Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report
Abstract
:1. Introduction
2. Case Presentation
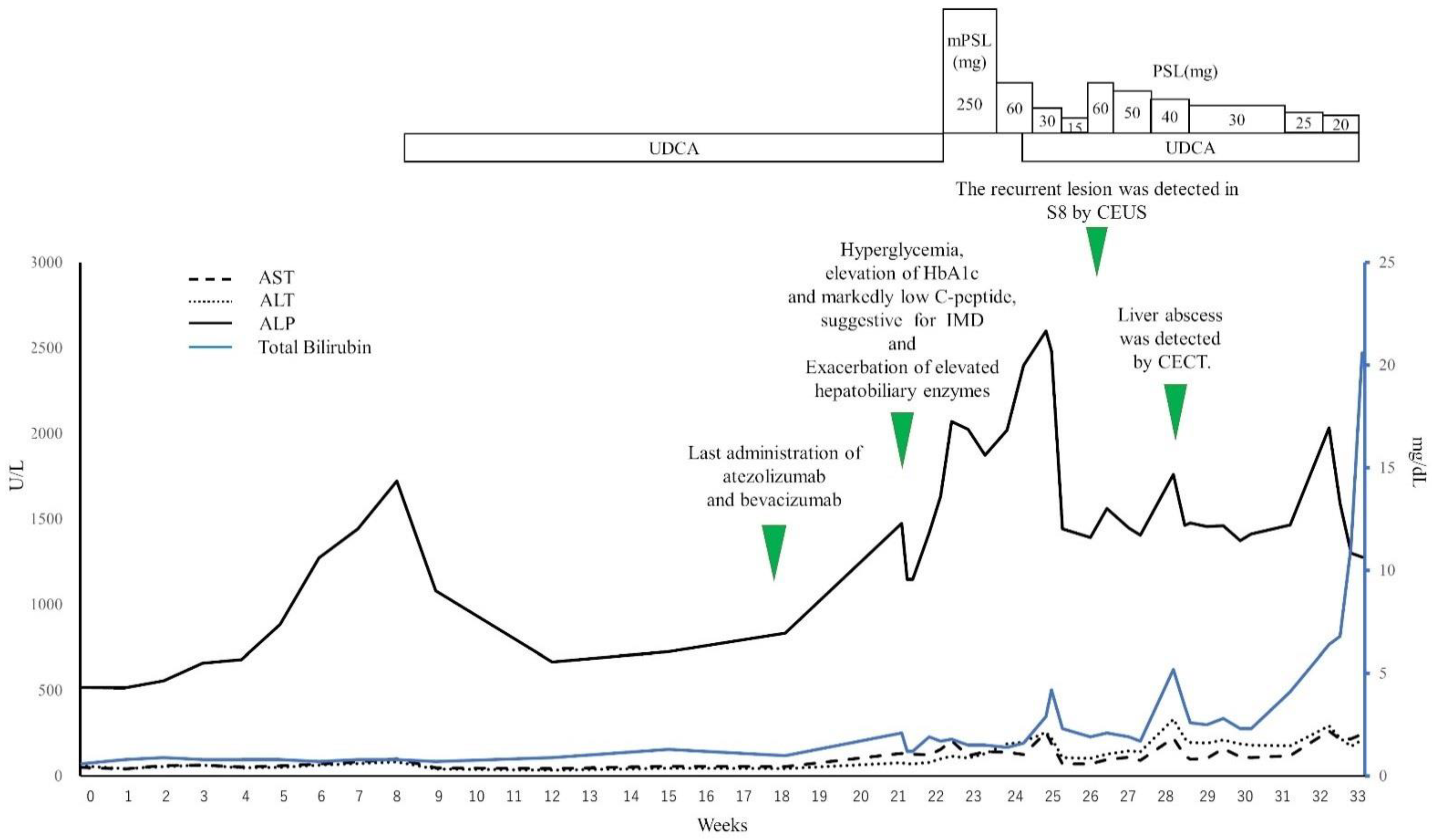
2.1. Clinical Course
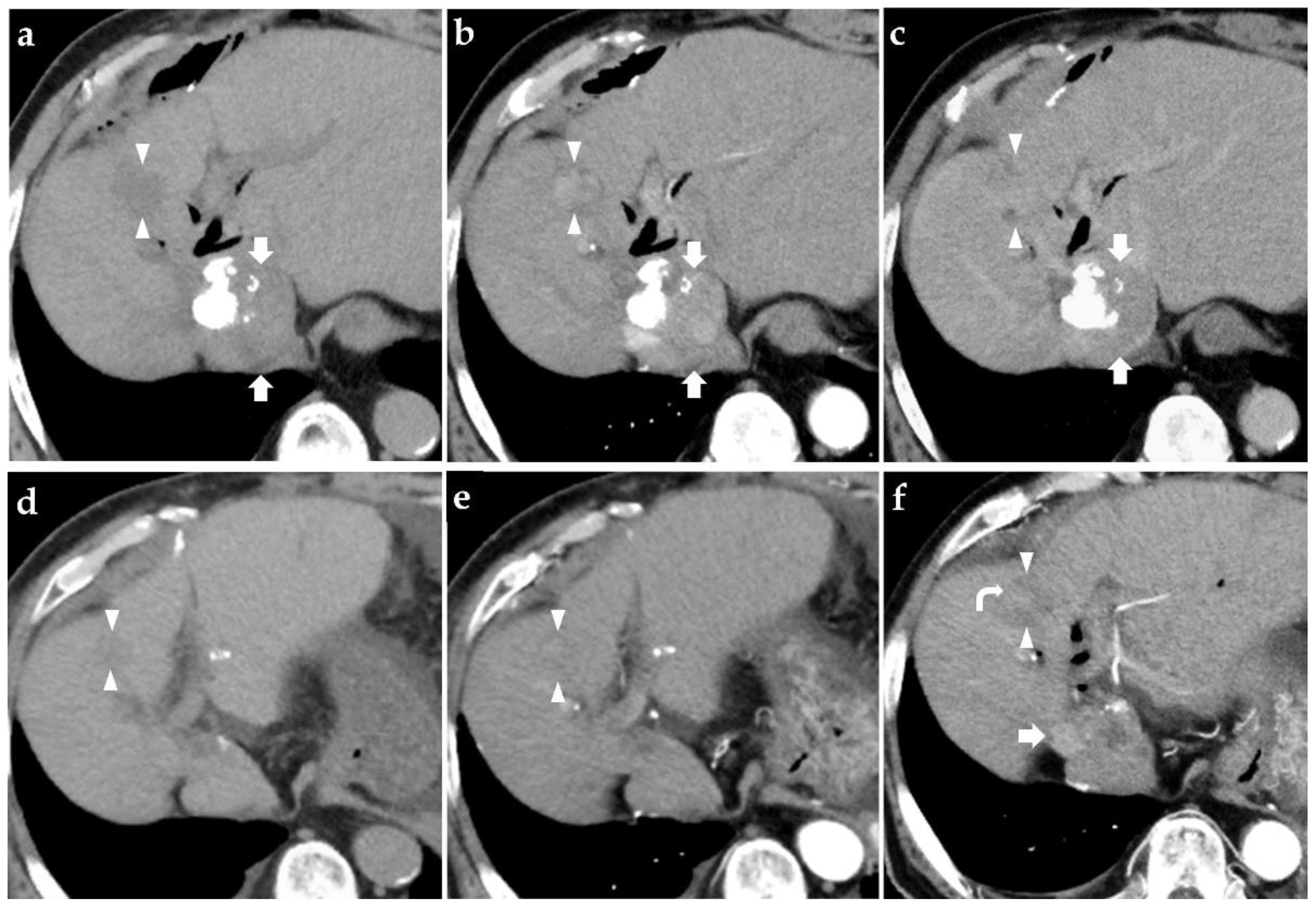
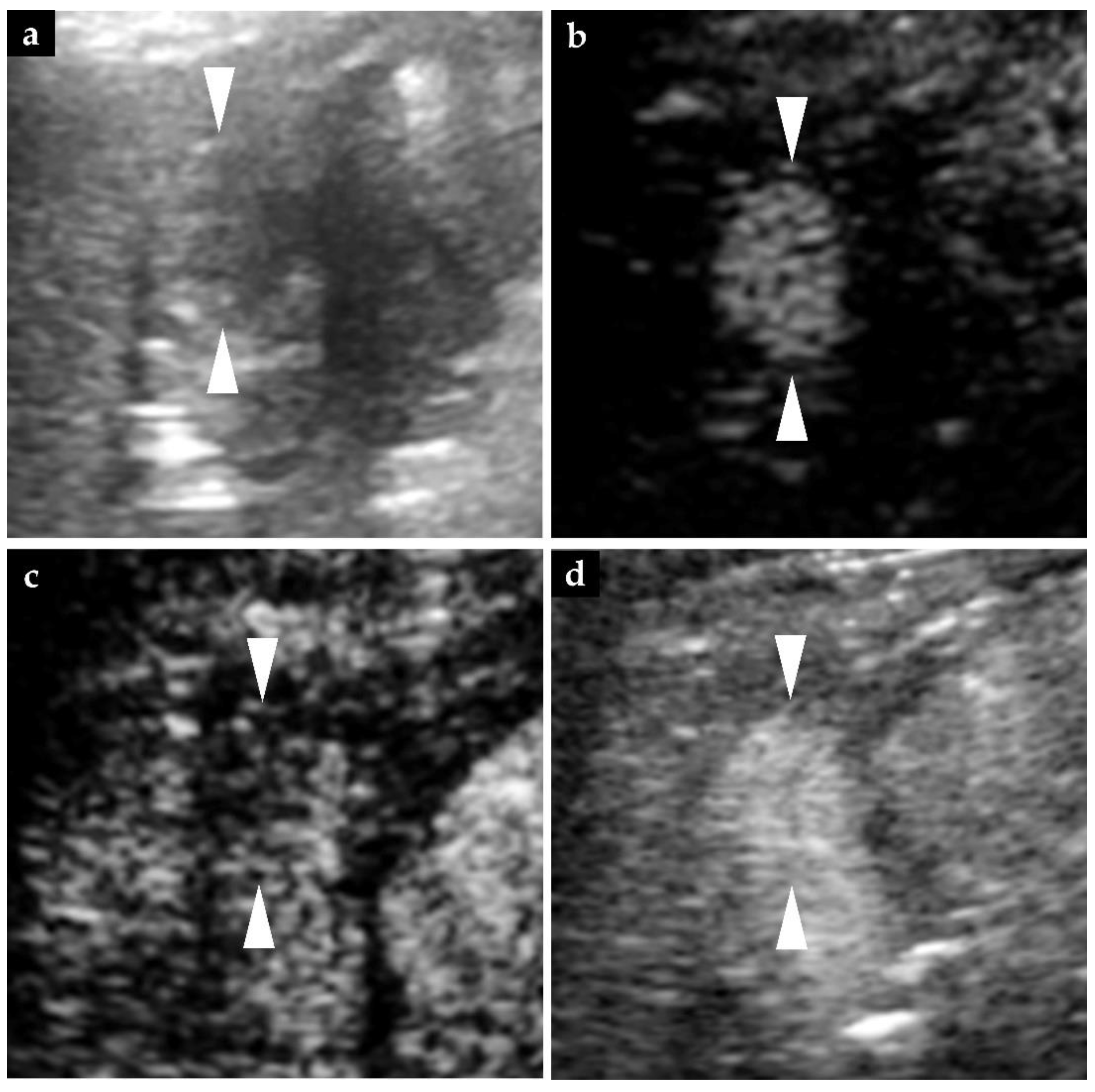
2.2. Findings of Diagnostic Imaging upon Intrahepatic Recurrence of HCC
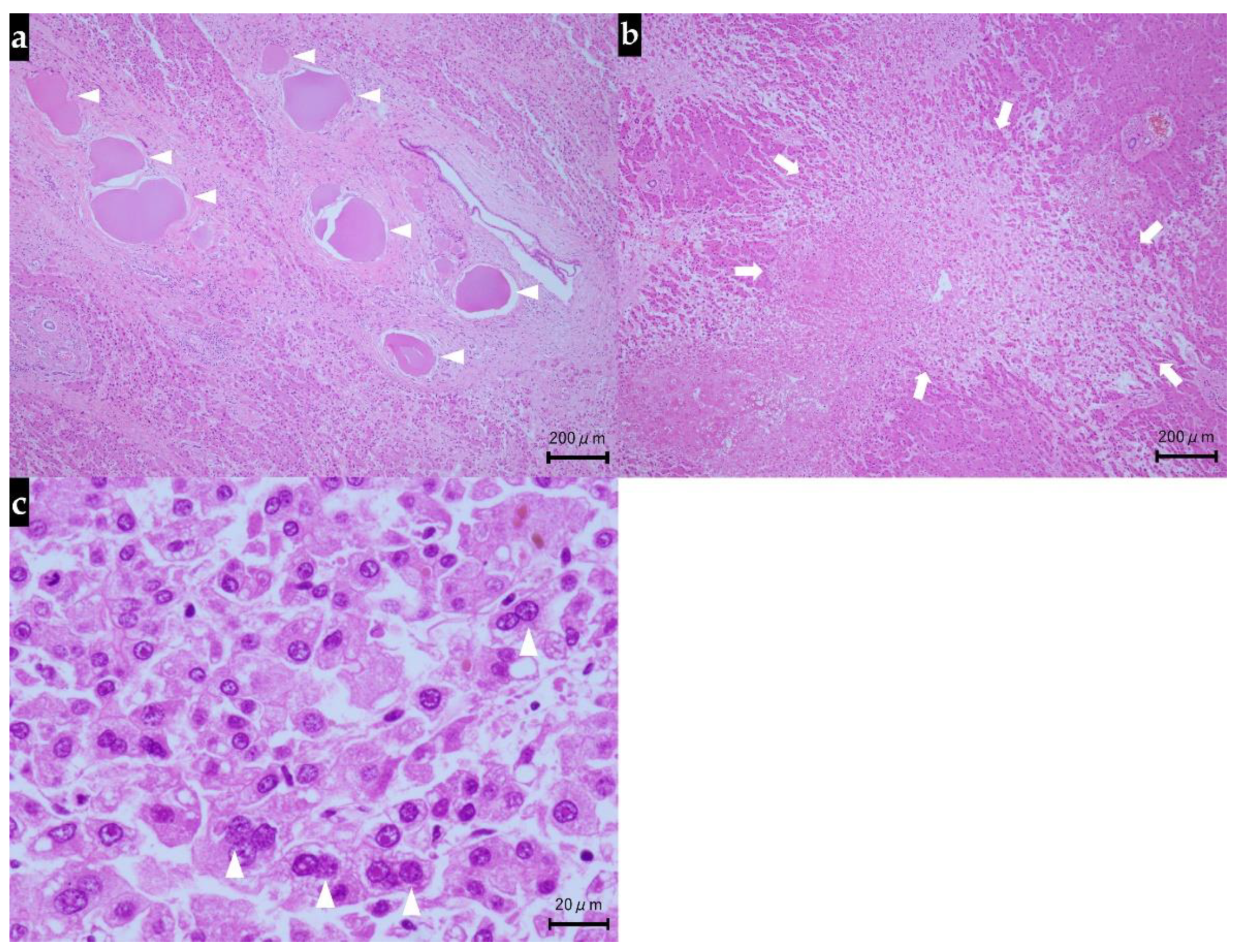
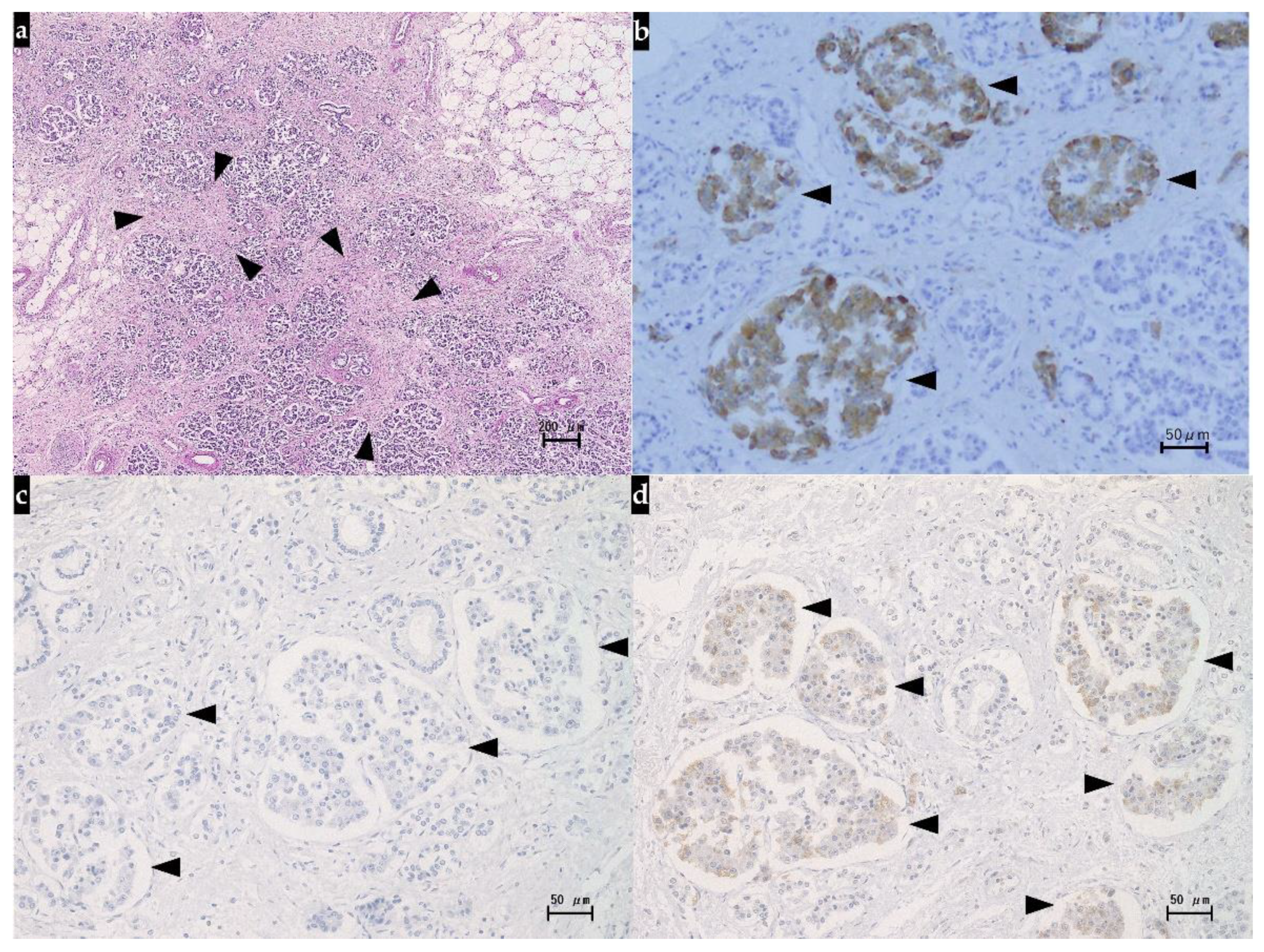
2.3. Autopsy Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 14 June 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs. best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). In Proceedings of the 2019 American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 31 May–4 June 2019; p. 4004. [Google Scholar]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs. sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). In Proceedings of the European Society for Medical Oncology 2019 Congress, Barcelona, Spain, 27 September–1 October 2019. [Google Scholar]
- Kim, J.M.; Chen, D.S. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann. Oncol. 2016, 27, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Hurwitz, H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef]
