Using Non-Contrast MRA to Discriminate between Obstructive and Nonobstructive Venous Diseases of the Legs
Abstract
1. Introduction
2. Materials and Methods
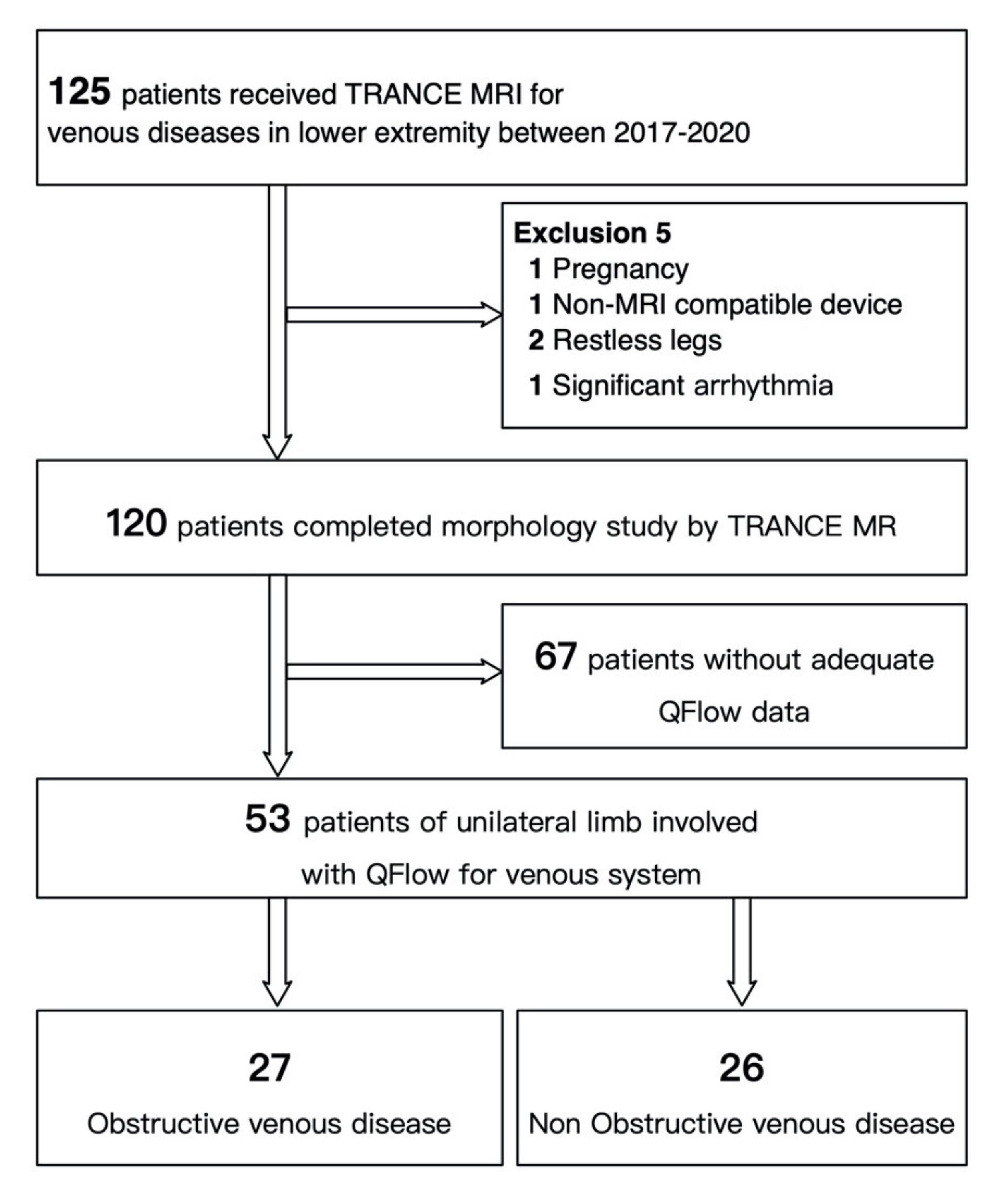
2.1. Patients
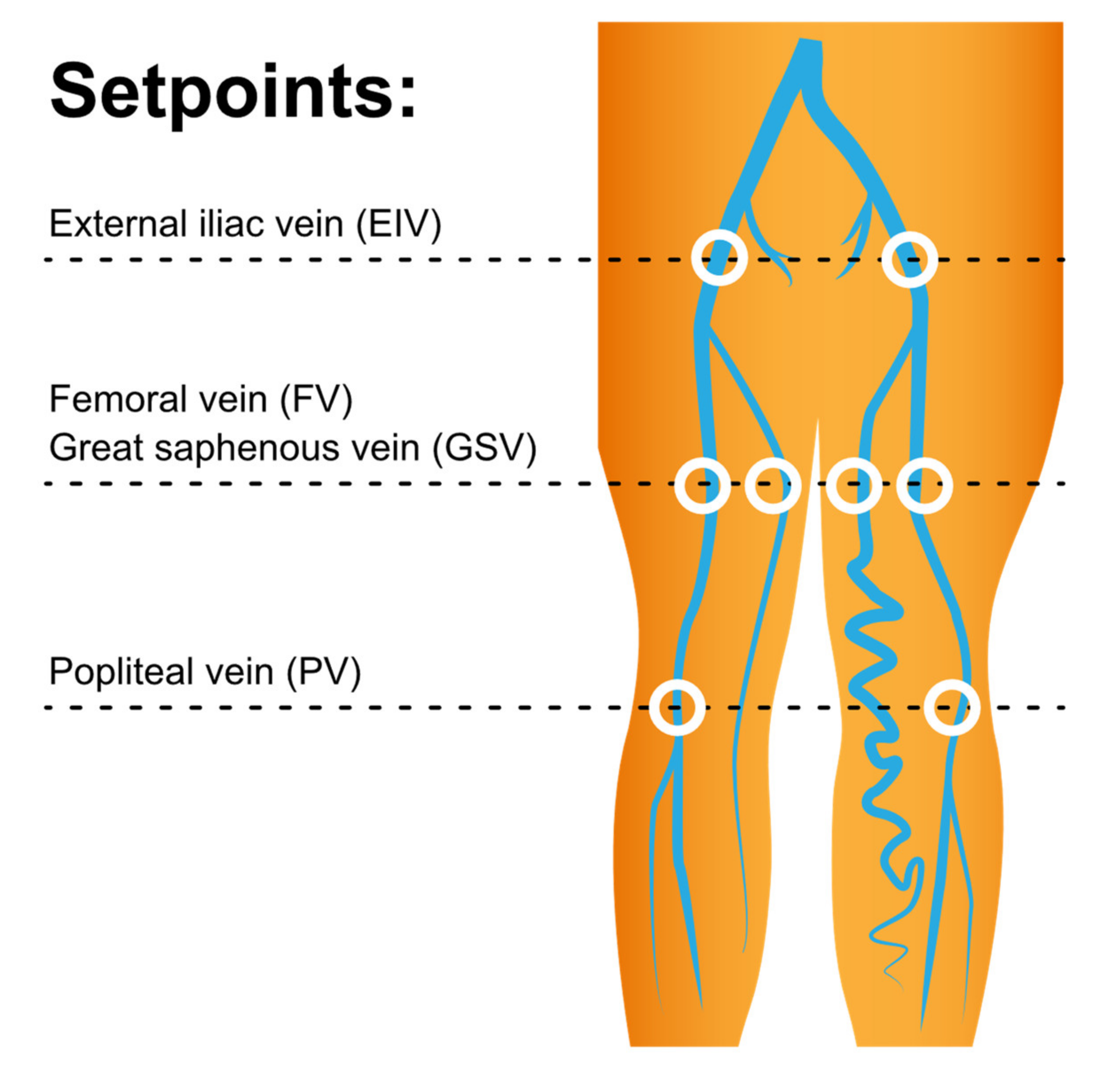
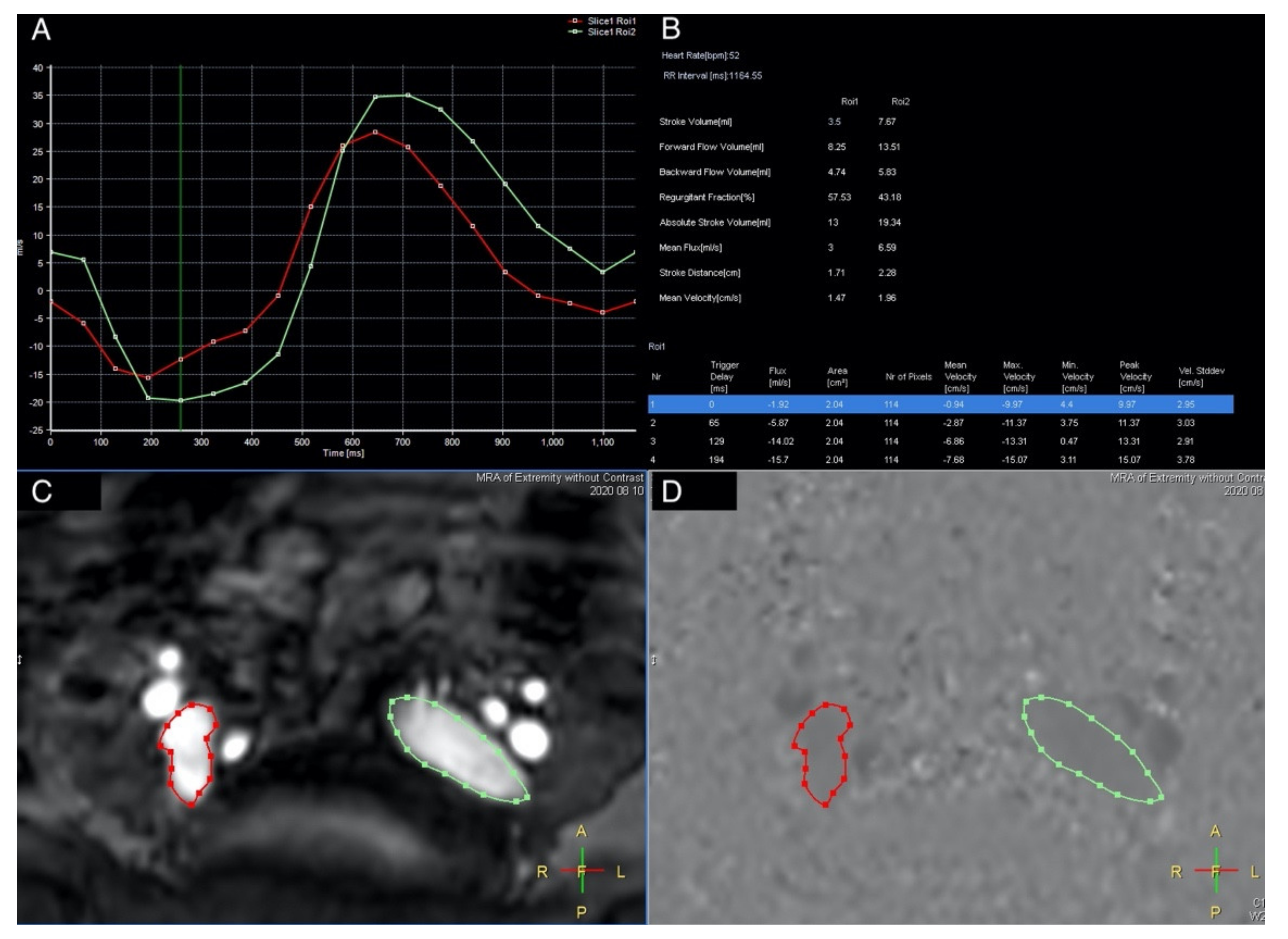
2.2. MRI Acquisition
2.3. Statistical Analysis
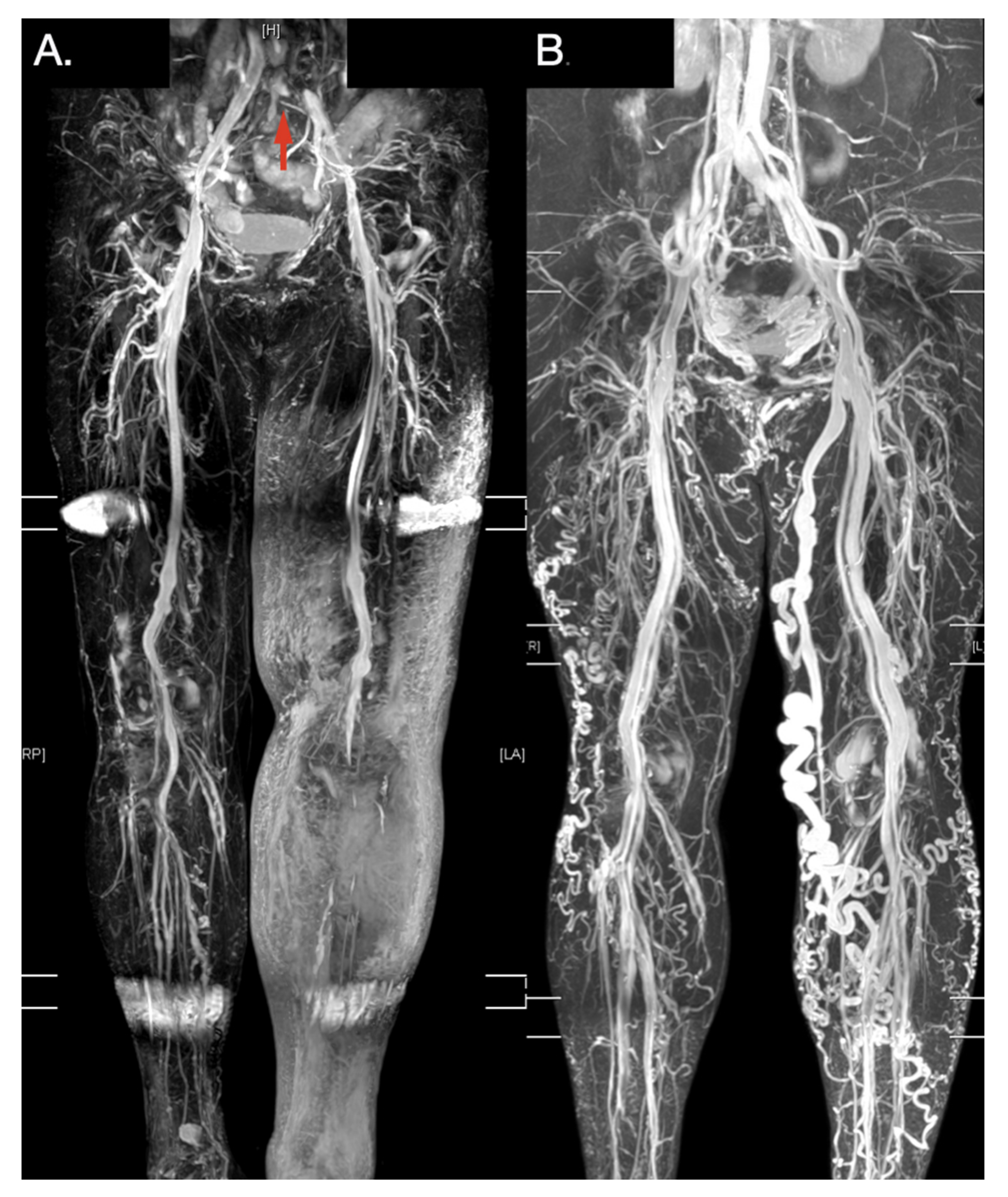
3. Results
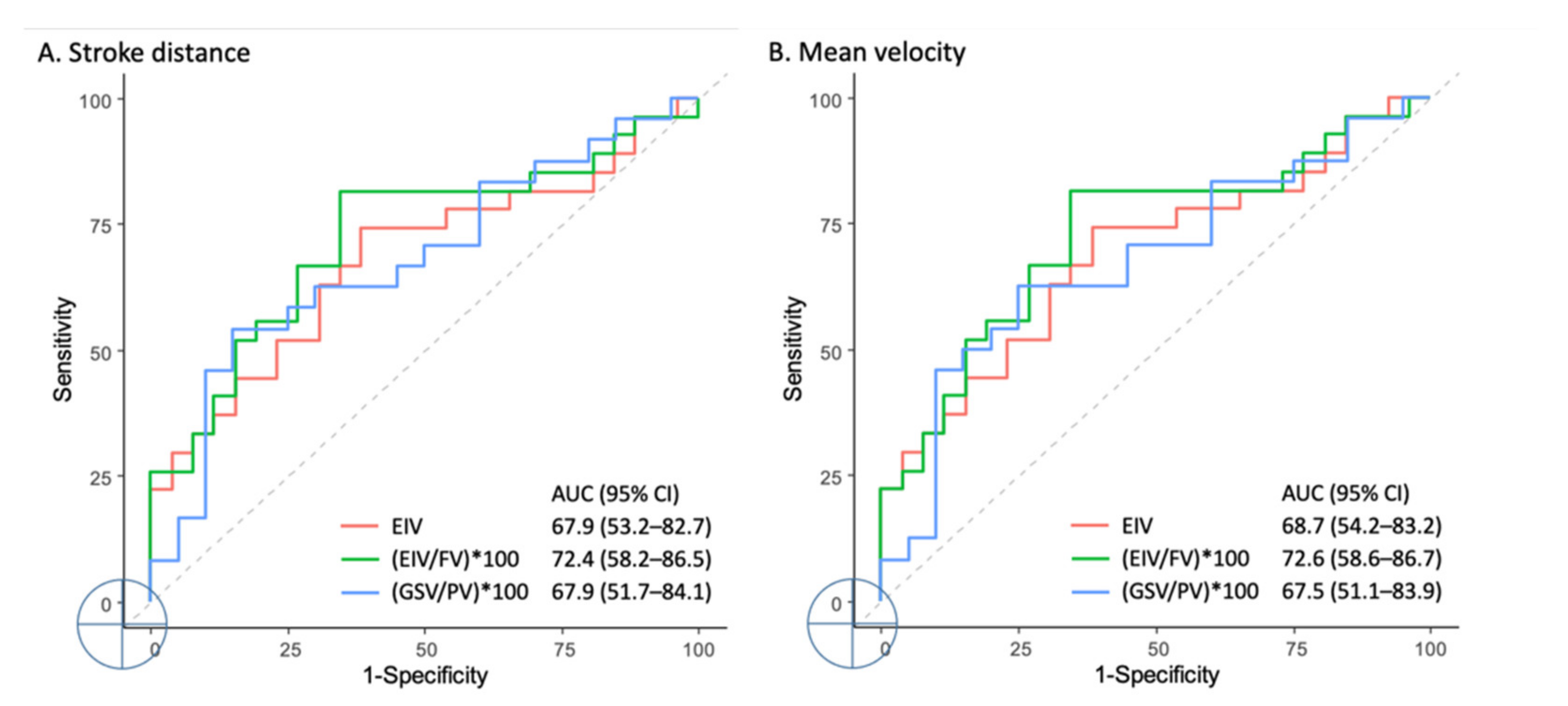
Discriminant Ability of QFlow According to TRANCE MRI
4. Discussion
Study Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| CT | computed tomography |
| CTA | computed tomography angiography |
| DVT | deep venous thrombosis |
| EIV | external iliac vein |
| FFV | forward flow volume |
| FOV | field of view |
| FV | femoral vein |
| GSV | great saphenous vein |
| IR | inversion recovery |
| IRB | institutional review board |
| MF | mean flux |
| MRI | magnetic resonance imaging |
| MRV | magnetic resonance venography |
| MV | mean velocity |
| NSF | nephrogenic systemic fibrosis |
| PV | popliteal vein |
| ROC | receiver operating characteristic |
| SD | stroke distance |
| STIR | short tau inversion recovery |
| SV | stroke volume |
| TE | echo time |
| TOF | time-of-flight |
| TR | repetition time |
| TRANCE-MRI | triggered angiography non-contrast-enhanced MRI |
| TSE | turbo spin-echo |
| US | ultrasonography |
References
- Tassiopoulos, A.K.; Golts, E.; Oh, D.S.; Labropoulos, N. Current concepts in chronic venous ulceration. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 227–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schleimer, K.; Barbati, M.E.; Grommes, J.; Hoeft, K.; Toonder, I.M.; Wittens, C.H.A.; Jalaie, H. Update on diagnosis and treatment strategies in patients with post-thrombotic syndrome due to chronic venous obstruction and role of endovenous recanalization. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 592–600. [Google Scholar] [CrossRef]
