The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging
2.3. Image Analyses
2.4. Outcome
2.5. Statistics
3. Results
Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beasley, N.J.P.; Lee, J.; Eski, S.; Walfish, P.; Witterick, I.; Freeman, J.L. Impact of Nodal Metastases on Prognosis in Patients with Well-Differentiated Thyroid Cancer. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 825–828. [Google Scholar] [CrossRef]
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising Thyroid Cancer Incidence in the United States by Demographic and Tumor Characteristics, 1980–2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M. Radioiodine Scintigraphy with SPECT/CT: An Important Diagnostic Tool for Thyroid Cancer Staging and Risk Stratification. J. Nucl. Med. 2012, 53, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Sellers, M.; Beenken, S.; Blankenship, A.; Soong, S.-J.; Turbat-Herrera, E.; Urist, M.; Maddox, W. Prognostic significance of cervical lymph node metastases in differentiated thyroid cancer. Am. J. Surg. 1992, 164, 578–581. [Google Scholar] [CrossRef]
- Hughes, C.J.; Shaha, A.R.; Shah, J.P.; Loree, T.R. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: A matched-pair analysis. Head Neck 1996, 18, 127–132. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Robyn, J. Postsurgical management of differentiated thyroid carcinoma. Otolaryngol. Clin. N. Am. 1996, 29, 637–662. [Google Scholar]
- Mercante, G.; Frasoldati, A.; Pedroni, C.; Formisano, D.; Renna, L.; Piana, S.; Gardini, G.; Valcavi, R.; Barbieri, V. Prognostic Factors Affecting Neck Lymph Node Recurrence and Distant Metastasis in Papillary Microcarcinoma of the Thyroid: Results of a Study in 445 Patients. Thyroid 2009, 19, 707–716. [Google Scholar] [CrossRef]
- Spanu, A.; Nuvoli, S.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Role of Diagnostic 131I SPECT/CT in Long-Term Follow-up of Patients with Papillary Thyroid Microcarcinoma. J. Nucl. Med. 2018, 59, 1510–1515. [Google Scholar] [CrossRef]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Neck lymph node metastasis detection in patients with differentiated thyroid carcinoma (DTC) in long-term follow-up: A 131I-SPECT/CT study. BMC Cancer 2020, 20, 239. [Google Scholar] [CrossRef]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef]
