Comparison of Accuracies between Real-Time Nonrigid and Rigid Registration in the MRI–US Fusion Biopsy of the Prostate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
- patients over 30 years of age;
- patients requested for the prostate fusion biopsy;
- patients with a PI-RADS score of 3 or higher;
- patients with signed informed consent.
- Contraindicated for prostate fusion biopsy
- ▪
- patients with bleeding tendency;
- ▪
- patients allergic to local analgesic agents;
- ▪
- patients intolerant of prostate biopsy;
- patients without multiparametric prostate MRI;
- patients with a PI-RADS score 1 or 2;
- technical errors from the image fusion;
- patients who were not given prophylactic antibiotics or enema;
- unwillingness or inability to sign an informed consent form.
2.2. MRI Acquisition and Categorization for Registration
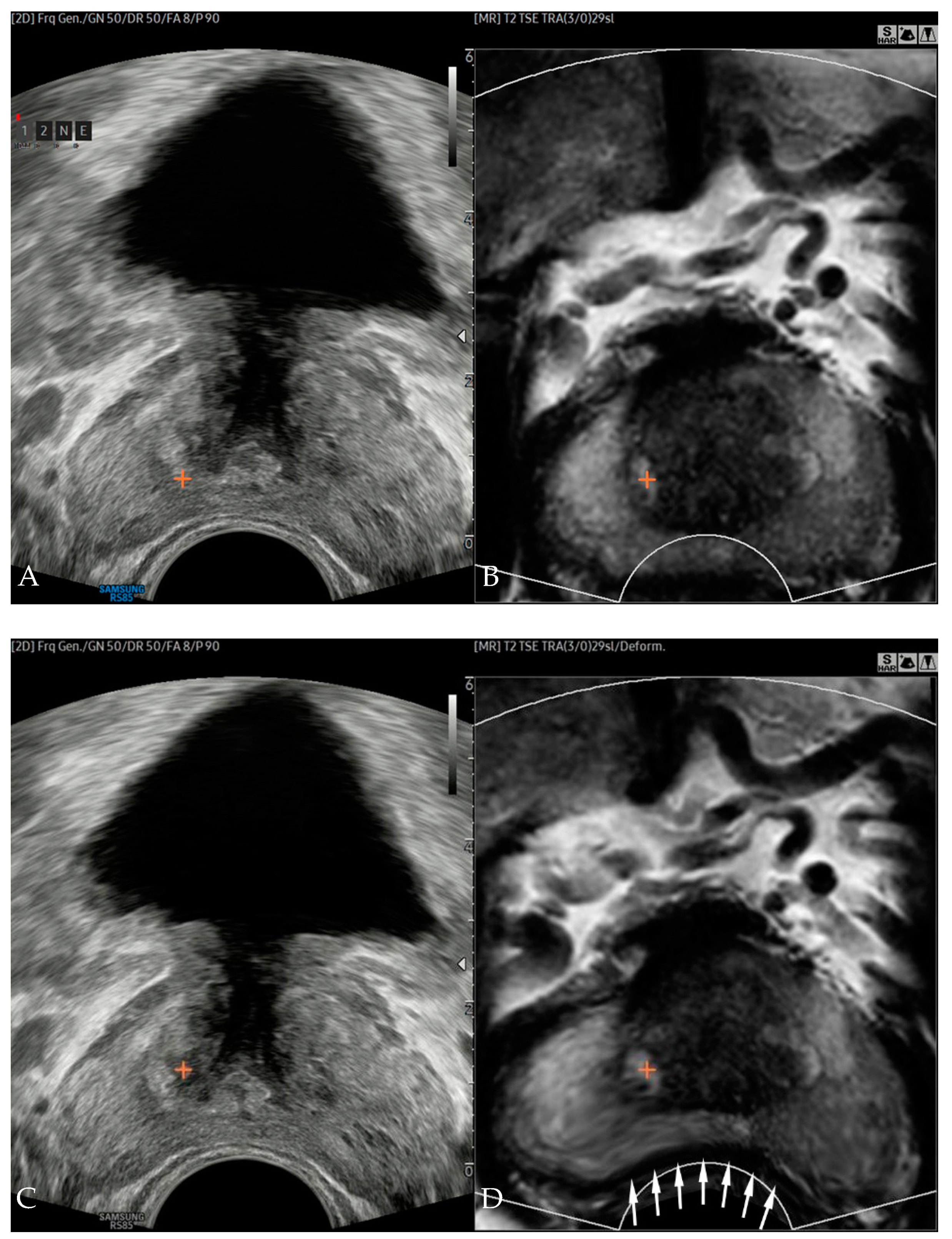
2.3. Image Fusion, Registration, and Error Measurements
2.4. Prostate Biopsy
2.5. Outcome Measures
2.6. Sample Number Calculation and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of Transregistration Error from Rigid and Nonrigid Registration
3.3. Per Patient and Per Core Cancer-Detection Rates
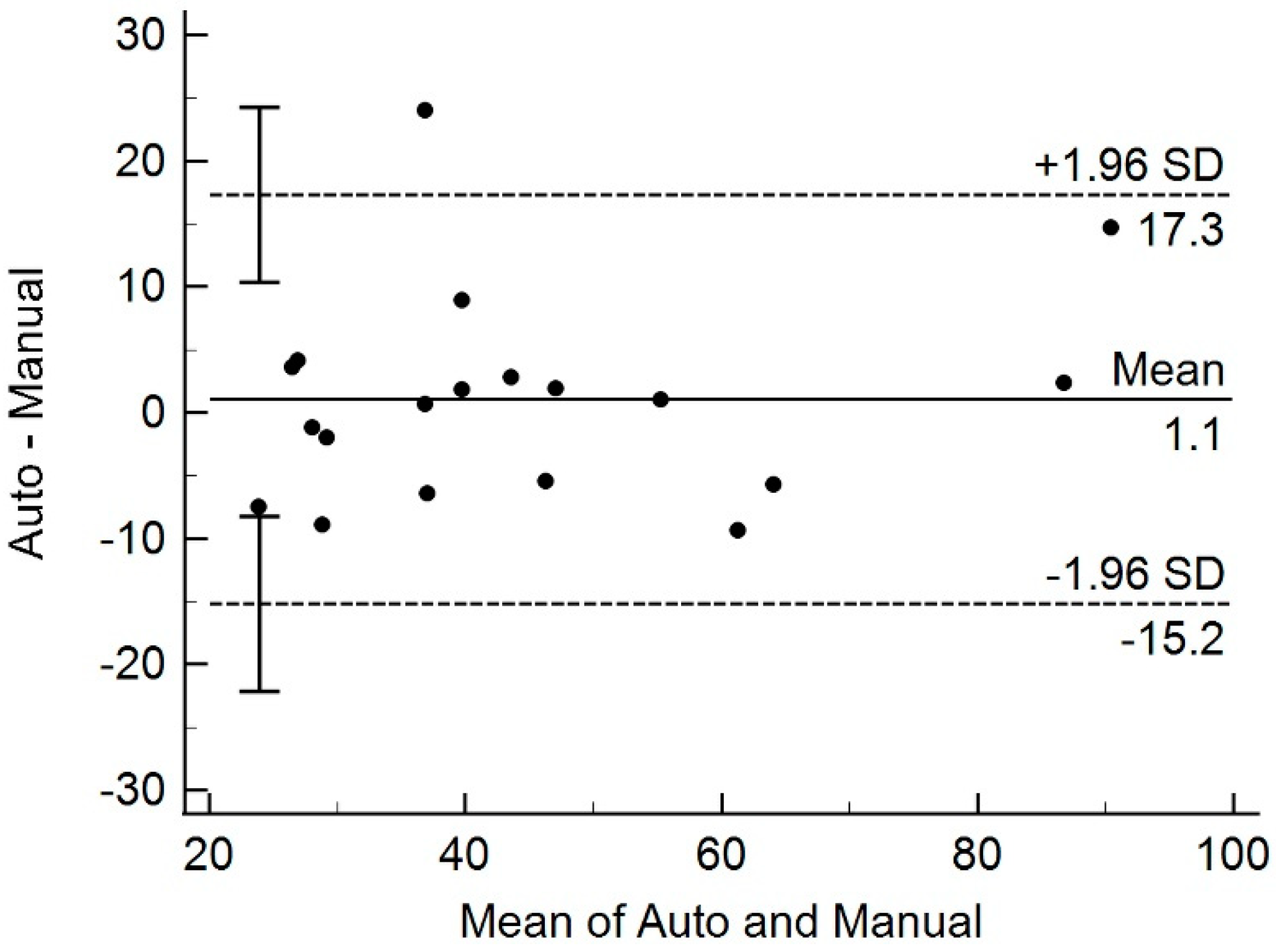
3.4. Comparison of Prostate Volume from MRI and Transrectal US
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, E.J.; Strup, S.E. Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR Am. J. Roentgenol. 2000, 174, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.B.J.; Briers, E.; Bolla, M.; Bourke, L.; Cornford, P.; De Santis, M.; Henry, A.; Joniau, S.; Lam, T.; Mason, M.D.; et al. EAU–ESTRO–ESUR–SIOG Guidelines on Prostate Cancer; Edn. Presented at the EAU Annual Congress Milan; EAU Guidelines Office: Arnhem, The Netherlands, 2021. [Google Scholar]