- Millan, S.B.; Gan, R.; Townsend, P.E. Venous Ulcers: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 298–305. [Google Scholar]
- Lin, B.S.; Chen, C.W.; Zhou, S.K.; Tseng, Y.H.; Wang, S.C.; Huang, Y.K. Evaluation of static ulcer on lower extremities using wireless wearable near-infrared spectroscopy device: Effect of deep venous thrombosis on TRiggered Angiography Non-Contrast-Enhanced sequence magnetic resonance imaging. Phlebology 2020, 35, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.C.; Chen, C.W.; Tseng, Y.H.; Tsai, Y.H.; Wang, S.C.; Huang, Y.K. Non-contrast-enhanced magnetic resonance imaging: Objective figures in differentiation between acute and chronic deep venous thrombosis in the lower extremities. Phlebology 2020, 35, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Tseng, Y.H.; Lin, C.C.; Kao, C.C.; Wong, M.Y.; Lin, B.S.; Huang, Y.K. Novel Diagnostic Options without Contrast Media or Radiation: Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging in Treating Different Leg Venous Diseases. Diagnostics 2020, 10, 355. [Google Scholar] [CrossRef]
- Asciutto, G.; Mumme, A.; Marpe, B.; Koster, O.; Asciutto, K.C.; Geier, B. MR venography in the detection of pelvic venous congestion. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Huang, Y.K.; Hsu, L.S.; Chen, P.Y.; Chen, C.W. The use of non-contrast-enhanced MRI to evaluate serial changes in endoleaks after aortic stenting: A case report. BMC Med. Imaging 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.K.; Tseng, Y.H.; Lin, C.H.; Tsai, Y.H.; Hsu, Y.C.; Wang, S.C.; Chen, C.W. Evaluation of venous pathology of the lower extremities with triggered angiography non-contrast-enhanced magnetic resonance imaging. BMC Med. Imaging 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Tseng, Y.H.; Fang, Y.F.; Wong, M.Y.; Lin, Y.H.; Huang, Y.K. Superficial Venous Reflux Intervention Guided by Triggered Angiography Noncontrast-Enhanced Sequence Magnetic Resonance Imaging: Different QFlow Pattern from Health Controls. J. Pers. Med. 2021, 11, 751. [Google Scholar] [CrossRef]