- Donohoe, K.J.; Aloff, J.; Avram, A.M.; Bennet, K.; Giovanella, L.; Greenspan, B.; Gulec, S.; Hassan, A.; Kloos, R.T.; Solórzano, C.C.; et al. Appropriate Use Criteria for Nuclear Medicine in the Evaluation and Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2020, 61, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Tomoda, C.; Uruno, T.; Takamura, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Kuma, K.; Miyauchi, A. Prognostic Significance of Extrathyroid Extension of Papillary Thyroid Carcinoma: Massive but Not Minimal Extension Affects the Relapse-free Survival. World J. Surg. 2006, 30, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Kobayashi, K.; Miya, A. Prognostic values of clinical lymph node metastasis and macroscopic extrathyroid extension in papillary thyroid carcinoma. Endocr. J. 2014, 61, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.E.; Kinsella, J.; Loree, T.R.; Shaha, A.R.; Shah, J.P. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am. J. Surg. 1995, 170, 467–470. [Google Scholar] [CrossRef]
- Ortiz, S.; Rodríguez, J.M.; Soria, T.; Pérez-Flores, D.; Piñero, A.; Moreno, J.; Parrilla, P. Extrathyroid Spread in Papillary Carcinoma of the Thyroid: Clinicopathological and Prognostic Study. Otolaryngol. Head Neck Surg. 2001, 124, 261–265. [Google Scholar] [CrossRef]
- Lombardi, C.P.; Bellantone, R.; De Crea, C.; Paladino, N.C.; Fadda, G.; Salvatori, M.; Raffaelli, M. Papillary Thyroid Microcarcinoma: Extrathyroidal Extension, Lymph Node Metastases, and Risk Factors for Recurrence in a High Prevalence of Goiter Area. World J. Surg. 2010, 34, 1214–1221. [Google Scholar] [CrossRef]
- Yin, D.-T.; Yu, K.; Lu, R.-Q.; Li, X.; Xu, J.; Lei, M. Prognostic impact of minimal extrathyroidal extension in papillary thyroid carcinoma. Medicine 2016, 95, e5794. [Google Scholar] [CrossRef]
- Youngwirth, L.M.; Adam, M.A.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Extrathyroidal Extension Is Associated with Compromised Survival in Patients with Thyroid Cancer. Thyroid 2017, 27, 626–631. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Chen, S.; Hu, D.; Wang, M.; Zhou, L.; Zhou, W.; Chen, D.; Feng, H.; Wei, W.; et al. Minimal extrathyroidal extension affects the prognosis of differentiated thyroid cancer: Is there a need for change in the AJCC classification system? PLoS ONE 2019, 14, e0218171. [Google Scholar] [CrossRef]
- Danilovic, D.L.; Castroneves, L.A.; Suemoto, C.K.; Elias, L.O.; Soares, I.C.; Camargo, R.Y.; Correa, F.D.A.; Hoff, A.O.; Marui, S. Is There a Difference Between Minimal and Gross Extension into the Strap Muscles for the Risk of Recurrence in Papillary Thyroid Carcinomas? Thyroid 2020, 30, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kim, E.-K.; Chung, W.Y.; Yoon, J.H.; Kwak, J.Y. Minimal Extrathyroidal Extension in Patients with Papillary Thyroid Microcarcinoma: Is It a Real Prognostic Factor? Ann. Surg. Oncol. 2011, 18, 1916–1923. [Google Scholar] [CrossRef]