- Stabile, A.; Giganti, F.; Emberton, M.; Moore, C.M. MRI in prostate cancer diagnosis: Do we need to add standard sampling? A review of the last 5 years. Prostate Cancer Prostatic Dis. 2018, 21, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, T.; Tempany-Afdhal, C.M. Prostate cancer detection and diagnosis: The role of MR and its comparison with other diagnostic modalities—A radiologist’s perspective. NMR Biomed. 2014, 27, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Elbuluk, O.; Mertan, F.; Sankineni, S.; Margolis, D.J.; Wood, B.J.; Pinto, P.A.; Choyke, P.L.; Turkbey, B. Recent advances in image-guided targeted prostate biopsy. Abdom. Imaging 2015, 40, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Cornud, F.; Roumiguie, M.; Barry de Longchamps, N.; Ploussard, G.; Bruguiere, E.; Portalez, D.; Malavaud, B. Precision Matters in MR Imaging-targeted Prostate Biopsies: Evidence from a Prospective Study of Cognitive and Elastic Fusion Registration Transrectal Biopsies. Radiology 2018, 287, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sokolakis, I.; Pyrgidis, N.; Koneval, L.; Krebs, M.; Thurner, A.; Kubler, H.; Hatzichristodoulou, G. Usability and diagnostic accuracy of different MRI/ultrasound-guided fusion biopsy systems for the detection of clinically significant and insignificant prostate cancer: A prospective cohort study. World J. Urol. 2021, 1–8. [Google Scholar] [CrossRef]
- Moldovan, P.; Udrescu, C.; Ravier, E.; Souchon, R.; Rabilloud, M.; Bratan, F.; Sanzalone, T.; Cros, F.; Crouzet, S.; Gelet, A.; et al. Accuracy of Elastic Fusion of Prostate Magnetic Resonance and Transrectal Ultrasound Images under Routine Conditions: A Prospective Multi-Operator Study. PLoS ONE 2016, 11, e0169120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, A.; Park, J.-S.; Mukhopadhyay, S.; Sharda, S.; Son, Y.; Ajani, B.; Kudavelly, S.R. Rigid and Deformable Corrections in Real-Time Using Deep Learning for Prostate Fusion Biopsy; SPIE: Bellingham, WA, USA, 2020; Volume 11315. [Google Scholar]