- Stalder, A.F.; Russe, M.; Frydrychowicz, A.; Bock, J.; Hennig, J.; Markl, M. Quantitative 2D and 3D phase contrast MRI: Optimized analysis of blood flow and vessel wall parameters. Magn. Reson. Med. 2008, 60, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.; Meier, C.; Leppert, A.; Galanski, M. Cardiovascular flow measurement with phase-contrast MR imaging: Basic facts and implementation. Radiographics 2002, 22, 651–671. [Google Scholar] [CrossRef]
- Chen, C.W.; Tseng, Y.H.; Wong, M.Y.; Wu, C.M.; Lin, B.S.; Huang, Y.K. Stasis Leg Ulcers: Venous System Revises by Triggered Angiography Non-Contrast-Enhanced Sequence Magnetic Resonance Imaging. Diagnostics 2020, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Tseng, Y.-H.; Lin, C.-C.; Kao, C.-C.; Wong, M.Y.; Ting, H.; Huang, Y.-K. Aortic dissection assessment by 4D phase-contrast MRI with hemodynamic parameters: The impact of stent type. Quant. Imaging Med. Surg. 2021, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Ting, H.; Chen, P.Y.; Weng, J.C.; Hsu, Y.C.; Wang, S.C.; Tseng, Y.H.; Huang, Y.K. Usefulness of triggered non-contrast-enhanced magnetic resonance angiography in assessing lower extremity venous disease. Medicine 2021, 100, e25809. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chen, C.W.; Wong, M.Y.; Yang, T.Y.; Lin, B.S.; Ting, H.; Huang, Y.K. Discriminating Reflux from Non-Reflux Diseases of Superficial Veins in Legs by Novel Non-Contrast MR with QFlow Technique. J. Pers. Med. 2021, 11, 242. [Google Scholar] [CrossRef]
- Coleridge-Smith, P.; Labropoulos, N.; Partsch, H.; Myers, K.; Nicolaides, A.; Cavezzi, A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs--UIP consensus document. Part I. Basic principles. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cavezzi, A.; Labropoulos, N.; Partsch, H.; Ricci, S.; Caggiati, A.; Myers, K.; Nicolaides, A.; Smith, P.C. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. Anatomy. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.R. Venous thromboembolic disease: CT evaluation. Q. J. Nucl. Med. 2001, 45, 302–310. [Google Scholar] [PubMed]