- Shin, J.H.; Ha, T.K.; Park, H.K.; Ahn, M.S.; Kim, K.H.; Bae, K.B.; Kim, T.H.; Choi, C.S.; Bae, S.K.; Kim, S.H. Implication of minimal extrathyroidal extension as a prognostic factor in papillary thyroid carcinoma. Int. J. Surg. 2013, 11, 944–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nixon, I.; Ganly, I.; Patel, S.; Palmer, F.L.; Whitcher, M.M.; Tuttle, R.M.; Shaha, A.R.; Shah, J.P. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery 2011, 150, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurayshi, Z.; Shama, M.A.; Randolph, G.W.; Kandil, E. Minimal extrathyroidal extension does not affect survival of well-differentiated thyroid cancer. Endocr.-Relat. Cancer 2017, 24, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Amit, M.; Boonsripitayanon, M.; Busaidy, N.L.; Cabanillas, M.E.; Waguespack, S.G.; Gross, N.D.; Grubbs, E.G.; Williams, M.D.; Lai, S.; et al. Effect of Tumor Size and Minimal Extrathyroidal Extension in Patients with Differentiated Thyroid Cancer. Thyroid 2018, 28, 982–990. [Google Scholar] [CrossRef]
- Castagna, M.G.; Forleo, R.; Maino, F.; Fralassi, N.; Barbato, F.; Palmitesta, P.; Pilli, T.; Capezzone, M.; Brilli, L.; Ciuoli, C.; et al. Small papillary thyroid carcinoma with minimal extrathyroidal extension should be managed as ATA low-risk tumor. J. Endocrinol. Investig. 2018, 41, 1029–1035. [Google Scholar] [CrossRef]
- Ahmaddy, F.; Wenter, V.; Ilhan, H.; Wacker, D.; Unterrainer, M.; Knösel, T.; Bartenstein, P.; Spitzweg, C.; Lehner, S.; Todica, A. Effects of the Minimal Extrathyroidal Extension on Early Response Rates after (Adjuvant) Initial Radioactive Iodine Therapy in PTC Patients. Cancers 2020, 12, 3357. [Google Scholar] [CrossRef]
- Hay, I.; Hutchinson, M.E.; Gonzalez-Losada, T.; McIver, B.; Reinalda, M.E.; Grant, C.S.; Thompson, G.B.; Sebo, T.J.; Goellner, J.R. Papillary thyroid microcarcinoma: A study of 900 cases observed in a 60-year period. Surgery 2008, 144, 980–988. [Google Scholar] [CrossRef]
- Wang, T.S.; Goffredo, P.; Sosa, J.A.; Roman, S.A. Papillary Thyroid Microcarcinoma: An Over-Treated Malignancy? World J. Surg. 2014, 38, 2297–2303. [Google Scholar] [CrossRef]
- Lubin, E.; Mechlis-Frish, S.; Zatz, S.; Shimoni, A.; Segal, K.; Avraham, A.; Levy, R.; Feinmesser, R. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J. Nucl. Med. 1994, 35, 257–262. [Google Scholar]
- Franceschi, M.; Kusić, Z.; Franceschi, D.; Lukinac, L.; Roncević, S. Thyroglobulin determination, neck ultrasonography and iodine-131 whole-body scintigraphy in differentiated thyroid carcinoma. J. Nucl. Med. 1996, 37, 446–451. [Google Scholar]
- Filesi, M.; Signore, A.; Ventroni, G.; Melacrinis, F.F.; Ronga, G. Role of initial iodine-131 whole-body scan and serum thyroglobulin in differentiated thyroid carcinoma metastases. J. Nucl. Med. 1998, 39, 1542–1546. [Google Scholar] [PubMed]
