The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Oral Rinse Sample Collection and DNA Extraction
2.3. Oral Investigation
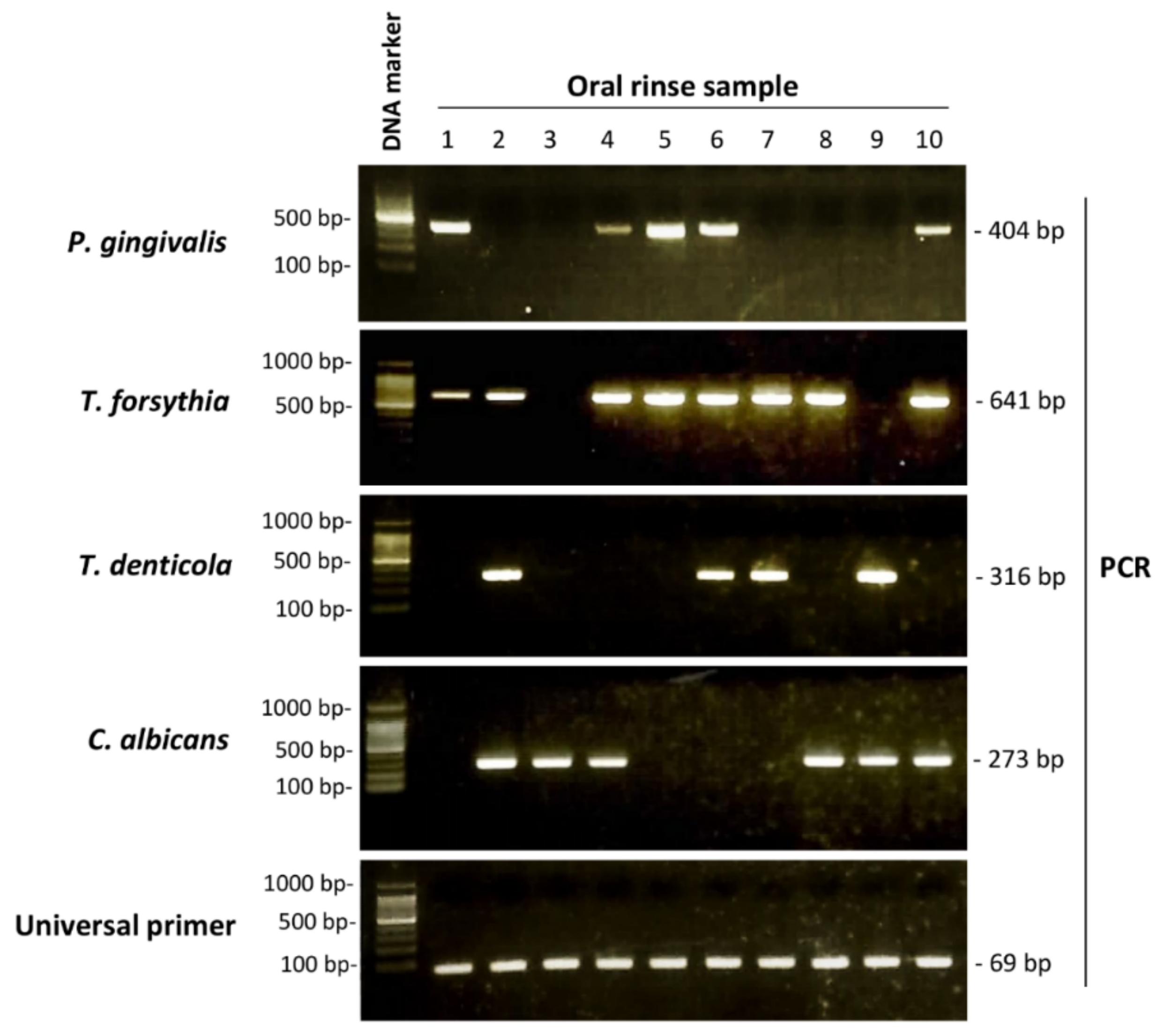
2.4. Periodontopathic Bacteria and Candida Detection by Polymerase Chain Reaction (PCR)
2.5. Statistical Analysis
3. Results
3.1. Associations of PISA and PESA with Clinical Factors in Older People
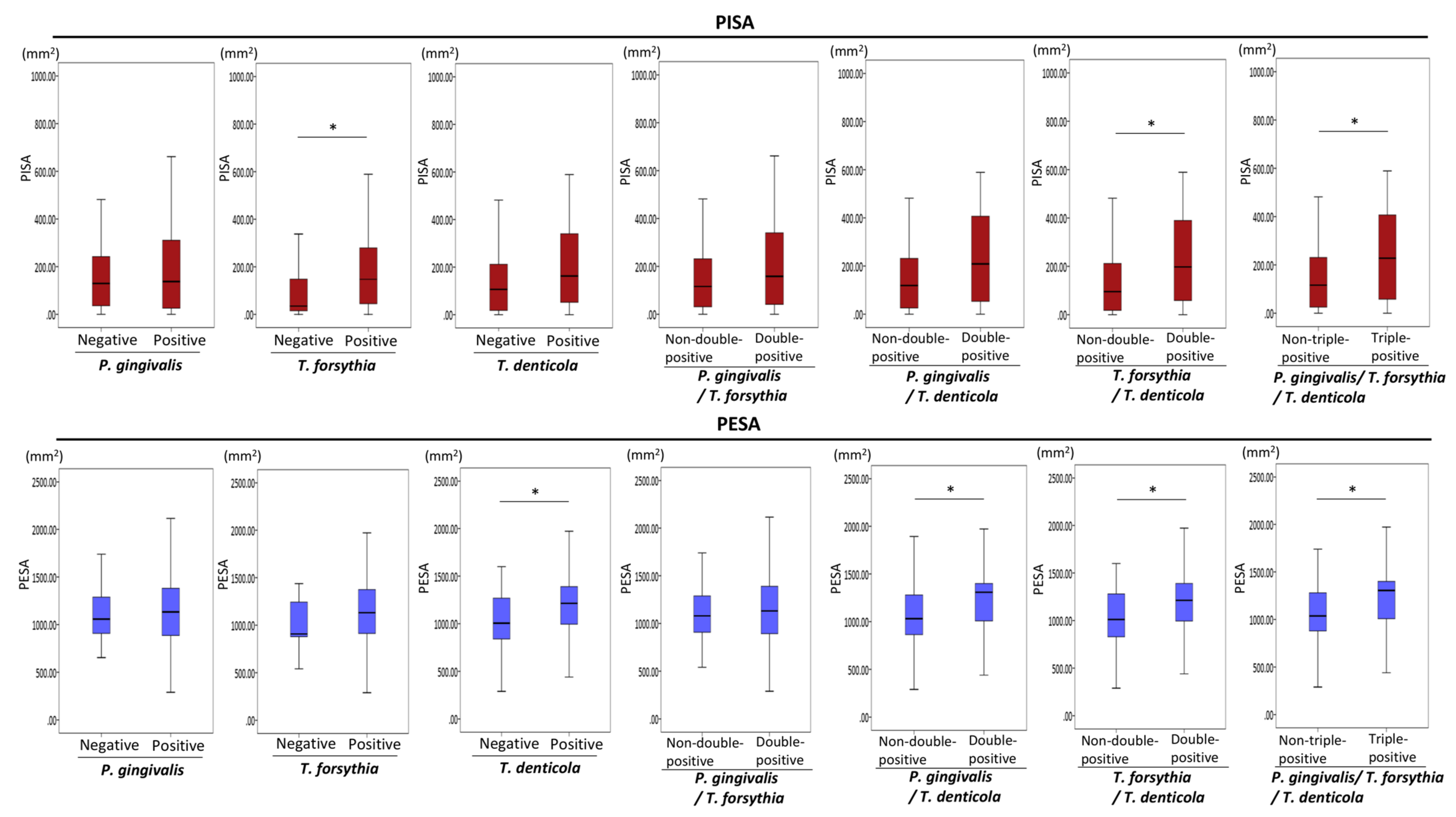
3.2. Associations of the Presence of Periodontopathic Bacteria with PISA and PESA in Older People
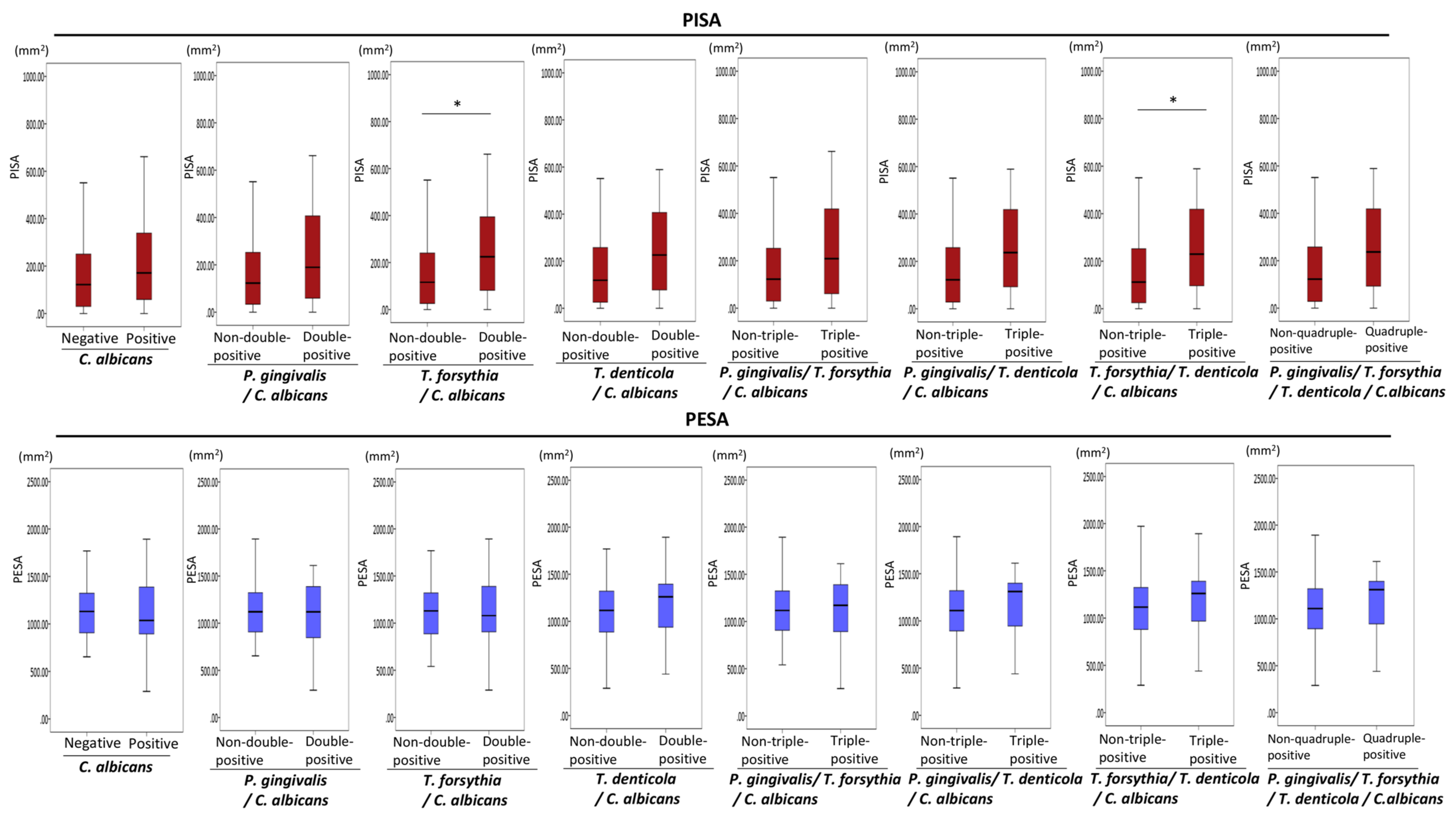
3.3. Associations of the Combined Presence of Periodontopathic Bacteria and C. albicans with PISA in Older People
3.4. Associations of the Presence of Periodontopathic Bacteria/Candida with Clinical Factors
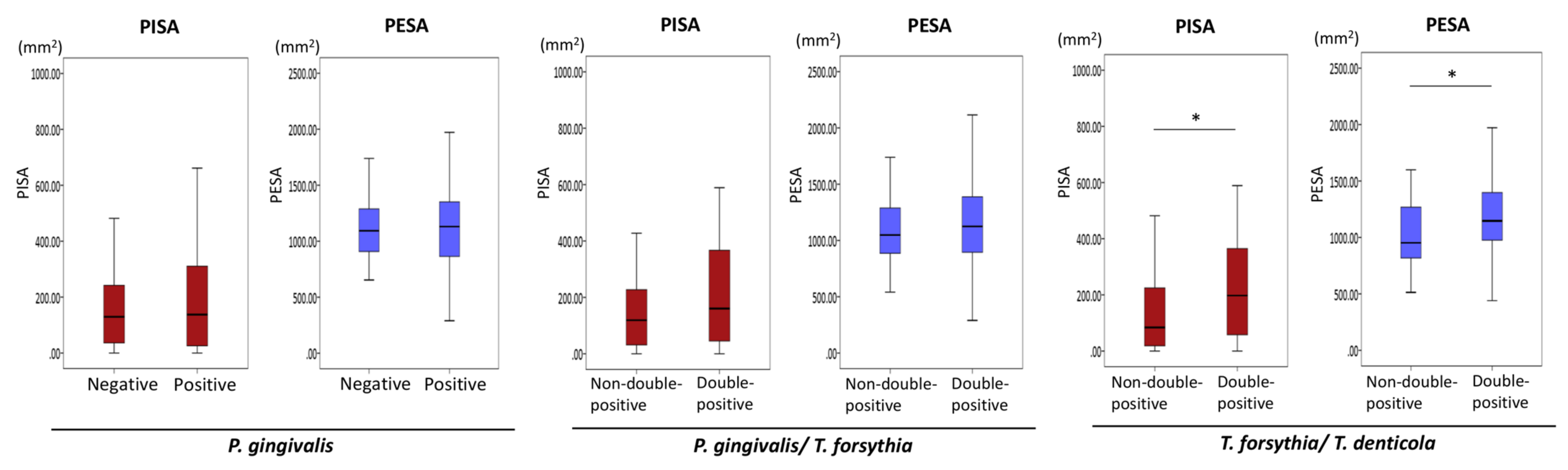
3.5. Associations of the Presence of Periodontopathic Bacteria with PISA and PESA in Propensity Score-Matched Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ulloa, P.C.; Van Der Veen, M.H.; Krom, B.P. Review: Modulation of the oral microbiome by the host to promote ecological balance. Odontology 2019, 107, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.; Haffajee, A.; Cugini, M.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kigure, T.; Saito, A.; Seida, K.; Yamada, S.; Ishihara, K.; Okuda, K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J. Periodontal. Res. 1995, 30, 332. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Maula, T.; Bao, K.; Lindholm, M.; Bostanci, N.; Oscarsson, J.; Ihalin, R.; Johansson, A. Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens 2019, 8, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canabarro, A.; Valle, C.; Farias, M.R.; Santos, F.B.; Lazera, M.; Wanke, B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J. Periodontal Res. 2013, 48, 428–432. [Google Scholar] [CrossRef]