- Yucel, E.K.; Kaufman, J.A.; Geller, S.C.; Waltman, A.C. Atherosclerotic occlusive disease of the lower extremity: Prospective evaluation with two-dimensional time-of-flight MR angiography. Radiology 1993, 187, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Suttmeyer, B.; Teichgraber, U.; Thomas, A.; Rathke, H.; Albrecht, L.; Jonczyk, M.; Verba, M.; Guttler, F.; Schnackenburg, B.; Hamm, B.; et al. Non-invasive ECG-triggered 2D TOF MR angiography of the pelvic and leg arteries in an open 1.0-tesla high-field MRI system in comparison to conventional DSA. Biomed. Tech. 2014, 59, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Okada, T.; Umeoka, S.; Nagayama, S.; Tanaka, E.; Fujimoto, K.; Kido, A.; Takeda, K.; Togashi, K.; Sakai, Y. Non-contrast-enhanced MR venography of the upper limb: A comparative study of acquisitions with fresh blood imaging vs. time-of-flight methods. Clin. Imaging 2012, 36, 496–501. [Google Scholar] [CrossRef]
- Steffens, J.C.; Link, J.; Schwarzenberg, H.; Mueller-Huelsbeck, S.; Brinkmann, G.; Heller, M. Lower extremity occlusive disease: Diagnostic imaging with a combination of cardiac-gated 2D phase-contrast and cardiac-gated 2D time-of-flight MRA. J. Comput. Assist. Tomogr. 1999, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gurel, K.; Gurel, S.; Karavas, E.; Buharalioglu, Y.; Daglar, B. Direct contrast-enhanced MR venography in the diagnosis of May-Thurner syndrome. Eur. J. Radiol. 2011, 80, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Ruehm, S.G.; Zimny, K.; Debatin, J.F. Direct contrast-enhanced 3D MR venography. Eur. Radiol. 2001, 11, 102–112. [Google Scholar] [CrossRef]