- Spies, W.G.; Wojtowicz, C.H.; Spies, S.M.; Shah, A.Y.; Zimmer, A.M. Value of Post-Therapy Whole-Body I-131 Imaging in the Evaluation of Patients with Thyroid Carcinoma Having Undergone High-Dose I-131 Therapy. Clin. Nucl. Med. 1989, 14, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Sutter, C.W.; Masilungan, B.G.; Stadalnik, R.C. False-positive results of I-131 whole-body scans in patients with thyroid cancer. Semin. Nucl. Med. 1995, 25, 279–282. [Google Scholar] [CrossRef]
- Shapiro, B.; Rufini, V.; Jarwan, A.; Geatti, O.; Kearfott, K.J.; Fig, L.M.; Kirkwood, I.D.; Gross, M.D. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin. Nucl. Med. 2000, 30, 115–132. [Google Scholar] [CrossRef]
- Mitchell, G.; Pratt, B.E.; Vini, L.; McCready, V.R.; Harmer, C.L. False positive 131I whole body scans in thyroid cancer. Br. J. Radiol. 2000, 73, 627–635. [Google Scholar] [CrossRef]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I Scans With SPECT/CT in Postoperative Thyroid Cancer Patients: What Is the Impact on Staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar] [CrossRef]
- Avram, A.M.; Esfandiari, N.H.; Wong, K.K. Preablation 131-I Scans with SPECT/CT Contribute to Thyroid Cancer Risk Stratification and 131-I Therapy Planning. J. Clin. Endocrinol. Metab. 2015, 100, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Kohlfuerst, S.; Igerc, I.; Lobnig, M.; Gallowitsch, H.J.; Gomez-Segovia, I.; Matschnig, S.; Mayr, J.; Mikosch, P.; Beheshti, M.; Lind, P. Posttherapeutic 131I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 886–893. [Google Scholar] [CrossRef]
- Schmidt, D.; Szikszai, A.; Linke, R.; Bautz, W.; Kuwert, T. Impact of 131I SPECT/Spiral CT on Nodal Staging of Differentiated Thyroid Carcinoma at the First Radioablation. J. Nucl. Med. 2009, 50, 18–23. [Google Scholar] [CrossRef][Green Version]
- Mustafa, M.; Kuwert, T.; Weber, K.; Knesewitsch, P.; Negele, T.; Haug, A.; Linke, R.; Bartenstein, P.; Schmidt, D. Regional lymph node involvement in T1 papillary thyroid carcinoma: A bicentric prospective SPECT/CT study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.K.; Tuttle, R.M.; Fox, J.; Borkar, S.; Chou, J.F.; Gonen, M.; Strauss, H.W.; Larson, S.M.; Schöder, H. The Effect of Posttherapy 131I SPECT/CT on Risk Classification and Management of Patients with Differentiated Thyroid Cancer. J. Nucl. Med. 2010, 51, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Ciappuccini, R.; Heutte, N.; Trzepla, G.; Rame, J.-P.; Vaur, D.; Aide, N.; Bardet, S. Postablation 131I scintigraphy with neck and thorax SPECT–CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2011, 164, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Abe, K.; Baba, S.; Isoda, T.; Sawamoto, H.; Tanabe, Y.; Sasaki, M.; Honda, H. Incremental Diagnostic Value of SPECT/CT with131I Scintigraphy after Radioiodine Therapy in Patients with Well-differentiated Thyroid Carcinoma. Radiology 2012, 265, 902–909. [Google Scholar] [CrossRef]