- Peters, B.; Wu, J.; Hayes, R.; Ahn, J. The oral fungal mycobiome: Characteristics and relation to periodontitis in a pilot study. BMC Microbiol. 2017, 17, 157. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Newbrun, E. Indices to Measure Gingival Bleeding. J. Periodontol. 1996, 67, 555–561. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Chen, K.-W.; Lo, H.-J.; Chen, Y.-C.; Liao, M.-H.; Lin, Y.-H.; Li, S.-Y. Species identification of medically important fungi by use of real-time LightCycler PCR. J. Med. Microbiol. 2003, 52, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, T.; Ohmori, M.; Sato, S. The relationship between physiologic halitosis and periodontopathic bacteria of the tongue and gingival sulcus. Odontology 2010, 98, 44–51. [Google Scholar] [CrossRef]
- Yoshida, A.; Suzuki, N.; Nakano, Y.; Oho, T.; Kawada, M.; Koga, T. Development of a 5′ Fluorogenic Nuclease-Based Real-Time PCR Assay for Quantitative Detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J. Clin. Microbiol. 2003, 41, 863–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Ahn, S.; Lee, J.T.; Yun, P.Y.; Lee, Y.J.; Lee, J.Y.; Song, Y.W.; Chang, Y.S.; Lee, H.J. Periodontal inflamed surface area as a novel numerical variable describing periodontal conditions. J. Periodontal. Implant. Sci. 2017, 47, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, Y.; Umeda, M.; Sakamoto, M.; Benno, Y.; Huang, Y.; Ishikawa, I. Treponema socranskii, Treponema denticola, and Porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J. Periodontol. 2001, 72, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Miura, T.; Kuramitsu, H.K.; Okuda, K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin). Infect. Immun. 1996, 64, 5178–5186. [Google Scholar] [CrossRef] [Green Version]