- Costa, D.N.; Pedrosa, I.; Donato, F., Jr.; Roehrborn, C.G.; Rofsky, N.M. MR Imaging-Transrectal US Fusion for Targeted Prostate Biopsies: Implications for Diagnosis and Clinical Management. Radiographics 2015, 35, 696–708. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.; Fenster, A.; Cool, D.W.; Gardi, L.; Romagnoli, C.; Samarabandu, J.; Ward, A.D. 2D–3D rigid registration to compensate for prostate motion during 3D TRUS-guided biopsy. Med. Phys. 2013, 40, 022904. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.N.; Lotan, Y.; Rofsky, N.M.; Roehrborn, C.; Liu, A.; Hornberger, B.; Xi, Y.; Francis, F.; Pedrosa, I. Assessment of Prospectively Assigned Likert Scores for Targeted Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsies in Patients with Suspected Prostate Cancer. J. Urol. 2016, 195, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Kim, S.H. Transrectal ultrasound-guided prostate biopsy versus combined magnetic resonance imaging-ultrasound fusion and systematic biopsy for prostate cancer detection in routine clinical practice. Ultrasonography 2020, 39, 137–143. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yuan, Z.; Liang, L.; Xie, X.I.; Yuan, J.; He, W.; Chen, J.; Kuang, Y. Endorectal power Doppler/grayscale ultrasound-guided biopsies vs. multiparametric MRI/ultrasound fusion-guided biopsies in males with high risk of prostate cancer: A prospective cohort study. Exp. Ther. Med. 2019, 18, 4765–4773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Kruecker, J.; Turkbey, B.; Glossop, N.; Singh, A.K.; Choyke, P.; Pinto, P.; Wood, B.J. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput. Aided Surg. 2008, 13, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Jiang, S.; Yang, Z.; Liu, R. 2D Ultrasound and 3D MR Image Registration of the Prostate for Brachytherapy Surgical Navigation. Medicine 2015, 94, e1643. [Google Scholar] [CrossRef] [PubMed]
- Venderink, W.; de Rooij, M.; Sedelaar, J.P.M.; Huisman, H.J.; Futterer, J.J. Elastic Versus Rigid Image Registration in Magnetic Resonance Imaging-transrectal Ultrasound Fusion Prostate Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The Comparison of Imaging and Clinical Methods to Estimate Prostate Volume: A Single-Centre Retrospective Study. Urol. Int. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Mean | Standard Deviation | |