- Tharp, K.; Israel, O.; Hausmann, J.; Bettman, L.; Martin, W.H.; Daitzchman, M.; Sandler, M.P.; Delbeke, D. Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1435–1442. [Google Scholar] [CrossRef]
- Spanu, A.; Solinas, M.E.; Chessa, F.; Sanna, D.; Nuvoli, S.; Madeddu, G. 131I SPECT/CT in the Follow-up of Differentiated Thyroid Carcinoma: Incremental Value Versus Planar Imaging. J. Nucl. Med. 2009, 50, 184–190. [Google Scholar] [CrossRef]
- Schmidt, D.; Linke, R.; Uder, M.; Kuwert, T. Five months’ follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by 131I-SPECT/CT at the first radioablation. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 699–705. [Google Scholar] [CrossRef]
- Menges, M.; Uder, M.; Kuwert, T.; Schmidt, D. 131I SPECT/CT in the Follow-Up of Patients with Differentiated Thyroid Carcinoma. Clin. Nucl. Med. 2012, 37, 555–560. [Google Scholar] [CrossRef]
- Xue, Y.-L.; Qiu, Z.-L.; Song, H.-J.; Luo, Q.-Y. Value of 131I SPECT/CT for the evaluation of differentiated thyroid cancer: A systematic review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2012, 40, 768–778. [Google Scholar] [CrossRef]
- Kuker, R.; Sztejnberg, M.; Gulec, S. I-124 Imaging and Dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26, 66–73. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Luttrell, B.; Reeve, T.; Wiseman, J.; Wilmshurst, E.; Stiel, J.; Donohoe, D.; Cooper, R.; Bridgman, M. Thyroglobulin may be undetectable in the serum of patients with metastatic disease secondary to differentiated thyroid carcinoma. Follow-up of differentiated thyroid carcinoma. Cancer 1984, 54, 1625–1628. [Google Scholar] [CrossRef]
- Miller, J.H.; Marcus, C.S. Metastatic Papillary Thyroid Carcinoma with Normal Serum Thyroglobulin Level. Clin. Nucl. Med. 1988, 13, 652–653. [Google Scholar] [CrossRef]
- Lee, S.-W. SPECT/CT in the Treatment of Differentiated Thyroid Cancer. Nucl. Med. Mol. Imaging 2017, 51, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Szujo, S.; Sira, L.; Bajnok, L.; Bodis, B.; Gyory, F.; Nemes, O.; Rucz, K.; Kenyeres, P.; Valkusz, Z.; Sepp, K.; et al. The impact of post-radioiodine therapy SPECT/CT on early risk stratification in differentiated thyroid cancer; a bi-institutional study. Oncotarget 2017, 8, 79825–79834. