenicht, A.; Sager, H.B.; Raes, G.; Weber, W.; et al. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using 68Ga-NOTA-anti-MMR nanobody: Non-invasive imaging of atherosclerotic plaques. EJNMMI Res. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Bala, G.; Crauwels, M.; Blykers, A.; Remory, I.; Marschall, A.L.J.; Dübel, S.; Dumas, L.; Broisat, A.; Martin, C.; Ballet, S.; et al. Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis–impact of radiochemistry on pharmacokinetics. Biol. Chem. 2019, 400, 323–332. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Yang, Y.; Sun, Y.; Wei, W.; Wang, C.; Wan, L.; Zhu, C.; Li, L.; Huang, G.; et al. ImmunoPET imaging of human CD8+ T cells with novel 68Ga-labeled nanobody companion diagnostic agents. J. Nanobiotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Hou, Y.N.; Liu, Q.; Zhang, D.; Zhao, H.; Zhang, Y.; An, S.; Li, L.; Hou, J.; et al. ImmunoPET imaging of multiple myeloma with [68Ga] Ga–NOTA–Nb1053. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, J.; Broos, K.; Lecocq, Q.; Debie, P.; Martin, C.; Ballet, S.; Raes, G.; Neyt, S.; Vanhove, C.; Breckpot, K.; et al. Anti-human PD-L1 nanobody for immuno-PET imaging: Validation of a conjugation strategy for clinical translation. Biomolecules 2020, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Sun, X.; Qiu, L.; Sun, Y.; Li, K.; Liu, Q.; Zhao, Q.; Qin, S.; Lin, J. PET imaging of tumor PD-l1 expression with a highly specific nonblocking single-domain antibody. J. Nucl. Med. 2020, 61, 117–122. [Google Scholar] [CrossRef]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Van der Heyden, J.; Lahoutte, T.; Tavernier, J.; Devoogdt, N. theranostic radiolabeled anti–CD20 sdAb for targeted radionuclide therapy of non-hodgkin lymphoma. Mol. Cancer Ther. 2017, 16, 2828–2839. [Google Scholar] [CrossRef]
- Demine, S.; Garcia Ribeiro, R.; Thevenet, J.; Marselli, L.; Marchetti, P.; Pattou, F.; Kerr-Conte, J.; Devoogdt, N.; Eizirik, D.L. A nanobody–based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice. Diabetologia 2020, 63, 825–836. [Google Scholar] [CrossRef]
- Chigoho, D.M.; Lecocq, Q.; Awad, R.M.; Breckpot, K.; Devoogdt, N.; Keyaerts, M.; Caveliers, V.; Xavier, C.; Bridoux, J. Site–specific Radiolabeling of a human PD-L1 nanobody via maleimide-cysteine chemistry. Pharmaceuticals (Basel) 2021, 14, 550. [Google Scholar] [CrossRef]
- Vosjan, M.J.W.D.; Perk, L.R.; Roovers, R.C.; Visser, G.W.M.; Stigter-van Walsum, M.; van Bergen En Henegouwen, P.M.P.; van Dongen, G.A.M.S. Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. 89Zr-Desferrioxamine p-Isothiocyanatobenzyl-Anti-EGFR Nanobody 7D12. In Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD), National Center for Biotechnology Information (US). 2004. Available online: http://www.ncbi.nlm.nih.gov/books/NBK97356/ (accessed on 18 May 2021).
