Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index
Abstract
:1. Introduction
2. Materials and Methods
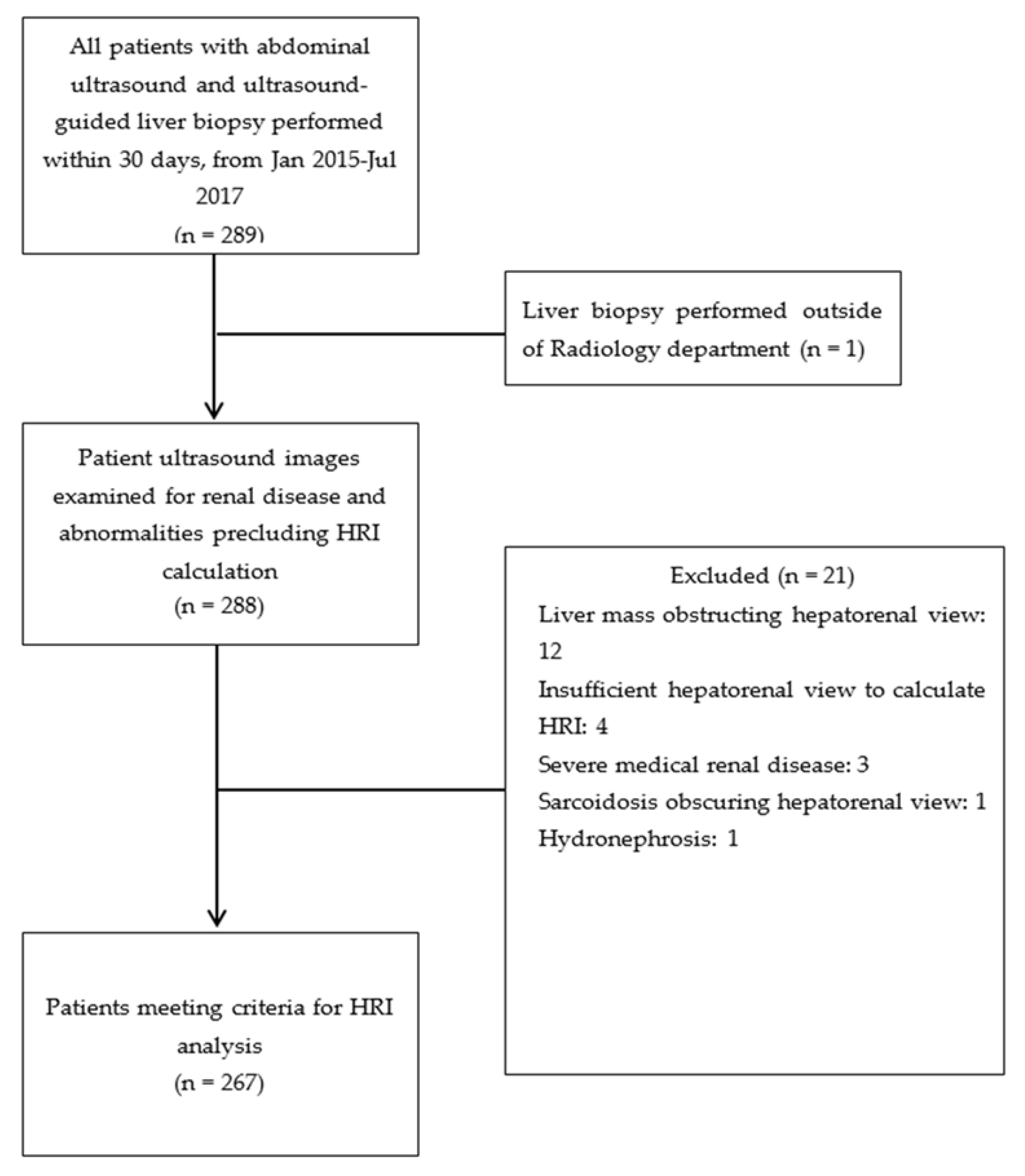
2.1. Patient Selection
2.2. Exclusion Criteria
2.3. Sonographic Examination
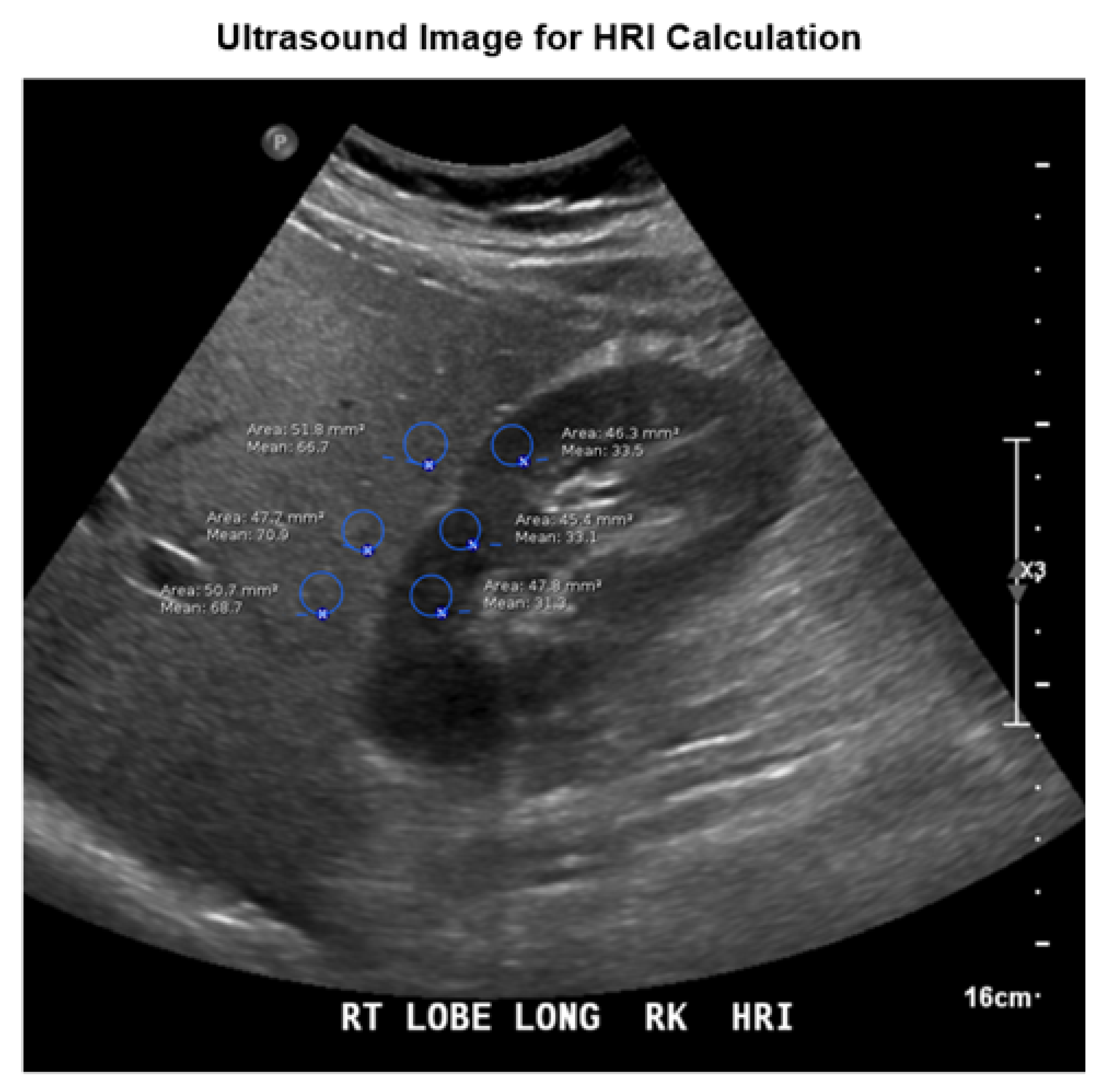
2.4. Image Analysis and HRI Calculation
2.5. Histologic Sampling
2.6. Analysis
3. Results
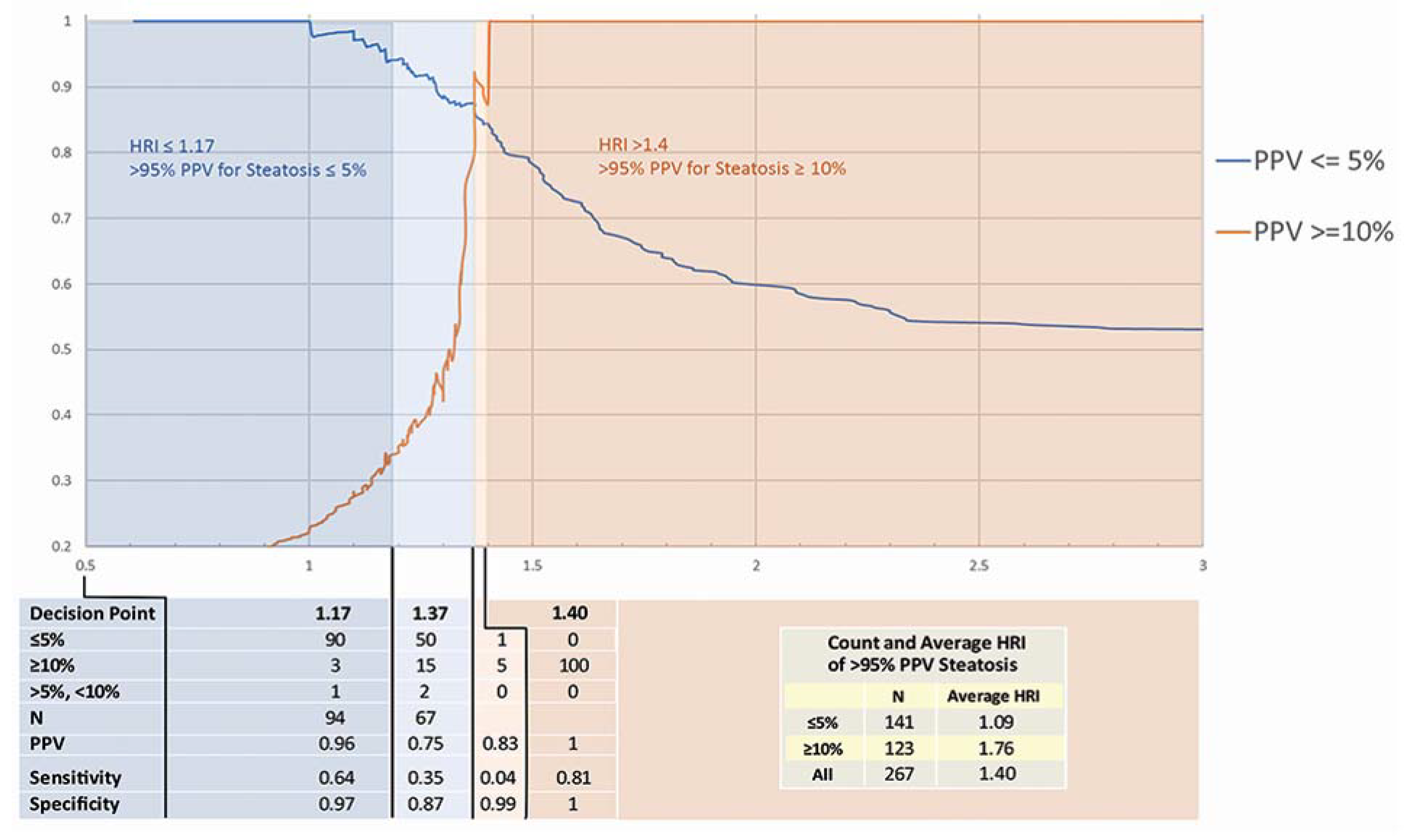
3.1. HRI Measurements
3.2. HRI Decision Point Analysis
4. Discussion
4.1. Accuracy of HRI and Decision Points
4.2. Evaluation of Steatosis by Other Modalities
4.3. Applications in Hepatology
4.4. Limitations
4.5. Key Take Home Message
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, K.; Bhalla, V.; El Regal, M.E.; A-Kader, H.H. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J. Gastroenterol. 2014, 20, 12082–12101. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Conca, P.; Pasanisi, F.; Ariello, M.; Mastrolia, M.; Arena, A.; Vecchione, R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur. J. Gastroenterol. Hepatol. 2009, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.; Yeshua, H.; Zelber-Sagi, S.; Santo, E.; Brazowski, E.; Halpern, Z.; Oren, R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. Am. J. Roentgenol. 