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L.J.; Starritt, H.C.; Hiscock, S.C.; Evans, M.J. Effective doses to patients from CT acquisitions on the GE Infinia Hawkeye: A comparison of calculation methods. Nucl. Med. Commun. 2008, 29, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, C.; Kim, S.W.; Park, T.; Chun, B.K.; Hong, J.C.; Lee, K.D. Correlation of minimal extrathyroidal extension with pathologic features of lymph node metastasis in patients with papillary thyroid carcinoma. J. Surg. Oncol. 2015, 112, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Garofalo, M.R.; Giuffrida, D.; Runello, F.; Filetti, S.; Fiumara, A.; Ippolito, O.; Vigneri, R. Increased Aggressiveness of Thyroid Cancer in Patients with Graves’ Disease. J. Clin. Endocrinol. Metab. 1990, 70, 830–835. [Google Scholar] [CrossRef]
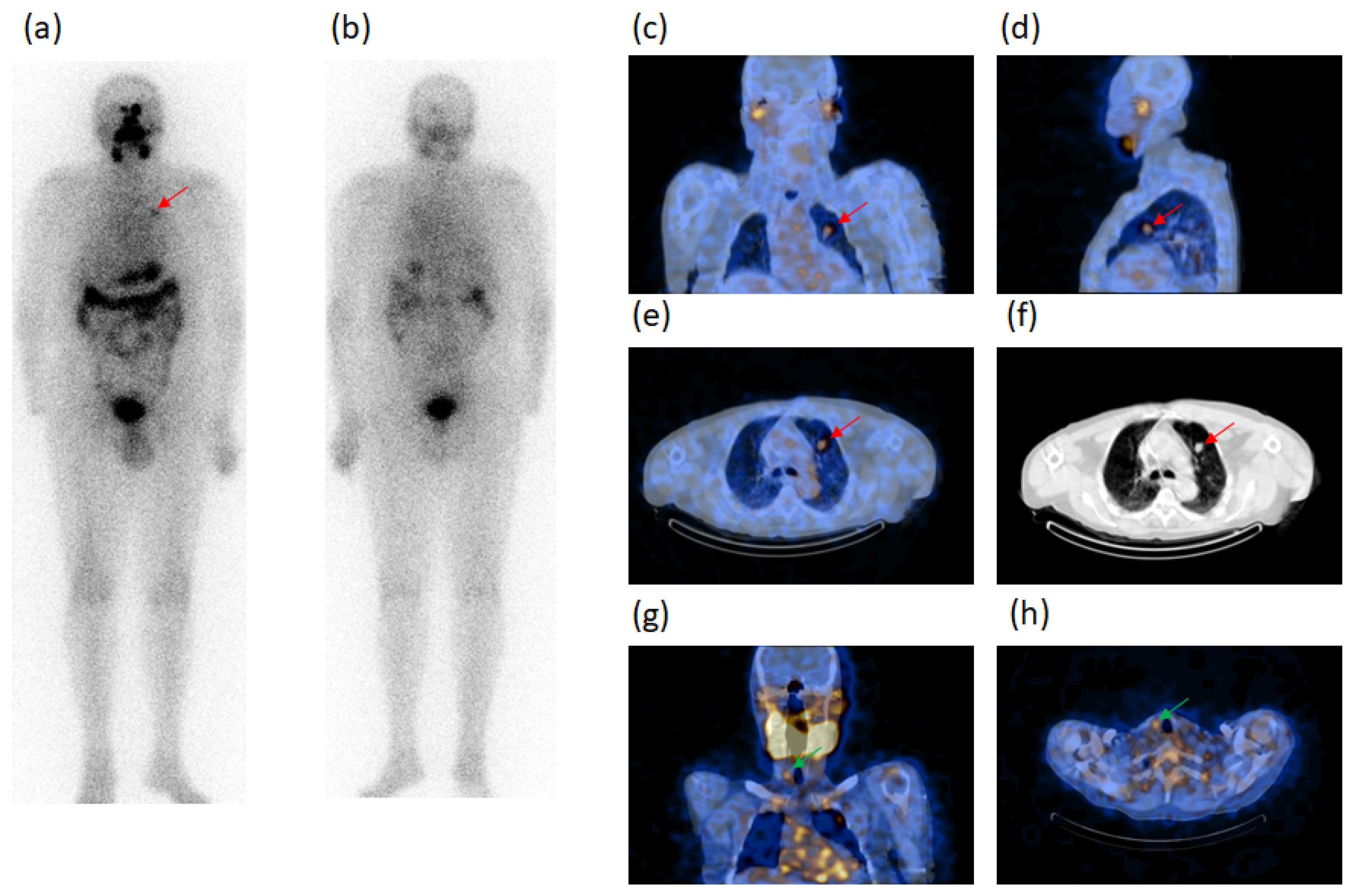



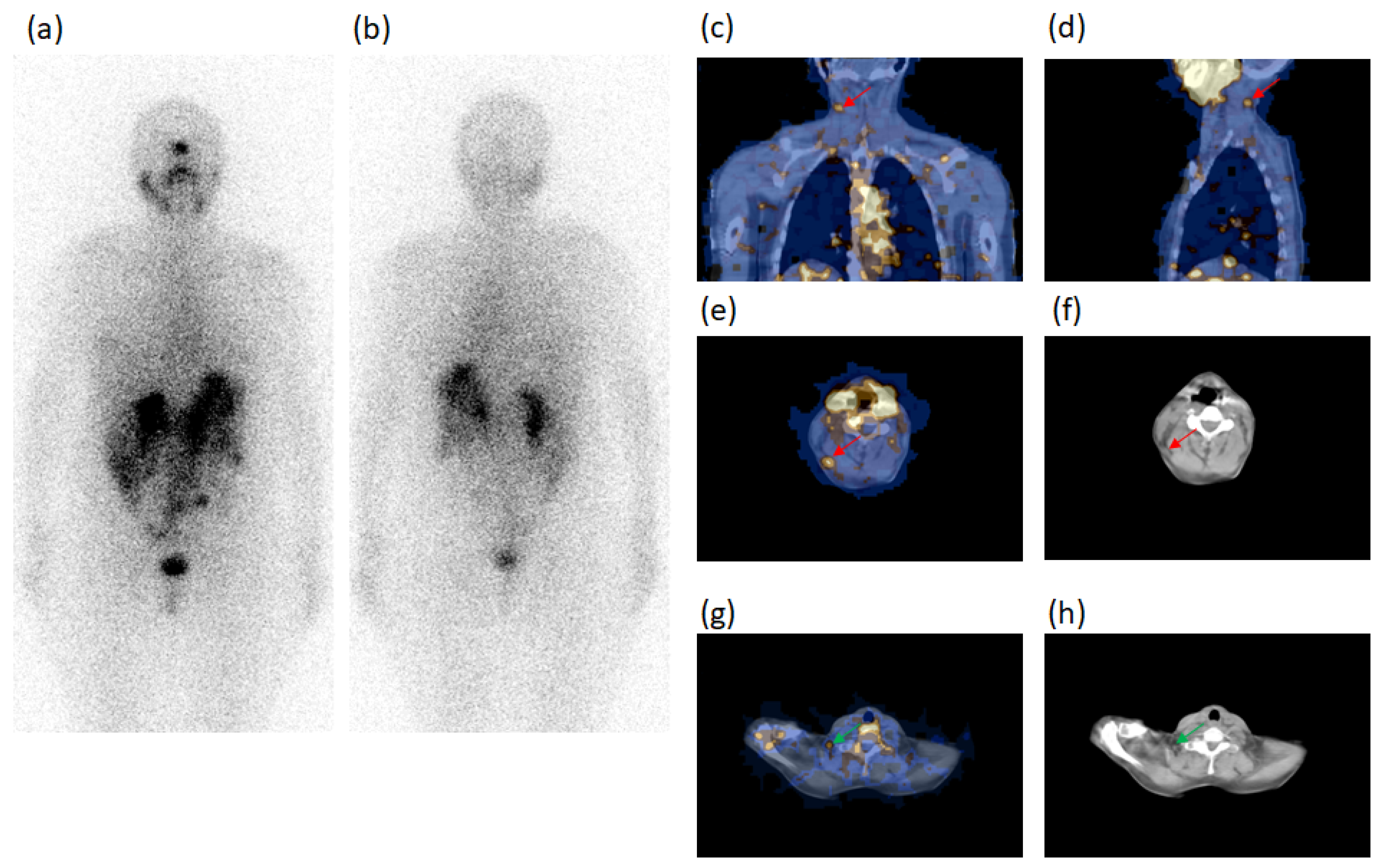
| Characteristics | Patients (n) | Characteristics | Patients (n) |
|---|---|---|---|
| Sex | Structural characteristics | ||
| Male | 29 | Unifocal carcinoma | 62 |
| Female | 77 | Multifocal carcinoma | 44 |
| Age | Minimal tumor extra-thyroid extension | 21 | |
| <45 y | 33 | Extended tumor extra-thyroid extension | 1 |
| ≥45 y | 73 | Lymph node metastasis | 14 |
| Histology | Distant metastasis | 2 | |
| Papillary carcinoma | 98 | Chronic thyroiditis | 20 |
| Follicular carcinoma | 3 | Graves/Basedow disease | 5 |
| Hürtle cell carcinoma | 5 | Risk stratification | |
| Size | High risk | 29 | |
| ≤10 mm | 42 | Low risk | 56 |
| >10 mm | 64 | Very low risk | 21 |
| Foci Concordantly Classified by WBS and SPECT/CT | Foci Unclear at WBS Characterized by SPECT/CT | Foci Occult (oc) at WBS Characterized by SPECT/CT | Foci Wrongly (W) Classified by WBS Correctly Characterized by SPECT/CT |
|---|---|---|---|
| n. 186 | n. 42 | n. 29 | n. 3 |
| 172 Residues 8 Lung metastases 5 Soft tissue metastases 1 Cutaneous contamination | 24 Residues 10 Neck lymph node metastases 6 Lymph node metastases outside the neck 1 Bone metastases 1 Physiologic uptake (stomach) | 12 Residues 12 Neck lymph node metastases 1 LN metastasis outside the neck 4 Lung metastases | 1 Neck lymph node metastasis 1 Thorax muscle metastasis 1 Benign vertebral disorder |
| AT SURGERY | AT RADIOIODINE ABLATION | AT FOLLOW-UP | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients n. | Sex | Age | Histology | Size (mm) | Focality | ETE | Metastases | HT | GB | TNM | Risk Stratification | Thyroglobulin (ng/mL) | Planar WBS (n. foci) | SPECT/CT (n. foci) | TNM | Risk Stratification | Thyroglobulin (ng/mL) | Planar WBS (n. foci) | SPECT/CT (n. foci) | TNM | Risk Stratification |
| 1 | F | <45 | PC | ≤10 | UF | HT | T1aN0M0 | VL | und + AbTg | 1 unclear | 2 LTC (1 oc) | T1aN1M0 | H | und + AbTg | 0 | 3 LTC (oc) | T1aN1M0 | H | |||