- Vosjan, M.J.W.D.; Vercammen, J.; Kolkman, J.A.; Stigter–van Walsum, M.; Revets, H.; van Dongen, G.A.M.S. Nanobodies targeting the hepatocyte growth factor: Potential new drugs for molecular cancer therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Behnam Azad, B.; Zwaenepoel, O.; Zawadzki, M.; Lisok, A.; Pomper, M.G.; Walczak, P.; Gettemans, J.; et al. PET imaging of distinct brain uptake of a nanobody and similarly–sized PAMAM dendrimers after intra–arterial administration. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Warnders, F.J.; van Terwisscha Scheltinga, A.G.T.; Knuehl, C.; van Roy, M.; de Vries, E.F.J.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub–de Hooge, M.N. Human epidermal growth factor receptor 3–specific tumor uptake and biodistribution of 89Zr–MSB0010853 visualized by real-time and noninvasive PET Imaging. J. Nucl. Med. 2017, 58, 1210–1215. [Google Scholar] [CrossRef]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef]
- Rashidian, M.; LaFleur, M.W.; Verschoor, V.L.; Dongre, A.; Zhang, Y.; Nguyen, T.H.; Kolifrath, S.; Aref, A.R.; Lau, C.J.; Paweletz, C.P.; et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc. Natl. Acad. Sci. USA 2019, 116, 16971–16980. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.S.; Veldhoven–Zweistra, J.; Bolkestein, M.; Hoeben, S.; Koning, G.A.; Boerman, O.C.; De Jong, M.; Van Weerden, W.M. A Novel ¹¹¹in–labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med. 2015, 56, 1094–1099. [Google Scholar] [CrossRef]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; De Backer, M.E.; Rip, J.; Nabuurs, R.J.A.; Gaillard, P.J.; Van Buchem, M.A.; Van der Maarel, S.M.; Van der Weerd, L. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Balhuizen, A.; Massa, S.; Mathijs, I.; Turatsinze, J.V.; De Vos, J.; Demine, S.; Xavier, C.; Villate, O.; Millard, I.; Egrise, D.; et al. A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef]
- Krasniqi, A.; Bialkowska, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Devoogdt, N.; D’Huyvetter, M. Pharmacokinetics of radiolabeled dimeric sdAbs constructs targeting human CD20. New Biotechnol. 2018, 45, 69–79. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Aerts, A.; Xavier, C.; Vaneycken, I.; Devoogdt, N.; Gijs, M.; Impens, N.; Baatout, S.; Ponsard, B.; Muyldermans, S.; et al. Development of 177Lu-nanobodies for radioimmunotherapy of HER2-positive breast cancer: Evaluation of different bifunctional chelators. Contrast Media Mol. Imaging 2012, 7, 254–264. [Google Scholar] [CrossRef]
- Romão, E.; Krasniqi, A.; Maes, L.; Vandenbrande, C.; Sterckx, Y.G.J.; Stijlemans, B.; Vincke, C.; Devoogdt, N.; Muyldermans, S. Identification of nanobodies against the acute myeloid leukemia marker CD33. Int. J. Mol. Sci. 2020, 21, 310. [Google Scholar] [CrossRef]
- Gainkam, L.O.T.; Huang, L.; Caveliers, V.; Keyaerts, M.; Hernot, S.; Vaneycken, I.; Vanhove, C.; Revets, H.; De Baetselier, P.; Lahoutte, T. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro–CT. J. Nucl. Med. 2008, 49, 788–795. [Google Scholar] [CrossRef]
- Movahedi, K.; Schoonooghe, S.; Laoui, D.; Houbracken, I.; Waelput, W.; Breckpot, K.; Bouwens, L.; Lahoutte, T.; de Baetselier, P.; Raes, G.; et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012, 72, 4165–4177. [Google Scholar] [CrossRef] [PubMed]
- Put, S.; Schoonooghe, S.; Devoogdt, N.; Schurgers, E.; Avau, A.; Mitera, T.; D’Huyvetter, M.; de Baetselier, P.; Raes, G.; Lahoutte, T.; et al. SPECT imaging of joint inflammation with Nanobodies targeting the macrophage mannose receptor in a mouse model for rheumatoid arthritis. J. Nucl. Med. 2013, 54, 807–814. [Google Scholar] [CrossRef]
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ. Res. 2012, 110, 927–937. [Google Scholar] [CrossRef]
- Broisat, A.; Toczek, J.; Dumas, L.S.; Ahmadi, M.; Bacot, S.; Perret, P.; Slimani, L.; Barone–Rochette, G.; Soubies, A.; Devoogdt, N.; et al. 99mTc-cAbVCAM1-5 imaging is a sensitive and reproducible tool for the detection of inflamed atherosclerotic lesions in mice. J. Nucl. Med. 2014, 55, 1678–1684. [Google Scholar] [CrossRef][Green Version]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017, 8, 41932–41946. [Google Scholar] [CrossRef]