- Alfano, G.; Fontana, F.; Ferrari, A.; Solazzo, A.; Perrone, R.; Giaroni, F.; Torricelli, P.; Cappelli, G. Incidence of nephrogenic systemic fibrosis after administration of gadoteric acid in patients on renal replacement treatment. Magn. Reson. Imaging 2020, 70, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Maralani, P.J.; Hurrell, C.; Tsampalieros, A.K.; Hiremath, S. Updated Clinical Practice Guideline on Use of Gadolinium-Based Contrast Agents in Kidney Disease Issued by the Canadian Association of Radiologists. Can. Assoc. Radiol. J. 2019, 70, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.R.; Pelc, N.J.; Enzmann, D.R. Qualitative phase contrast MRA in the normal and abnormal circle of Willis. Am. J. Neuroradiol. 1993, 14, 19–25. [Google Scholar] [PubMed]
- Giner, J.F.; Sanz-Requena, R.; Florez, N.; Alberich-Bayarri, A.; Garcia-Marti, G.; Ponz, A.; Marti-Bonmati, L. Quantitative phase-contrast MRI study of cerebrospinal fluid flow: A method for identifying patients with normal-pressure hydrocephalus. Neurologia 2014, 29, 68–75. [Google Scholar] [CrossRef]
- Gutzeit, A.; Sutter, R.; Froehlich, J.M.; Roos, J.E.; Sautter, T.; Schoch, E.; Giger, B.; Wyss, M.; Graf, N.; von Weymarn, C.; et al. ECG-triggered non-contrast-enhanced MR angiography (TRANCE) versus digital subtraction angiography (DSA) in patients with peripheral arterial occlusive disease of the lower extremities. Eur. Radiol. 2011, 21, 1979–1987. [Google Scholar] [CrossRef]
- Suttmeyer, B.; Teichgraber, U.; Rathke, H.; Albrecht, L.; Guttler, F.; Schnackenburg, B.; Hamm, B.; de Bucourt, M. Initial experience with imaging of the lower extremity arteries in an open 1.0 Tesla MRI system using the triggered angiography non-contrast-enhanced sequence (TRANCE) compared to digital subtraction angiography (DSA). Biomed. Tech. 2016, 61, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Radlbauer, R.; Salomonowitz, E.; van der Riet, W.; Stadlbauer, A. Triggered non-contrast enhanced MR angiography of peripheral arteries: Optimization of systolic and diastolic time delays for electrocardiographic triggering. Eur. J. Radiol. 