2009, 192, 909–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Bydder, M.; Hamilton, G.; Middleton, M.S.; Gamst, A.C.; Wolfson, T.; Hassanein, T.; Patton, H.M.; LaVine, J.E.; Schwimmer, J.; et al. Nonalcoholic fatty liver disease: Diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 2009, 251, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H. Imaging evaluation of non-alcoholic fatty liver disease: Focused on quantification. Clin. Mol. Hepatol. 2017, 23, 290–301. [Google Scholar] [CrossRef]
- Marshall, R.H.; Eissa, M.; Bluth, E.I.; Gulotta, P.M.; Davis, N.K. Hepatorenal index as an accurate, simple, and effective tool in screening for steatosis. Am. J. Roentgenol. 2012, 199, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Shiralkar, K.; Johnson, S.; Bluth, E.I.; Marshall, R.H.; Dornelles, A.; Gulotta, P.M. Improved method for calculating hepatic steatosis using the hepatorenal index. J. Ultrasound Med. 2015, 34, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Holalkere, N.S.; Kambadakone, R.A.; Mino-Kenudson, M.; Hahn, P.F.; Sahani, D.V. Imaging-based quantification of hepatic fat: Methods and clinical applications. Radiographics 2009, 29, 1253–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, Y.; Ng, C.S.; Wu, T.T.; Ayers, G.D.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N.; Charnsangavej, C. Comparison of CT methods for determining the fat content of the liver. Am. J. Roentgenol. 2007, 188, 1307–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idilman, I.S.; Aniktar, H.; Idilman, R.; Kabacam, G.; Savas, B.; Elhan, A.H.; Celik, A.; Bahar, K.; Karcaaltincaba, M. