Polyprev: Randomized, Multicenter, Controlled Trial Comparing Fecal Immunochemical Test with Endoscopic Surveillance after Advanced Adenoma Resection in Colorectal Cancer Screening Programs: A Study Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Hypothesis
2.2. Objectives
- -
- To compare the diagnostic yield for CRC and advanced adenomas detection between both groups at a 3 year interval.
- -
- To evaluate the effect of the two surveillance strategies on the following variables: mortality (global and associated with CRC), colonic lesion detection, participation in the surveillance strategy, adverse effects, use of health resources and cost-effectiveness.
- -
- To determine the values and preferences of subjects with advanced adenomas resected endoscopically regarding the type of surveillance and the risk of CRC.
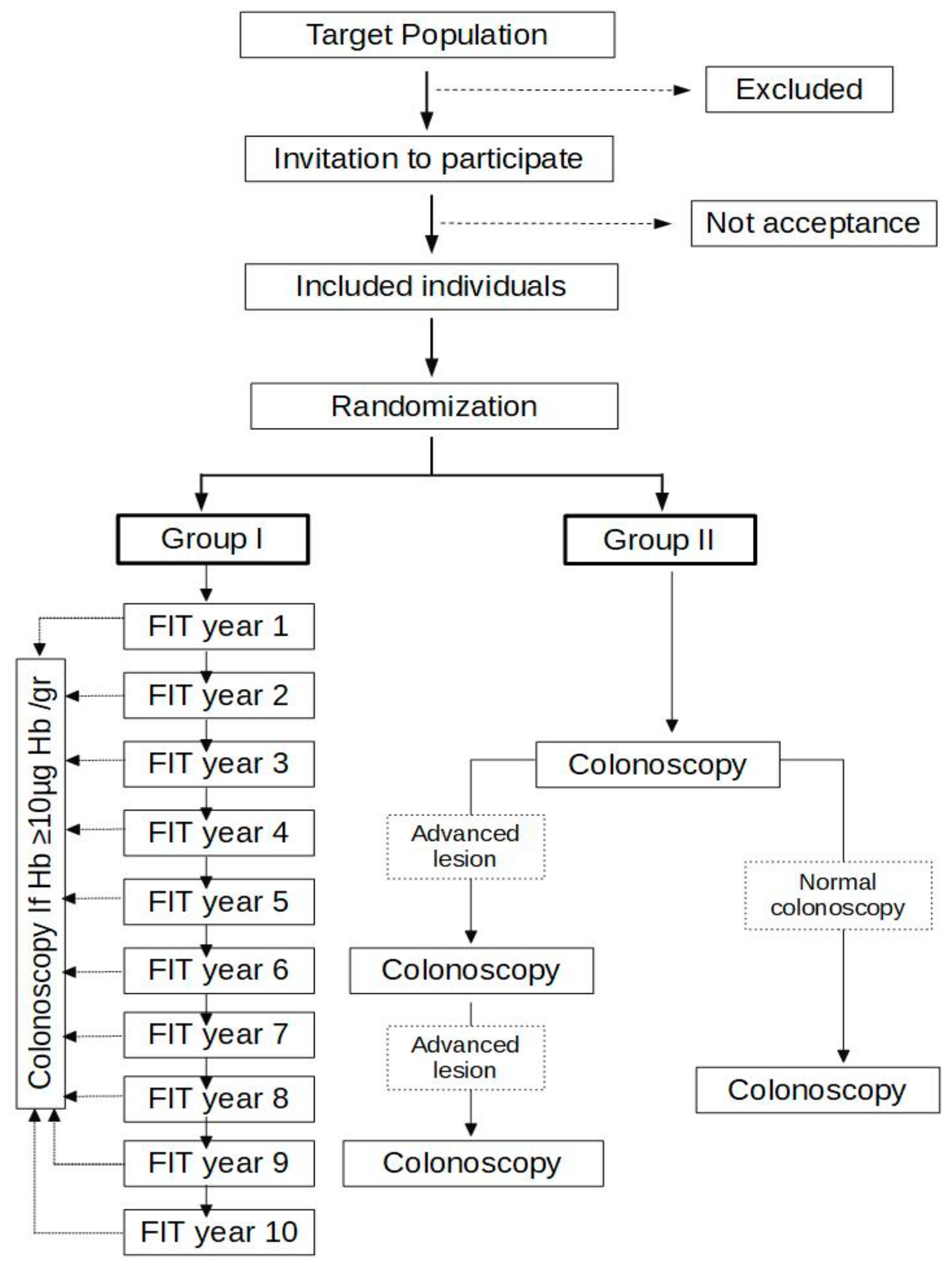
2.3. Study Design
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Study Development: Inclusion
2.7. Study Development: Group I
- -
- After one year if the colonoscopy was incomplete or a lesion requiring endoscopic surveillance was completely resected.