- Fenno, J.C.; Müller, K.H.; McBride, B.C. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J. Bacteriol. 1996, 178, 2489–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, J.V.; Frederick, J.; Stamm, L.; Marconi, R.T. Identification of the Gene Encoding the FhbB Protein of Treponema denticola, a Highly Unique Factor H-Like Protein 1 Binding Protein. Infect. Immun. 2007, 75, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Fenno, J.C.; Hannam, P.M.; Leung, W.K.; Tamura, M.; Uitto, V.-J.; McBride, B.C. Cytopathic Effects of the Major Surface Protein and the Chymotrypsinlike Protease of Treponema denticola. Infect. Immun. 1998, 66, 1869–1877. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sojar, H.T.; Glurich, I.; Homma, K.; Kuramitsu, H.K.; Genco, R.J. Cloning, Expression, and Sequencing of a Cell Surface Antigen Containing a Leucine-Rich Repeat Motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 1998, 66, 5703–5710. [Google Scholar] [CrossRef] [Green Version]
- Grenier, D. Effect of proteolytic enzymes on the lysis and growth of oral bacteria. Oral Microbiol. Immunol. 1994, 9, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Braham, P.H.; Moncla, B.J. Rapid presumptive identification and further characterization of Bacteroides forsythus. J. Clin. Microbiol. 1992, 30, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, T.; Tomi, N.; Fukuyo, Y.; Ishikura, H.; Ohno, Y.; Arvind, R.; Arai, T.; Ishikawa, I.; Arakawa, S. Isolation and identification of a cytopathic activity in Tannerella forsythia. Biochem. Biophys Res. Commun. 2006, 351, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Higuchi, N.; Nakamura, H.; Yoshimura, F.; Oppenheim, F.G. Bacteroides forsythus hemagglutinin is inhibited by N-acetylneuraminyllactose. Oral Microbiol. Immunol. 2002, 17, 125–128. [Google Scholar] [CrossRef]