2011, 80, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miyati, T.; Noda, T.; Alperin, N.; Hamaguchi, T.; Ohno, M.; Matsushita, T.; Mase, M.; Gabata, T.; Kobayashi, S. Fast Phase-Contrast Cine MRI for Assessing Intracranial Hemodynamics and Cerebrospinal Fluid Dynamics. Diagnostics 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Altaha, M.A.; Jaskolka, J.D.; Tan, K.; Rick, M.; Schmitt, P.; Menezes, R.J.; Wintersperger, B.J. Non-contrast-enhanced MR angiography in critical limb ischemia: Performance of quiescent-interval single-shot (QISS) and TSE-based subtraction techniques. Eur. Radiol. 2017, 27, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
| Total | Obstructive | Nonobstructive | p-Value | |
|---|---|---|---|---|
| Patient number | 53 | 27 | 26 | |
| Male | 15 | 7 | 8 | 0.696 |
| Age (years old) | 59.7 ± 14.1 | 64.7 ± 5.2 | 54.5 ± 5.4 | 0.007 * |
| Substance | ||||
| Smoking | 10 | 5 | 5 | 0.947 |
| Alcohol | 3 | 2 | 1 | 0.575 |
| Betelnuts | 3 | 1 | 2 | 0.53 |
| Comorbidities | ||||
| Hypertension | 15 | 11 | 4 | 0.041 * |
| Diabetes | 8 | 5 | 3 | 0.478 |
| Stroke | 0 | 0 | 0 | NA |
| Coronary disease | 1 | 0 | 1 | 0.322 |
| DVT history | 6 | 6 | 0 | 0.011 * |
| Cancer | 8 | 7 | 1 | 0.025 * |
| Left leg involved | 32 | 23 | 9 | <0.001 * |
| TRANCE MRI proved varicose veins | 36 | 14 | 22 | 0.011 * |
| Chronic leg ulcer | 8 | 3 | 5 | 0.409 |
| Variable | Total (n = 53) | Obstruction (n = 27) | Non-Obstruction (n = 26) | p-Value |
|---|---|---|---|---|
| Stroke volume (SV) | ||||
| External iliac vein (EIV) | 100 (60, 124) | 72 (39, 124) | 105 (80, 132) | 0.059 |
| Femoral vein (FV) | 91 (60, 136) | 80 (41, 126) | 113 (66, 174) | 0.084 |
| (EIV/FV) × 100 | 86 (58, 147) | 72 (45, 124) | 99 (66, 162) | 0.247 |
| Great saphenous vein (GSV) | 130 (72, 766) | 116 (59, 706) | 168 (94, 848) | 0.239 |
| Popliteal vein (PV) | 89 (53, 120) | 92 (50, 114) | 78 (60, 207) | 0.631 |
| (GSV/PV) × 100 | 154 (79, 518) | 135 (51, 664) | 242 (86, 463) | 0.423 |
| Forward flow volume (FFV) | ||||