- -
- After five years if the colonoscopy evaluated the entire mucosa, was normal or had lesions that do not require endoscopic surveillance (1–2 non-advanced adenomas).
2.8. Study Development: Group II
2.9. Sample Size Calculation
2.10. Endpoints
- -
- Invasive CRC: this is the main endpoint of the study and is defined as a colonic adenocarcinoma that invades the submucosa. We will not consider adenocarcinomas in situ and intramucosal carcinomas as invasive CRC.
- -
- Interval CRC: this is defined as the CRC detected between two organized surveillance (FIT or colonoscopy).
- -
- Mortality: we will collect the deaths and their cause (associated with CRC, associated with adverse effects or unrelated).
- -
- Colonic lesions: we will classify colonic lesions as advanced or not advanced. We will define advanced lesions as advanced adenomas (some of at least 10 mm, hairy histology or high grade dysplasia) or advanced serrated lesions (some of at least 10 mm or with dysplasia). The rest will be classified as not advanced.
- -
- Participation in the surveillance strategy: we will define three categories to evaluate the participation: non-participation, irregular and regular participation.
- -
- Adverse effects: adverse effects associated with surveillance are defined as complications that require hospitalization. Those related to the surgical treatment of benign colonic lesions will be also included as adverse effects.
2.11. Exit from the Study
2.12. Data Management
2.13. Study Monitoring
2.14. Ethical and Legal Aspects
2.15. Statistical Analysis
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estimaciones de la Incidencia y la Supervivencia del Cáncer en España y su Situación en Europa. Available online: https://redecan.org/redecan.org/es/page1b34.html?id=196&title=estimaciones-de-la-incidencia-y-la-supervivencia-del-cancer-en-espana-y-su-situacion-en-europa (accessed on 15 October 2014).
- Holleczek, B.; Rossi, S.; Domenic, A.; Innos, K.; Minicozzi, P.; Francisci, S.; Hackl, M.; Eisemann, N.; Brenner, H.; EUROCARE-5 Working Group. On-going improvement and persistent differences in the survival for patients with lon and rectum cancer across Europe 1999-2007- Results from the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.S.; Valori, R.; Kuipers, E.J.; Hoff, G.; Senore, C.; Segnan, N.; Jover, R.; Schmielgel, W.; Lambert, R.; Pox, C. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition-Colonoscopic surveillance following adenoma removal. Endoscopy 2012, 44, SE151–SE163. [Google Scholar] [CrossRef]
- Cubiella, J.; Marzo-Castillejo, M.; Mascort-Roca, J.J.; Amador-Romero, F.J.; Bellas-Ceiro, B.; Clogent-Vilaplana, J.; Crballal, S.; Ferrándiz-Santos, J.; Gimeno-García, A.Z.; Jover, R.; et al. Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 Update. Gastroenterol. Hepatol. 2018, 41, 585–596. [Google Scholar] [CrossRef]
- Levin, T.R.; Corley, D.A.; Jensen, C.D.; Schottinger, J.E.; Quinn, V.P.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Udaltsova, N.; Ghai, N.R.; et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018, 155, 1383–1391.e5. [Google Scholar] [CrossRef] [PubMed]
- Situación Actual de Los Programas de Cribado de Cáncer Colorrectal en España. Available online: http://cribadocancer.org/images/archivos/LolaSalas_8.pdf (accessed on 21 December 2017).