- Dzink, J.L.; Socransky, S.S.; Haffajee, A.D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J. Clin. Periodontol. 1998, 15, 316–323. [Google Scholar] [CrossRef]
- Grossi, S.G.; Genco, R.J.; Machtei, E.E.; Ho, A.W.; Koch, G.; Dunford, R.; Zambon, J.J.; Hausmann, E. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J. Periodontol. 1995, 66, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Dzink, J.L.; Smith, C.M.; Socransky, S.S. Development of a broth medium for Bacteroides forsythus. J. Clin. Microbiol. 1987, 25, 925. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, S.H. Reducing the bioactivity of Tannerella forsythia lipopolysaccharide by Porphyromonas gingivalis. J. Microbiol. 2014, 52, 702–708. [Google Scholar] [CrossRef]
- Lanza, E.; Magan-Fernandez, A.; Bermejo, B.; de Rojas, J.; Marfil-Alvarez, R.; Mesa, F. Complementary clinical effects of red complex bacteria on generalized periodontitis in a caucasian population. Oral Dis. 2016, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef]
- Rodier, M.-H.; El Moudni, B.; Kauffmann-Lacroix, C.; Daniault, G.; Jacquemin, J.-L. A Candida albicans metallopeptidase degrades constitutive proteins of extracellular matrix. FEMS Microbiol. Lett. 1999, 177, 205–210. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Marcelino, S.L.; Feitosa, A.C.R.; Romito, G.A.; Avila-Campos, M.J. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J. Med. Microbiol. 2009, 58 Pt 12, 1568–1575. [Google Scholar] [CrossRef]