- Oyama, T.; Ran, S.; Ishida, T.; Nadaf, S.; Kerr, L.; Carbone, D.P.; Gabrilovich, D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998, 160, 1224–1232. [Google Scholar]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.K.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham 3rd, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, S.; Ooka, Y.; Koroki, K.; Maruta, S.; Kanzaki, H.; Kanayama, K.; Kobayashi, K.; Kiyono, S.; Nakamura, M.; Kanogawa, N.; et al. Switching to systemic therapy after locoregional treatment failure: Definition and best timing. Clin. Mol. Hepatol. 2020, 26, 155–162. [Google Scholar] [CrossRef]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M.; Hatanaka, K.; Maekawa, K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology 2010, 78, 40–45. [Google Scholar] [CrossRef]
- Guiu, B.; Deschamps, F.; Aho, S.; Munck, F.; Dromain, C.; Boige, V.; Malka, D.; Leboulleux, S.; Ducreux, M.; Schlumberger, M.; et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: Lipiodol vs. drug-eluting beads. J. Hepatol. 2012, 56, 609–617. [Google Scholar] [CrossRef]
- Monier, A.; Guiu, B.; Duran, R.; Aho, S.; Bize, P.; Deltenre, P.; Dunet, V.; Denys, A. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: Comparison between drug-eluting beads and lipiodol emulsion. Eur. Radiol. 2017, 27, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef] [Green Version]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Noureldein, M.; Daoud, G.; Eid, A.A. Immune checkpoint inhibitors and diabetes: Mechanisms and predictors. Diabetes Metab. Diabetes Metab. 2021, 47, 101193. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, M.A.; Maclaren, N.K. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 1994, 331, 1428–1436. [Google Scholar] [PubMed]
- Marchand, L.; Disse, E.; Dalle, S.; Reffet, S.; Vouillarmet, J.; Fabien, N.; Thivolet, C.; Cugnet-Anceau, C. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol. 2019, 56, 1239–1245. [Google Scholar] [CrossRef]
- Marchand, L.; Thivolet, A.; Dalle, S.; Chikh, K.; Reffet, S.; Vouillarmet, J.; Fabien, N.; Cugnet-Anceau, C.; Thivolet, C. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: Description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. 2019, 56, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73, 158–191. [Google Scholar] [CrossRef]