- Martínez, M.E.; Baron, J.A.; Lieberman, D.A.; Schatzkin, A.; Lanza, E.; Winawer, S.J.; Zauber, A.G.; Jiang, R.; Ahnen, D.J.; Bond, J.H.; et al. A Pooled Analysis of Advanced Colorectal Neoplasia Diagnoses After Colonoscopic Polypectomy. Gastroenterology 2009, 136, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Quintero, E.; Dumonceau, J.-M.; Regula, J.; Brandao, C.; Chaussade, S.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; Gimeno-García, A.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013, 45, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Quintero, E.; Dumonceau, J.-M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar]
- Mangas-Sanjuan, C.; Jover, R.; Cubiella, J.; Marzo-Castillejo, M.; Balaguer, F.; Bessa, X.; Bujanda, L.; Bustamante, M.; Castells, A.; Diaz-Tasende, J.; et al. Endoscopic surveillance after colonic polyps and colorrectal cancer resection. 2018 update. Gastroenterol. Hepatol. 2019, 42, 188–201. [Google Scholar] [CrossRef]
- Rutter, M.D.; East, J.; Rees, C.J.; Cripps, N.; Docherty, J.; Dolwani, S.; Kaye, P.V.; Monahan, K.J.; Novelli, M.R.; Plumb, A.; et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut 2020, 69, 201–223. [Google Scholar] [CrossRef]
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burker, C.A.; Dominitz, J.A.; Kalenbach, T.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc. 2020, 91, 463–485.e5. [Google Scholar] [CrossRef]
- Greuter, M.J.E.; Klerk, C.M.; Meijer, G.A.; Dekker, E.; Coupé, V.M.H. Screening for Colorectal Cancer With Fecal Immunochemical Testing with and without Postpolypectomy Surveillance Colonoscopy: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2017, 167, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.; Cross, A.J.; Kralj-Hans, I.; MacRae, E.; Piggott, C.; Pearson, S.; Wooldrage, K.; Brown, J.; Lucas, F.; Prendergast, A. Faecal immunochemical tests versus colonoscopy for post-polypectomy surveillance: An accuracy, acceptability and economic study. Health Technol. Assess. 2019, 23. [Google Scholar] [CrossRef] [PubMed]
- Bonello, B.; Ghanouni, A.; Bowyer, H.L.; MacRae, E.; Atkin, W.; Halloran, S.P.; Wardle, J.; von Wagner, C. Using a hypothetical scenario to assess public preferences for colorectal surveillance following screening-detected, intermediate-risk adenomas: Annual home-based stool test vs. triennial colonoscopy. BMC Gastroenterol. 2016, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Jensen, C.D.; Levin, T.R.; Doubeni, C.A.; Zauber, A.G.; Chubak, K.; Kamineni, A.S.; Sachottinger, J.E.; Ghai, N.R.; Udaltsova, N.; et al. Long-term Risk of Colorectal Cancer and Related Death after Adenoma Removal in a Large, Community-based Population. Gastroenterology 2019, 158, 884–894. [Google Scholar] [CrossRef]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C.; Agoritsas, T.; Lytvyn, L.; Anderson, J.C.; Auer, R.; Murphy, S.B.; Almadi, M.A.; Corley, D.A.; et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, D.; Castilla-Rodríguez, I.; Salgado-Fernández, M.; Gougil, M.; Ponce, M.; Ocaña, T.; Bujanda, L.; Sopena, F.; González-López, N.; Castaño, O.; et al. Annual Fecal Immunochemical Testing is as Effective as Colonoscopy Every 5 Years for Familial Colorectal Cancer Screening. Gastroenterology 2017, 152, S542. [Google Scholar] [CrossRef]
- Rutter, M.D.; Bretthauer, M.; Hassan, C.; Jover, R.; WEO Surveillance Working Group. Principles for Evaluation of Surveillance after Removal of Colorectal Polyps: Recommendations from the World Endoscopy Organization. Gastroenterology 2020, 158, 529–1533. [Google Scholar] [CrossRef]