- Xing, Y.; Chand, G.; Liu, C.; Cook, G.J.R.; O’Doherty, J.; Zhao, L.; Wong, N.C.L.; Meszaros, L.K.; Ting, H.H.; Zhao, J. Early phase i study of a 99mTc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J. Nucl. Med. 2019, 60, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Put, S.; Bouwens, L.; Lahoutte, T.; Matthys, P.; Muyldermans, S.; de Baetselier, P.; Devoogdt, N.; Raes, G.; Schoonooghe, S. Molecular imaging with macrophage CRIg-targeting nanobodies for early and preclinical diagnosis in a mouse model of rheumatoid arthritis. J. Nucl. Med. 2014, 55, 824–829. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.; Mathijs, I.; Xavier, C.; Massa, S.; Wernery, U.; Bouwens, L.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Specific targeting of atherosclerotic plaques in ApoE(-/-) mice using a new Camelid sdAb binding the vulnerable plaque marker LOX-1. Mol. Imaging Biol. 2014, 16, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Verhelle, A.; Van Overbeke, W.; Peleman, C.; Smet, R.D.; Zwaenepoel, O.; Lahoutte, T.; Van Dorpe, J.; Devoogdt, N.; Gettemans, J. Non–invasive imaging of amyloid deposits in a mouse model of agel using 99mTc–modified nanobodies and SPECT/CT. Mol. Imaging Biol. 2016, 18, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Verhelle, A.; Nair, N.; Everaert, I.; Van Overbeke, W.; Supply, L.; Zwaenepoel, O.; Peleman, C.; Van Dorpe, J.; Lahoutte, T.; Devoogdt, N.; et al. AAV9 delivered bispecific nanobody attenuates amyloid burden in the gelsolin amyloidosis mouse model. Hum. Mol. Genet. 2017, 26, 1353–1364. [Google Scholar] [CrossRef][Green Version]
- Evazalipour, M.; D’Huyvetter, M.; Tehrani, B.S.; Abolhassani, M.; Omidfar, K.; Abdoli, S.; Arezumand, R.; Morovvati, H.; Lahoutte, T.; Muyldermans, S.; et al. Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer. Contrast Media Mol. Imaging 2014, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs, R.J.A.; Rutgers, K.S.; Welling, M.M.; Metaxas, A.; De Backer, M.E.; Rotman, M.; Bacskai, B.J.; Van Buchem, M.A.; Van der Maarel, S.M.; Van der Weerd, L. In vivo detection of amyloid-β deposits using heavy chain antibody fragments in a transgenic mouse model for Alzheimer’s disease. PLoS ONE 2012, 7, e38284. [Google Scholar] [CrossRef]
- Vaneycken, I.; Govaert, J.; Vincke, C.; Caveliers, V.; Lahoutte, T.; De Baetselier, P.; Raes, G.; Bossuyt, A.; Muyldermans, S.; Devoogdt, N. In vitro analysis and in vivo tumor targeting of a humanized, grafted nanobody in mice using pinhole SPECT/micro–CT. J. Nucl. Med. 2010, 51, 1099–1106. [Google Scholar] [CrossRef]
- Montemagno, C.; Bacot, S.; Ahmadi, M.; Kerfelec, B.; Baty, D.; Debiossat, M.; Soubies, A.; Perret, P.; Riou, L.; Fagret, D.; et al. Preclinical evaluation of mesothelin-specific ligands for SPECT imaging of triple-negative breast cancer. J. Nucl. Med. 2018, 59, 1056–1062. [Google Scholar] [CrossRef]
- De Groeve, K.; Deschacht, N.; de Koninck, C.; Caveliers, V.; Lahoutte, T.; Devoogdt, N.; Muyldermans, S.; de Baetselier, P.; Raes, G. Nanobodies as tools for in vivo imaging of specific immune cell types. J. Nucl. Med. 2010, 51, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Sparkes, A.; De Baetselier, P.; Schoonooghe, S.; Stijlemans, B.; Muyldermans, S.; Flamand, V.; Van Ginderachter, J.A.; Devoogdt, N.; Raes, G.; et al. molecular imaging with Kupffer cell-targeting nanobodies for diagnosis and prognosis in mouse models of liver pathogenesis. Mol. Imaging Biol. 2017, 19, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Awad, R.M.; De Vlaeminck, Y.; De Mey, W.; Ertveldt, T.; Goyvaerts, C.; Raes, G.; Thielemans, K.; Keyaerts, M.; Devoogdt, N.; et al. Nanobody nuclear imaging allows noninvasive quantification of LAG-3 expression by tumor-infiltrating leukocytes and predicts response of immune checkpoint blockade. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Zeven, K.; De Vlaeminck, Y.; Martens, S.; Massa, S.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; Breckpot, K.; Devoogdt, N. Noninvasive imaging of the immune checkpoint LAG-3 using nanobodies, from development to pre-clinical use. Biomolecules 2019, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Randolph, T.W. The two faces of His-tag: Immune response versus ease of protein purification. Biotechnol. J. 2012, 7, 18–19. [Google Scholar] [CrossRef]
- Khan, F.; Legler, P.M.; Mease, R.M.; Duncan, E.H.; Bergmann-Leitner, E.S.; Angov, E. Histidine affinity tags affect MSP1(42) structural stability and immunodominance in mice. Biotechnol. J. 2012, 7, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Waibel, R.; Alberto, R.; Willuda, J.; Finnern, R.; Schibli, R.; Stichelberger, A.; Egli, A.; Abram, U.; Mach, J.P.; Plückthun, A.; et al. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat. Biotechnol. 1999, 17, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wu, Y.; Shi, J.; Zhang, X.; Liu, T.; Hu, B.; Jia, B.; Wan, Y.; Liu, Z.; Wang, F. Nuclear imaging-guided PD-L1 blockade therapy increases effectiveness of cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001156. [Google Scholar] [CrossRef]