- Edeline, J.; Boucher, E.; Rolland, Y.; Vauléon, E.; Pracht, M.; Perrin, C.; Le Roux, C.; Raoul, J.L. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 2012, 118, 147–156. [Google Scholar] [CrossRef]
- Paul, S.B.; Dhamija, E.; Gamanagatti, S.R.; Sreenivas, V.; Yadav, D.P.; Jain, S.; Shalimar; Acharya, S.K. Evaluation of tumor response to intra-arterial chemoembolization of hepatocellular carcinoma: Comparison of contrast-enhanced ultrasound with multiphase computed tomography. Diagn. Interv. Imaging 2017, 98, 253–260. [Google Scholar] [CrossRef]
- Takizawa, K.; Numata, K.; Morimoto, M.; Kondo, M.; Nozaki, A.; Moriya, S.; Ishii, T.; Oshima, T.; Fukuda, H.; Okada, M.; et al. Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur. J. Radiol. 2013, 82, 1471–1480. [Google Scholar] [CrossRef]
- Ishii, T.; Numata, K.; Hao, Y.; Doba, N.; Hara, K.; Kondo, M.; Tanaka, K.; Maeda, S. Evaluation of hepatocellular carcinoma tumor vascularity using contrast-enhanced ultrasonography as a predictor for local recurrence following radiofrequency ablation. Eur. J. Radiol. 2017, 89, 234–241. [Google Scholar] [CrossRef]