- Susanto, H.; Nesse, W.; Dijkstra, P.U.; Hoedemaker, E.; van Reenen, Y.H.; Agustina, D.; Vissink, A.; Abbas, F. Periodontal inflamed surface area and C-reactive protein as predictors of HbA1c: A study in Indonesia. Clin. Oral Investig. 2012, 16, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Marzo, G.; Giannoni, M.; Ortu, E.; Monaco, A. Association between periodontal inflammation and hypertension using periodontal inflamed surface area and bleeding on probing. J. Clin. Periodontol. 2020, 47, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kimura, Y.; Ogawa, H.; Yamaga, T.; Ansai, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fujisawa, M.; Okumiya, K.; et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J. Periodontal Res. 2019, 54, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Leira, Y.; Rodríguez-Yáñez, M.; Arias, S.; López-Dequidt, I.; Campos, F.; Sobrino, T.; D’Aiuto, F.; Castillo, J.; Blanco, J. Periodontitis as a risk indicator and predictor of poor outcome for lacunar infarct. J. Clin. Periodontol. 2018, 46, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef] [PubMed]

| Periodontal Conditions | Number of Patients (%) |
|---|---|
| Probing depth | |
| <4 mm | 36 (36.0%) |
| ≥4 mm and <6 mm | 32 (32.0%) |
| ≥6 mm | 32 (32.0%) |
| ≥4 mm periodontal pocket with BOP | |
| No | 62 (62.0%) |
| Yes | 38 (38.0%) |
| ≥6 mm periodontal pocket with BOP | |
| No | 77 (77.0%) |
| Yes | 23 (23.0%) |
| Clinical Factor (n) | PISA | p-Value | PESA | p-Value |
|---|---|---|---|---|
| Age in years | ||||
| 60–69 (42) | 203.8 ± 172.5 | 0.70 a | 1180.8 ± 207.2 | 0.40 a |
| 70–79 (37) | 208.0 ± 313.4 | 1086.5 ± 361.0 | ||
| 80–89 (20) | 191.4 ± 268.0 | 1062.0 ± 435.3 | ||
| 90–99 (1) | 165.5 | 1036.3 | ||
| Sex | ||||
| Men (44) | 208.2 ± 271.2 | 0.83 b | 1122.9 ± 367.4 | 0.68 b |
| Women (56) | 197.9 ± 231.9 | 1119.0 ± 323.4 | ||
| Cardiovascular disease | ||||