| 2 | M | <45 | PC | >10 | UF | mETE | T3N0M0 | H | >10.0 | 2 unclear | 2 LTC | T3N1M0 | H | >10.0 | 0 | 3 LTC (oc) | T3N1M0 | H | |||
| 3 | F | ≥45 | PC | ≤10 | MF | T1aN0M0 | L | und | 1 unclear | 1 M | T1aN0M1 | H | >5.0–10.0 | 1 unclear | 1 M, 1 LTC (oc) | T1aN1M1 | H | ||||
| 4 | F | <45 | PC | >10 | UF | mETE | LN | T3N1M0 | H | >10.0 | 2 unclear | 2 LTC | T3N1M0 | H | >10.0 | 2 unclear | 2 LTC, 1 SM (oc), 1 lung (oc), 1 bone (oc) | T3N1M1 | H | ||
| 5 | F | ≥45 | PC | ≤10 | MF | T1aN0M0 | L | 2.5–5.0 | 1 unclear | 1 PT | T1aN1M0 | H | 2.5–5.0 | 0 | 1 PT (oc) | T1aN1M0 | H | ||||
| 6 | F | <45 | PC | >10 | MF | LN | T1bN1M0 | H | >10.0 | 0 | 1 PT (oc) | T1bN1M0 | H | >5.0–10.0 | 0 | 1 PT (oc) | T1bN1M0 | H | |||
| 7 | M | ≥45 | PC | >10 | UF | eETE | LN + DM | T4N1M1 | H | >10.0 | 6 unclear, 1 lung, 5 ST | 1 LTC, 1 bone, 5 AP (1 oc), 1 lung, 5 ST | T4N1M1 | H | >10.0 | 6 unclear, 1 lung, 5 ST | 1 LTC, 1 bone, 5 AP (1 oc), 1 lung, 5 ST | T4N1M1 | H | ||
| 8 | M | ≥45 | PCFV | >10 | UF | mETE | LN | T3N1M0 | H | >10.0 | 1 lung | 1 lung, 1 PT (oc) | T3N1M1 | H | >10.0 | 1 lung | 1 lung, 1 PT (oc) | T3N1M1 | H | ||
| 9 | F | <45 | PC | >10 | MF | HT | T1bN0M0 | L | >10.0 | 0 | 3 LTC (oc) | T1bN1M0 | H | >5.0–10.0 | 0 | 2 LTC (oc) | T1bN1M0 | H | |||
| 10 | F | <45 | PC | >10 | UF | mETE | LN | HT | T3N1M0 | H | >10.0 | 1 unclear | 2 LTC (1 oc) | T3N1M0 | H | <2.5 | 0 | 1 LTC (oc) | T3N1M0 | H | |
| 11 | F | ≥45 | PC | ≤10 | MF | HT | T1aN0M0 | L | <2.5 | 0 | 3 LTC (oc), 1 PT (oc) | T1aN1M0 | H | und | 0 | 1 PT (oc) | T1aN1M0 | H | |||
| 12 | M | ≥45 | PCFV | >10 | UF | DM | GB | T4N0M1 | H | >10.0 | 5 lung, 1 W | 5 lung, 1 TM (W) | T4N0M1 | H | >5.0–10.0 | 1 lung | 1 lung | T4N0M1 | H | ||
| 13 | F | ≥45 | PC | ≤10 | UF | T1aN0M0 | VL | >10.0 | 1 unclear | 1 SM | T1aN1M0 | H | und | 0 | 0 | T1aN0M0 | VL | ||||
| 14 | F | <45 | PC | ≤10 | MF | T1aN0M0 | L | <2.5 | 0 | 1 lung (oc) | T1aN0M1 | H | |||||||||
| 15 | M | ≥45 | PC | >10 | UF | mETE | LN | T3N1M0 | H | >10.0 | 1 lung, 1 unclear | 4 lung (3 oc), 1 M | T3N0M1 | H | |||||||
| 16 | F | ≥45 | PC | >10 | UF | T1bN0M0 | L | und | 1 unclear | 1 SC | T1bN1M0 | H | |||||||||
| 17 | M | ≥45 | PC | >10 | MF | mETE | LN | T3N1M0 | H | <2.5 | 1 W | 1 SM (W), 1 LTC (oc) | T3N1M0 | H | |||||||
| AT SURGERY | AT RADIOIODINE | AT FOLLOW-UP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient s n. | Sex | Age | Histology | Size (mm) | Focality | ETE | GB | TNM | Risk Stratification | Thyroglobulin (ng/mL) | Planar WBS (n. foci) | SPECT/CT (n. foci) | Thyroglobulin (ng/mL) | Planar WBS (n. foci) | SPECT/CT (n. foci) | TNM | Risk Stratification |