- Numata, K.; Fukuda, H.; Miwa, H.; Ishii, T.; Moriya, S.; Kondo, M.; Nozaki, A.; Morimoto, M.; Okada, M.; Takebayashi, S.; et al. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur. J. Radiol. 2014, 83, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef] [Green Version]
- Pinato, D.J.; Kaneko, T.; Saeed, A.; Pressiani, T.; Kaseb, A.; Wang, Y.; Szafron, D.; Jun, T.; Dharmapuri, S.; Naqash, A.R.; et al. Immunotherapy in Hepatocellular Cancer Patients with Mild to Severe Liver Dysfunction: Adjunctive Role of the ALBI Grade. Cancers 2020, 12, 1862. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Tajiri, K.; Toyoda, H.; Tada, T.; Ochi, H.; et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig. Dis. 2017, 35, 602–610. [Google Scholar] [CrossRef] [PubMed]
| Values | Normal Range | |
|---|---|---|
| Peripheral blood | ||
| White blood cell count (/μL) | 4910 | 3300–9400 |
| Red blood cell count (104/μL) | 532 | 414–534 |
| Hemoglobin (g/dL) | 15.1 | 13.8–17.2 |
| Platelet count (104/μL) | 11.0 | 18.0–39.0 |
| Prothrombin time (INR) | 1.02 | 0.90–1.10 |
| Blood chemistry | ||