- Jover, R.; Bretthauer, M.; Dekker, E.; Holme, O.; Kaminski, M.F.; Loberg, M.; Zauber, A.G.; Herán, M.A.; Lansdorp-Vogelaar, I.; Sunde, A.; et al. Rationale and design of the European Polyp Surveillance (EpoS) trials. Endoscopy 2016, 48, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Cubiella, J.; Castro, I.; Hernandez, V.; González-Mao, C.; Rivera, C.; Iglesias, F.; Cid, L.; Soto, S.; De-Castro, L.; Vega, P.; et al. Characteristics of adenomas detected by fecal immunochemical test in colorectal cancer screening. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1884–1892. [Google Scholar] [CrossRef][Green Version]
- Quintero, E.; Castells, A.; Bujanda, L.; Cubiella, J.; Salas, D.; Lanas, A.; Andreu, M.; Carballo, F.; Morillas, J.D.; Herández, C.; et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N. Engl. J. Med. 2012, 366, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Crotta, S.; Segnan, N.; Paganin, S.; Dagnes, B.; Rosset, R.; Senore, C. High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin. Gastroenterol. Hepatol. 2012, 10, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Cottet, V.; Jooste, V.; Fournel, I.; Bouvier, A.M.; Faivre, J.; Bonithon-Kopp, C. Long-term risk of colorectal cancer after adenoma removal: A population-based cohort study. Gut 2012, 61, 1180–1186. [Google Scholar] [CrossRef]
- Wieszcy, P.; Waldmann, E.; Løberg, M.; Regula, J.; Rupinski, M.; Bugajski, M.; Gray, K.; Kalager, M.; Ferlitsch, M.; Kaminski, M.F.; et al. Colonoscopist Performance and Colorectal Cancer Risk after Adenoma Removal to Stratify Surveillance: Two Nationwide Observational Studies. Gastroenterology 2021, 160, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Cubiella, J.; González, A.; Almazán, R.; Rodríguez-Camacho, E.; Zubizarreta, R.; Lorenzo, I.P.-R. Overtreatment in Nonmalignant Lesions Detected in a Colorectal Cancer Screening Program: A Cross-Sectional Analysis. BMC Cancer 2020, 21, 869. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regueiro, C.; Almazán, R.; Portillo, I.; Besó, M.; Tourne-Garcia, C.; Rodríguez-Camacho, E.; Ono, A.; Gómez-Amorín, Á.; Cubiella, J. Polyprev: Randomized, Multicenter, Controlled Trial Comparing Fecal Immunochemical Test with Endoscopic Surveillance after Advanced Adenoma Resection in Colorectal Cancer Screening Programs: A Study Protocol. Diagnostics 2021, 11, 1520. https://doi.org/10.3390/diagnostics11091520
Regueiro C, Almazán R, Portillo I, Besó M, Tourne-Garcia C, Rodríguez-Camacho E, Ono A, Gómez-Amorín Á, Cubiella J. Polyprev: Randomized, Multicenter, Controlled Trial Comparing Fecal Immunochemical Test with Endoscopic Surveillance after Advanced Adenoma Resection in Colorectal Cancer Screening Programs: A Study Protocol. Diagnostics. 2021; 11(9):1520. https://doi.org/10.3390/diagnostics11091520
Chicago/Turabian StyleRegueiro, Cristina, Raquel Almazán, Isabel Portillo, María Besó, Carlos Tourne-Garcia, Elena Rodríguez-Camacho, Akiko Ono, Ángel Gómez-Amorín, and Joaquín Cubiella. 2021. "Polyprev: Randomized, Multicenter, Controlled Trial Comparing Fecal Immunochemical Test with Endoscopic Surveillance after Advanced Adenoma Resection in Colorectal Cancer Screening Programs: A Study Protocol" Diagnostics 11, no. 9: 1520. https://doi.org/10.3390/diagnostics11091520
APA StyleRegueiro, C., Almazán, R., Portillo, I., Besó, M., Tourne-Garcia, C., Rodríguez-Camacho, E., Ono, A., Gómez-Amorín, Á., & Cubiella, J. (2021). Polyprev: Randomized, Multicenter, Controlled Trial Comparing Fecal Immunochemical Test with Endoscopic Surveillance after Advanced Adenoma Resection in Colorectal Cancer Screening Programs: A Study Protocol. Diagnostics, 11(9), 1520. https://doi.org/10.3390/diagnostics11091520