| 1 | F | <45 | PC | ≤10 | UF | T1aN0M0 | VL | und | 2 R | 2 R | <2.5 | 1 unclear | 1 SM | T1aN1M0 | H | ||
| 2 | F | ≥45 | PC | ≤10 | UF | T1aN0M0 | VL | und | 1 R | 1 R | <2.5 | 0 | 1 SM (oc) | T1aN1M0 | H | ||
| 3 | F | <45 | PC | ≤10 | UF | T1aN0M0 | VL | und + AbTg | 1 R | 1 R | und + AbTg | 0 | 1 SM (oc) | T1aN1M0 | H | ||
| 4 | F | <45 | PC | ≤10 | UF | mETE | GB | T3N0M0 | H | 2.5–5.0 | 2 R | 2 R | 2.5–5.0 | 0 | 1 SM (oc) | T3N1M0 | H |
| 5 | F | ≥45 | PC | >10 | UF | mETE | T3N0M0 | H | 2.5–5.0 | 1 R | 1 R | 2.5–5.0 | 0 | 1 SM (oc) | T3N1M0 | H | |
| 6 | M | ≥45 | PC | >10 | MF | mETE | T3N0M0 | H | 2.5–5.0 | 1 R | 1 R | 2.5–5.0 | 0 | 1 SM (oc) | T3N1M0 | H | |
| 7 | F | <45 | PC | >10 | UF | T1bN0M0 | L | und | 1 R | 1 R | <2.5 | 0 | 1 LTC (oc) | T1bN1M0 | H | ||
| 8 | F | ≥45 | PC | >10 | UF | T1bN0M0 | L | und + AbTg | 2 R | 2 R | und + AbTg | 1 unclear | 1 LTC | T1bN1M0 | H | ||
| 9 | F | <45 | PC | >10 | UF | T1bN0M0 | L | und + AbTg | 3 unclear | 3 R | und + AbTg | 0 | 1 LTC (oc) | T1bN1M0 | H | ||
| 10 | F | <45 | PC | >10 | UF | T1bN0M0 | L | <2.5 | 4 R | 4 R | >5.0–10.0 | 1 unclear | 1 LTC | T1bN1M0 | H | ||
| 11 | F | ≥45 | PC | >10 | UF | mETE | T3N0M0 | H | und | 3 R | 4 R | und | 0 | 2 LTC (oc) | T3N1M0 | H | |
| 12 | F | ≥45 | PC | >10 | MF | T1bN0M0 | L | und | 1 R | 2 R | <2.5 | 0 | 1 LTC (oc), 1 SM (oc) | T1bN1M0 | H | ||
| 13 | F | ≥45 | PC | >10 | MF | T1bN0M0 | L | 2.5–5.0 | 2 R | 2 R | >10.0 | 0 | 1 LTC (oc), 1 SC (oc) | T1bN1M0 | H | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; De Vito, A.; Rondini, M.; Madeddu, G. The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance. Diagnostics 2021, 11, 1504. https://doi.org/10.3390/diagnostics11081504
Spanu A, Nuvoli S, Marongiu A, Gelo I, Mele L, De Vito A, Rondini M, Madeddu G. The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance. Diagnostics. 2021; 11(8):1504. https://doi.org/10.3390/diagnostics11081504
Chicago/Turabian StyleSpanu, Angela, Susanna Nuvoli, Andrea Marongiu, Ilaria Gelo, Luciana Mele, Andrea De Vito, Maria Rondini, and Giuseppe Madeddu. 2021. "The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance" Diagnostics 11, no. 8: 1504. https://doi.org/10.3390/diagnostics11081504
APA StyleSpanu, A., Nuvoli, S., Marongiu, A., Gelo, I., Mele, L., De Vito, A., Rondini, M., & Madeddu, G. (2021). The Diagnostic Usefulness of 131I-SPECT/CT at Both Radioiodine Ablation and during Long-Term Follow-Up in Patients Thyroidectomized for Differentiated Thyroid Carcinoma: Analysis of Tissue Risk Factors Ascertained at Surgery and Correlated with Metastasis Appearance. Diagnostics, 11(8), 1504. https://doi.org/10.3390/diagnostics11081504