| No (91) | 208.1 ± 258.0 | 0.99 b | 1126.0 ± 349.0 | 0.61 b |
| Yes (9) | 145.1 ± 113.7 | 1067.5 ± 267.0 | ||
| Hypertension | ||||
| No (75) | 191.8 ± 235.7 | 0.52 b | 1100.2 ± 358.9 | 0.30 b |
| Yes (25) | 234.4 ± 287.1 | 1182.3 ± 281.1 | ||
| Diabetes | ||||
| No (87) | 185.0 ± 207.3 | 0.32 b | 1115.0 ± 334.5 | 0.88 b |
| Yes (13) | 319.2 ± 430.8 | 1158.6 ± 399.1 | ||
| Dyslipidemia | ||||
| No (79) | 207.6 ± 264.1 | 0.91 b | 1110.3 ± 363.8 | 0.39 b |
| Yes (21) | 183.0 ± 183.3 | 1159.1 ± 244.4 | ||
| Smoking | ||||
| Non-smoker (90) | 183.7 ± 221.2 | 0.15 b | 1099.2 ± 332.2 | 0.04 b |
| Current/former smoker (10) | 371.2 ± 401.7 | 1314.5 ± 382.4 | ||
| Number of remaining teeth | ||||
| ≥20 (81) | 221.4 ± 265.2 | 0.07 a | 1210.4 ± 286.7 | <0.01 a |
| 10–19 (17) | 134.4 ± 141.0 | 802.7 ± 232.2 | ||
| 0–9 (2) | 12.5 ± 17.7 | 191.1 ± 139.5 |
| Clinical Factor (n) | P. gingivalis | p-Value | |
|---|---|---|---|
| Negative (36) | Positive (36) | ||
| Age | 72.1 ± 7.9 | 72.1 ± 7.7 | 0.93 a |
| Age in years | |||
| 60–69 (32) | 15 (41.7%) | 17 (47.2%) | 0.77 b |
| 70–79 (27) | 15 (41.7%) | 12 (33.3%) | |
| 80–89 (13) | 6 (16.7%) | 7 (19.4%) | |
| Sex | |||
| Men (30) | 13 (36.1%) | 17 (47.2%) | 0.43 c |
| Women (42) | 23 (63.9%) | 19 (52.8%) | |
| Cardiovascular disease | |||
| No (65) | 33 (91.7%) | 32 (88.9%) | 0.50 c |
| Yes (7) | 3 (8.3%) | 4 (11.1%) | |
| Hypertension | |||
| No (54) | 28 (77.8%) | 26 (72.2%) | 0.39 c |
| Yes (18) | 8 (22.2%) | 10 (27.8%) | |
| Diabetes | |||
| No (66) | 32 (88.9%) | 34 (94.4%) | 0.34 c |
| Yes (6) | 4 (11.1%) | 2 (5.6%) | |
| Dyslipidemia | |||
| No (58) | 29 (80.6%) | 29 (80.6%) | 0.62 c |
| Yes (14) | 7 (19.4%) | 7 (19.4%) | |
| Smoking | |||
| Non-smoker (66) | 34 (94.4%) | 32 (88.9%) | 0.34 c |