| Sodium (mmol/L) | 140 | 138–144 |
| Potassium (mmol/L) | 4.4 | 3.7–5.0 |
| Chloride (mmol/L) | 105 | 100–108 |
| Calcium (mg/dL) | 9.2 | 8.8–10.1 |
| Total protein (g/dL) | 6.7 | 6.9–8.3 |
| Albumin (g/dL) | 4.1 | 4.2–5.4 |
| Total bilirubin (mg/dL) | 0.6 | 0.4–1.8 |
| Aspartate aminotransferase (U/L) | 50 | 14–32 |
| Alanine aminotransferase (U/L) | 59 | 11–45 |
| Alkaline phosphatase (U/L) | 518 | 109–312 |
| Gamma-glutamyl transpeptidase (U/L) | 351 | 10–58 |
| Lactate dehydrogenase (U/L) | 243 | 116–199 |
| Amylase (U/L) | 242 | 43–130 |
| Total cholesterol (mg/dL) | 156 | <219 |
| Triglyceride (mg/dL) | 124 | <149 |
| Blood urea nitrogen (mg/dL) | 13 | 8–20 |
| Creatinine (mg/dL) | 0.84 | 0.68–1.04 |
| Glucose (mg/dL) | 114 | <110 |
| HbA1c (%) | 6.9 | 4.6–6.2 |
| Immunological tests | ||
| C-reactive protein (mg/dL) | 0.401 | <0.20 |
| Hepatitis B surface antigen/antibody | both negative | |
| Hepatitis B core antigen/antibody | both negative | |
| Hepatitis C virus antibody | negative | |
| Tumor markers | ||
| Alpha-fetoprotein (ng/mL) | 6 | <10 |
| Protein induced by vitamin K absence or antagonists-II (mAU/mL) | 27 | <40 |