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013, 267, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterson, Y.B.; Grimaldi, G.M. Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin. Liver Dis. 2018, 22, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The controlled attenuation parameter (CAP): A novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- de Lédinghen, V.; Vergniol, J.; Capdepont, M.; Chermak, F.; Hiriart, J.-B.; Cassinotto, C.; Merrouche, W.; Foucher, J.; Brigitte, L.B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 2014, 60, 1026–1031. [Google Scholar] [CrossRef]
| Parameter | Number | Percent | |
|---|---|---|---|
| Sex | |||
| Male | 121 | 45.3% | |
| Female | 146 | 54.7% | |
| Age (average) | 52.19 ± 13.13 | ||
| Indication | Number of Patients with Indication Listed | Percent of Subjects with Indication Listed | |
| Patients with ≤5% steatosis | (Total 141 patients) | ||
| elevated liver function tests | 62 | 44.0% | |
| hepatitis C | 23 | 16.3% | |
| hepatitis B | 14 | 9.9% | |
| liver mass | 9 | 6.4% | |
| hyperbilirubinemia | 6 | 4.3% | |
| unspecified cirrhosis | 5 | 3.5% | |
| liver failure | 3 | 2.1% | |
| autoimmune hepatitis | 3 | 2.1% | |
| liver disease (unspecified) | 3 | 2.1% | |
| Other indications (i.e., abdominal pain, cholangitis) | ≤ 2 | ≤1.4% | |
| Patients with ≥10% Steatosis | (Total 123 Patients) | ||
| elevated liver function tests | 57 | 46.3% | |
| Hepatitis C | 15 | 12.2% | |
| NAFLD 1 | 11 | 8.9% | |
| NASH 2 | 7 | 5.7% | |