|---|---|---|
| Age (years) | 67.7 | 8.0 |
| PSA (ng/mL) | 9.7 | 7.3 |
| Prostate colume by TRUS (mL) | 44.1 | 19.0 |
| Prostate volume by automatic measurement (mL) | 45.2 | 20.5 |
| Frequency | Percent | |
| Preoperative Gleason score (n = 11) | ||
| 3 + 3 | 2 | 18.2 |
| 3 + 4 | 2 | 18.2 |
| 4 + 3 | 5 | 45.5 |
| 4 + 4 | 2 | 18.2 |
| Postoperative Gleason score (n = 7) | ||
| 3 + 4 | 1 | 14.3 |
| 4 + 3 | 5 | 71.4 |
| 4 + 5 | 1 | 14.3 |
| pT stage (n = 7) | ||
| 2c | 2 | 28.6 |
| 3a | 4 | 57.1 |
| 3b | 1 | 14.3 |
| PI-RADS Score | CSC Patient | SB Positive | TB Positive |
|---|---|---|---|
| 3 (n = 8) | 0 (0%) | 0 (0%) | 0 (0%) |
| 4 (n = 5) | 4 (80.0%) | 4 (80.0%) | 4 (80.0%) |
| 5 (n = 6) | 6 (100%) | 6 (100%) | 5 (83.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.I.; Ahn, H.; Lee, H.J.; Hong, S.K.; Byun, S.-S.; Lee, S.; Choe, G.; Park, J.-S.; Son, Y. Comparison of Accuracies between Real-Time Nonrigid and Rigid Registration in the MRI–US Fusion Biopsy of the Prostate. Diagnostics 2021, 11, 1481. https://doi.org/10.3390/diagnostics11081481
Hwang SI, Ahn H, Lee HJ, Hong SK, Byun S-S, Lee S, Choe G, Park J-S, Son Y. Comparison of Accuracies between Real-Time Nonrigid and Rigid Registration in the MRI–US Fusion Biopsy of the Prostate. Diagnostics. 2021; 11(8):1481. https://doi.org/10.3390/diagnostics11081481
Chicago/Turabian StyleHwang, Sung Il, Hyungwoo Ahn, Hak Jong Lee, Sung Kyu Hong, Seok-Soo Byun, Sangchul Lee, Gheeyoung Choe, Jun-Sung Park, and Yuri Son. 2021. "Comparison of Accuracies between Real-Time Nonrigid and Rigid Registration in the MRI–US Fusion Biopsy of the Prostate" Diagnostics 11, no. 8: 1481. https://doi.org/10.3390/diagnostics11081481
APA StyleHwang, S. I., Ahn, H., Lee, H. J., Hong, S. K., Byun, S.-S., Lee, S., Choe, G., Park, J.-S., & Son, Y. (2021). Comparison of Accuracies between Real-Time Nonrigid and Rigid Registration in the MRI–US Fusion Biopsy of the Prostate. Diagnostics, 11(8), 1481. https://doi.org/10.3390/diagnostics11081481