| Current/former smoker (6) | 2 (5.6%) | 4 (11.1%) | |
| Remaining teeth | 24.0 ± 6.1 | 23.2 ± 5.1 | 0.22 a |
| PISA (mm2) | 149.5 ± 134.0 | 199.0 ± 292.0 | 0.36 a |
| PESA (mm2) | 1093.8 ± 345.5 | 1113.7 ± 334.8 | 0.81 a |
| Clinical Factor (n) | P. gingivalis/T.forsythia Positive | p-Value | |
|---|---|---|---|
| No (36) | Yes (36) | ||
| Age | 72.1 ± 8.3 | 71.9 ± 7.0 | 0.91 a |
| Age in years | |||
| 60–69 (31) | 16 (44.4%) | 15 (41.7%) | 0.88 b |
| 70–79 (28) | 13 (36.1%) | 15 (41.7%) | |
| 80–89 (13) | 7 (19.4%) | 6 (16.7%) | |
| Sex | |||
| Men (29) | 15 (41.7%) | 14 (38.9%) | 0.50 c |
| Women (43) | 21 (58.3%) | 22 (61.1%) | |
| Cardiovascular disease | |||
| No (68) | 33 (91.7%) | 35 (97.2%) | 0.31 c |
| Yes (4) | 3 (8.3%) | 1 (2.8%) | |
| Hypertension | |||
| No (56) | 28 (77.8%) | 28 (77.8%) | 0.61 c |
| Yes (16) | 8 (22.2%) | 8 (22.2%) | |
| Diabetes | |||
| No (60) | 31 (86.1%) | 29 (80.6%) | 0.38 c |
| Yes (12) | 5 (13.9%) | 7 (19.4%) | |
| Dyslipidemia | |||
| No (58) | 29 (80.6%) | 29 (80.6%) | 0.62 c |
| Yes (14) | 7 (19.4%) | 7 (19.4%) | |
| Smoking | |||
| Non-smoker (66) | 34 (94.4%) | 32 (88.9%) | 0.34 c |
| Current/former smoker (6) | 2 (5.6%) | 4 (11.1%) | |
| Remaining teeth | 23.6 ± 6.2 | 23.0 ± 5.1 | 0.91 a |
| PISA (mm2) | 150.4 ± 136.8 | 274.1 ± 347.5 | 0.53 a |
| PESA (mm2) | 1073.6 ± 351.5 | 1144.4 ± 377.5 | 0.41 a |
| Clinical Factor (n) | T. forsythia/T. denticola Positive | p-Value | |
|---|---|---|---|
| No (36) | Yes (36) | ||
| Age | 71.7 ± 7.5 | 71.1 ± 7.8 | 0.71 a |
| Age in years | |||
| 60–69 (35) | 17 (47.2%) | 18 (50.0%) | 0.97 b |
| 70–79 (25) | 13 (36.1%) | 12 (33.3%) | |
| 80–89 (12) | 6 (16.7%) | 6 (16.7%) | |
| Sex | |||
| Men (30) | 16 (44.4%) | 14 (38.9%) | 0.41 c |
| Women (42) | 20 (55.6%) | 22 (61.1%) | |
| Cardiovascular disease | |||