| liver mass | 7 | 5.7% | |
| hepatitis B | 6 | 4.9% | |
| hepatic steatosis | 5 | 4.1% | |
| cirrhosis | 4 | 3.3% | |
| HCC 3 screening | 3 | 2.4% | |
| hyperbilirubinemia | 3 | 2.4% | |
| Other indications (i.e., jaundice, elevated ALP 4) | ≤2 | ≤1.6% | |
| Patients with >5% to <10% steatosis | (Total 3 patients) | ||
| hepatitis B | 1 | ||
| HCC 3 screening | 1 | ||
| RUQ 5 pain | 1 | ||
| hepatosplenomegaly | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, S.I.; Fort, D.; Shortt, K.J.; Therapondos, G.; Galliano, G.E.; Nguyen, T.; Bluth, E.I. Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index. Diagnostics 2021, 11, 1443. https://doi.org/10.3390/diagnostics11081443
Johnson SI, Fort D, Shortt KJ, Therapondos G, Galliano GE, Nguyen T, Bluth EI. Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index. Diagnostics. 2021; 11(8):1443. https://doi.org/10.3390/diagnostics11081443
Chicago/Turabian StyleJohnson, Stephen I., Daniel Fort, Kenneth J. Shortt, George Therapondos, Gretchen E. Galliano, Theresa Nguyen, and Edward I. Bluth. 2021. "Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index" Diagnostics 11, no. 8: 1443. https://doi.org/10.3390/diagnostics11081443
APA StyleJohnson, S. I., Fort, D., Shortt, K. J., Therapondos, G., Galliano, G. E., Nguyen, T., & Bluth, E. I. (2021). Ultrasound Stratification of Hepatic Steatosis Using Hepatorenal Index. Diagnostics, 11(8), 1443. https://doi.org/10.3390/diagnostics11081443