- Ye, Y.; Toczek, J.; Gona, K.; Kim, H.Y.; Han, J.; Razavian, M.; Golestani, R.; Zhang, J.; Wu, T.L.; Ghosh, M.; et al. Novel arginine-containing macrocyclic MMP inhibitors: Synthesis, 99mTc-labeling, and evaluation. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Duclos, V.; Iep, A.; Gomez, L.; Goldfarb, L.; Besson, F.L. PET molecular imaging: A holistic review of current practice and emerging perspectives for diagnosis, therapeutic evaluation and prognosis in clinical oncology. Int. J. Mol. Sci. 2021, 22, 4159. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Johnson, G.B.; Pomper, M.G.; Gorin, M.A.; Behr, S.C. Recent updates and developments in PET imaging of prostate cancer. Abdom. Radiol. N. Y. 2020, 45, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Goel, S.; Lan, X.; Cai, W. ImmunoPET of CD38 with a radiolabeled nanobody: Promising for clinical translation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2683–2686. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, D.; Pyka, T.; Scheidhauer, K.; Lütje, S.; Essler, M.; Bundschuh, R.A. Textural features in FDG-PET/CT can predict outcome in melanoma patients to treatment with Vemurafenib and Ipililumab. Nuklearmedizin 2020, 59, 228–234. [Google Scholar] [CrossRef]
- Hartmann, L.; Bundschuh, L.; Zsótér, N.; Essler, M.; Bundschuh, R.A. Tumorheterogenität zur differenzierung zwischen lebertumoren und gesundem lebergewebe in 18F-FDG-PET/CT. Nuklearmedizin 2021, 60, 25–32. [Google Scholar] [CrossRef]
- Eary, J.F.; Brenner, W. Brauchen wir quantitative Bildgebung? Nuklearmedizin 2020, 59, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Pektor, S.; Lawaczeck, L.; Tenzer, S.; Bausbacher, N.; Hoffmann, M.A.; Schreckenberger, M.; Miederer, M. Charakterisierung der aktivierungsinduzierten 18F-FDG-Aufnahme in dendritische Zellen. Nuklearmedizin 2021, 60, 90–98. [Google Scholar] [CrossRef]
- Nahrendorf, M.; McCarthy, J.R.; Libby, P. Over a hump for imaging atherosclerosis: Nanobodies visualize vascular cell adhesion molecule-1 in inflamed plaque. Circ. Res. 2012, 110, 902–903. [Google Scholar] [CrossRef]



| Radiolabeling Strategies | Positive Aspects | Negative Aspects | |
|---|---|---|---|
| Radiohalogens | Iodogen | sufficiently mild method to directly radioiodinate sensitive proteins at low temperatures | solely unspecific labeling possible limited to radioiodines |
| Prosthetic groups | labeling with different radiohalogens feasible huge variety in their design realizable | their preparation and purification are usually sophisticated resulting in a long labeling procedure and low chemical yields | |
| Chelation | simplified labeling process leading to good radiochemical yields as well as high molar activities | only applicable to fluorine-18 radioactivity is not introduced in the final step | |
| Radiometals | Synthetic chelators | radiolabel is inserted at the very end different radiometals are introducible to the same chelator | consistently metal-free conditions essential can affect the physicochemical properties of the nanobody |
| Proteinogenic chelator | chelator is often engineered for purification reasons enables site-specific labeling with technetium-99m | induction of immune responses 99mTc-tricarbonyl # required, which has to be prepared | |
| Heteroleptic complex | no conversion of technetium-99m necessary | radiocomplex needs to be formed prior to attachment | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küppers, J.; Kürpig, S.; Bundschuh, R.A.; Essler, M.; Lütje, S. Radiolabeling Strategies of Nanobodies for Imaging Applications. Diagnostics 2021, 11, 1530. https://doi.org/10.3390/diagnostics11091530
Küppers J, Kürpig S, Bundschuh RA, Essler M, Lütje S. Radiolabeling Strategies of Nanobodies for Imaging Applications. Diagnostics. 2021; 11(9):1530. https://doi.org/10.3390/diagnostics11091530
Chicago/Turabian StyleKüppers, Jim, Stefan Kürpig, Ralph A. Bundschuh, Markus Essler, and Susanne Lütje. 2021. "Radiolabeling Strategies of Nanobodies for Imaging Applications" Diagnostics 11, no. 9: 1530. https://doi.org/10.3390/diagnostics11091530
APA StyleKüppers, J., Kürpig, S., Bundschuh, R. A., Essler, M., & Lütje, S. (2021). Radiolabeling Strategies of Nanobodies for Imaging Applications. Diagnostics, 11(9), 1530. https://doi.org/10.3390/diagnostics11091530