| External iliac vein (EIV) | 92 (56, 121) | 76 (22, 121) | 104 (78, 132) | 0.072 |
| Femoral vein (FV) | 95 (64, 136) | 91 (52, 126) | 113 (66, 174) | 0.135 |
| (EIV/FV) × 100 | 81 (49, 145) | 66 (41, 113) | 99 (61, 149) | 0.145 |
| Great saphenous vein (GSV) | 125 (81, 332) | 111 (63, 217) | 170 (94, 588) | 0.083 |
| Popliteal vein (PV) | 92 (63, 120) | 97 (63, 114) | 78 (60, 207) | 0.852 |
| (GSV/PV) × 100 | 147 (73, 312) | 103 (54, 281) | 203 (96, 342) | 0.157 |
| Mean flux (MF) | ||||
| External iliac vein (EIV) | 100 (59, 124) | 72 (39, 124) | 105 (80, 133) | 0.070 |
| Femoral vein (FV) | 95 (64, 136) | 89 (48, 126) | 113 (67, 171) | 0.126 |
| (EIV/FV) × 100 | 83 (55, 146) | 67 (45, 114) | 96 (65, 163) | 0.140 |
| Great saphenous vein (GSV) | 129 (67, 778) | 116 (59, 694) | 173 (93, 824) | 0.239 |
| Popliteal vein (PV) | 88 (61, 120) | 92 (52, 114) | 77 (61, 209) | 0.709 |
| (GSV/PV) × 100 | 160 (80, 538) | 126 (52, 608) | 238 (86, 466) | 0.370 |
| Stroke distance (SD) | ||||
| External iliac vein (EIV) | 98 (71, 123) | 77 (29, 107) | 104 (80, 132) | 0.025 * |
| Femoral vein (FV) | 94 (76, 128) | 94 (70, 124) | 95 (76, 129) | 0.594 |
| (EIV/FV) × 100 | 94 (64, 122) | 68 (19, 96) | 109 (77, 132) | 0.005 *,b |
| Great saphenous vein (GSV) | 126 (73, 423) | 93 (46, 349) | 147 (101, 763) | 0.131 |
| Popliteal vein (PV) | 91 (57, 119) | 97 (61, 130) | 79 (54, 118) | 0.533 |
| (GSV/PV) × 100 | 135 (66, 333) | 100 (48, 314) | 204 (119, 726) | 0.043 * |
| Mean velocity (MV) | ||||
| External iliac vein (EIV) | 99 (71, 128) | 77 (29, 107) | 104 (80, 133) | 0.020 * |
| Femoral vein (FV) | 94 (76, 128) | 94 (70, 123) | 95 (76, 129) | 0.581 |
| (EIV/FV) × 100 | 94 (63, 129) | 68 (19, 96) | 112 (77, 135) | 0.005 *,b |
| Great saphenous vein (GSV) | 126 (73, 382) | 93 (58, 351) | 148 (102, 601) | 0.172 |
| Popliteal vein (PV) | 91 (57, 119) | 98 (61, 130) | 79 (54, 118) | 0.510 |
| (GSV/PV) × 100 | 135 (66, 332) | 100 (49, 316) | 206 (119, 502) | 0.048 * |