| Carcinoembryonic antigen (ng/mL) | 3.4 | <5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komiyama, S.; Numata, K.; Ogushi, K.; Chuma, M.; Tanaka, R.; Chiba, S.; Otani, M.; Inayama, Y.; Nakano, M.; Maeda, S. Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report. Diagnostics 2021, 11, 1394. https://doi.org/10.3390/diagnostics11081394
Komiyama S, Numata K, Ogushi K, Chuma M, Tanaka R, Chiba S, Otani M, Inayama Y, Nakano M, Maeda S. Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report. Diagnostics. 2021; 11(8):1394. https://doi.org/10.3390/diagnostics11081394
Chicago/Turabian StyleKomiyama, Satoshi, Kazushi Numata, Katsuaki Ogushi, Makoto Chuma, Reiko Tanaka, Sawako Chiba, Masako Otani, Yoshiaki Inayama, Masayuki Nakano, and Shin Maeda. 2021. "Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report" Diagnostics 11, no. 8: 1394. https://doi.org/10.3390/diagnostics11081394
APA StyleKomiyama, S., Numata, K., Ogushi, K., Chuma, M., Tanaka, R., Chiba, S., Otani, M., Inayama, Y., Nakano, M., & Maeda, S. (2021). Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report. Diagnostics, 11(8), 1394. https://doi.org/10.3390/diagnostics11081394