| No (67) | 33 (91.7%) | 34 (94.4%) | 0.50 c |
| Yes (5) | 3 (8.3%) | 2 (5.6%) | |
| Hypertension | |||
| No (56) | 28 (77.8%) | 28 (77.8%) | 0.61 c |
| Yes (16) | 8 (22.2%) | 8 (22.2%) | |
| Diabetes | |||
| No (65) | 33 (91.7%) | 32 (88.9%) | 0.50 c |
| Yes (7) | 3 (8.3%) | 4 (11.1%) | |
| Dyslipidemia | |||
| No (61) | 31 (86.1%) | 30 (83.3%) | 0.50 c |
| Yes (11) | 5 (13.9%) | 6 (16.7%) | |
| Smoking | |||
| Non-smoker (64) | 33 (91.7%) | 31 (86.1%) | 0.36 c |
| Current/former smoker (8) | 3 (8.3%) | 5 (13.9%) | |
| Remaining teeth | 23.1 ± 5.8 | 23.3 ± 5.2 | 0.95 a |
| PISA (mm2) | 130.0 ± 134.2 | 259.6 ± 286.3 | 0.02 a |
| PESA (mm2) | 1014.1 ± 318.6 | 1187.2 ± 347.6 | 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeishi, H.; Nakamura, M.; Oka, I.; Su, C.-Y.; Yano, K.; Ishikawa, M.; Kaneyasu, Y.; Sugiyama, M.; Ohta, K. The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy. Diagnostics 2021, 11, 1397. https://doi.org/10.3390/diagnostics11081397
Shigeishi H, Nakamura M, Oka I, Su C-Y, Yano K, Ishikawa M, Kaneyasu Y, Sugiyama M, Ohta K. The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy. Diagnostics. 2021; 11(8):1397. https://doi.org/10.3390/diagnostics11081397
Chicago/Turabian StyleShigeishi, Hideo, Mariko Nakamura, Iori Oka, Cheng-Yih Su, Kanako Yano, Momoko Ishikawa, Yoshino Kaneyasu, Masaru Sugiyama, and Kouji Ohta. 2021. "The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy" Diagnostics 11, no. 8: 1397. https://doi.org/10.3390/diagnostics11081397
APA StyleShigeishi, H., Nakamura, M., Oka, I., Su, C.-Y., Yano, K., Ishikawa, M., Kaneyasu, Y., Sugiyama, M., & Ohta, K. (2021). The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy. Diagnostics, 11(8), 1397. https://doi.org/10.3390/diagnostics11081397