| Variable | AUC, % (95% CI) * | Cutoff # | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| Stroke distance (SD) | ||||
| External iliac vein | 67.9 (53.2–82.7) * | ≤100.3 | 74.1 (53.7–88.9) | 61.5 (40.6–79.8) |
| (External iliac vein/Femoral vein) × 100 | 72.4 (58.2–86.5) * | ≤101.0 | 81.5 (61.9–93.7) | 65.4 (44.3–82.8) |
| (Great saphenous vein/Popliteal vein) × 100 | 67.9 (51.7–84.1) * | ≤110.2 | 54.2 (32.8–74.4) | 85.0 (62.1–96.8) |
| Mean velocity (MV) | ||||
| External iliac vein | 68.7 (54.2–83.2) * | ≤100.3 | 74.1 (53.7–88.9) | 61.5 (40.6–79.8) |
| (External iliac vein/Femoral vein) × 100 | 72.6 (58.6–86.7) * | ≤100.9 | 81.5 (61.9–93.7) | 65.4 (44.3–82.8) |
| (Great saphenous vein/Popliteal vein) × 100 | 67.5 (51.1–83.9) * | ≤122.9 | 62.5 (40.6–81.2) | 75.0 (50.9–91.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Tseng, Y.-H.; Wong, M.Y.; Lin, Y.-H.; Yang, T.-Y.; Hsu, Y.-C.; Lin, B.-S.; Huang, Y.-K. Using Non-Contrast MRA to Discriminate between Obstructive and Nonobstructive Venous Diseases of the Legs. Diagnostics 2021, 11, 1392. https://doi.org/10.3390/diagnostics11081392
Chen C-W, Tseng Y-H, Wong MY, Lin Y-H, Yang T-Y, Hsu Y-C, Lin B-S, Huang Y-K. Using Non-Contrast MRA to Discriminate between Obstructive and Nonobstructive Venous Diseases of the Legs. Diagnostics. 2021; 11(8):1392. https://doi.org/10.3390/diagnostics11081392
Chicago/Turabian StyleChen, Chien-Wei, Yuan-Hsi Tseng, Min Yi Wong, Yu-Hui Lin, Teng-Yao Yang, Yin-Chen Hsu, Bor-Shyh Lin, and Yao-Kuang Huang. 2021. "Using Non-Contrast MRA to Discriminate between Obstructive and Nonobstructive Venous Diseases of the Legs" Diagnostics 11, no. 8: 1392. https://doi.org/10.3390/diagnostics11081392
APA StyleChen, C.-W., Tseng, Y.-H., Wong, M. Y., Lin, Y.-H., Yang, T.-Y., Hsu, Y.-C., Lin, B.-S., & Huang, Y.-K. (2021). Using Non-Contrast MRA to Discriminate between Obstructive and Nonobstructive Venous Diseases of the Legs. Diagnostics, 11(8), 1392. https://doi.org/10.3390/diagnostics11081392






