The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location
Abstract
1. Introduction
2. Materials and Methods
2.1. IL-6, sCD40L, sL-Selectin, sE-Selectin and sP-Selectin Concentrations Evaluation
2.2. PLT, MPV and MCP Evaluation
2.3. CRP, CEA and CA 19-9 Evaluation
2.4. Statistical Analysis
3. Results
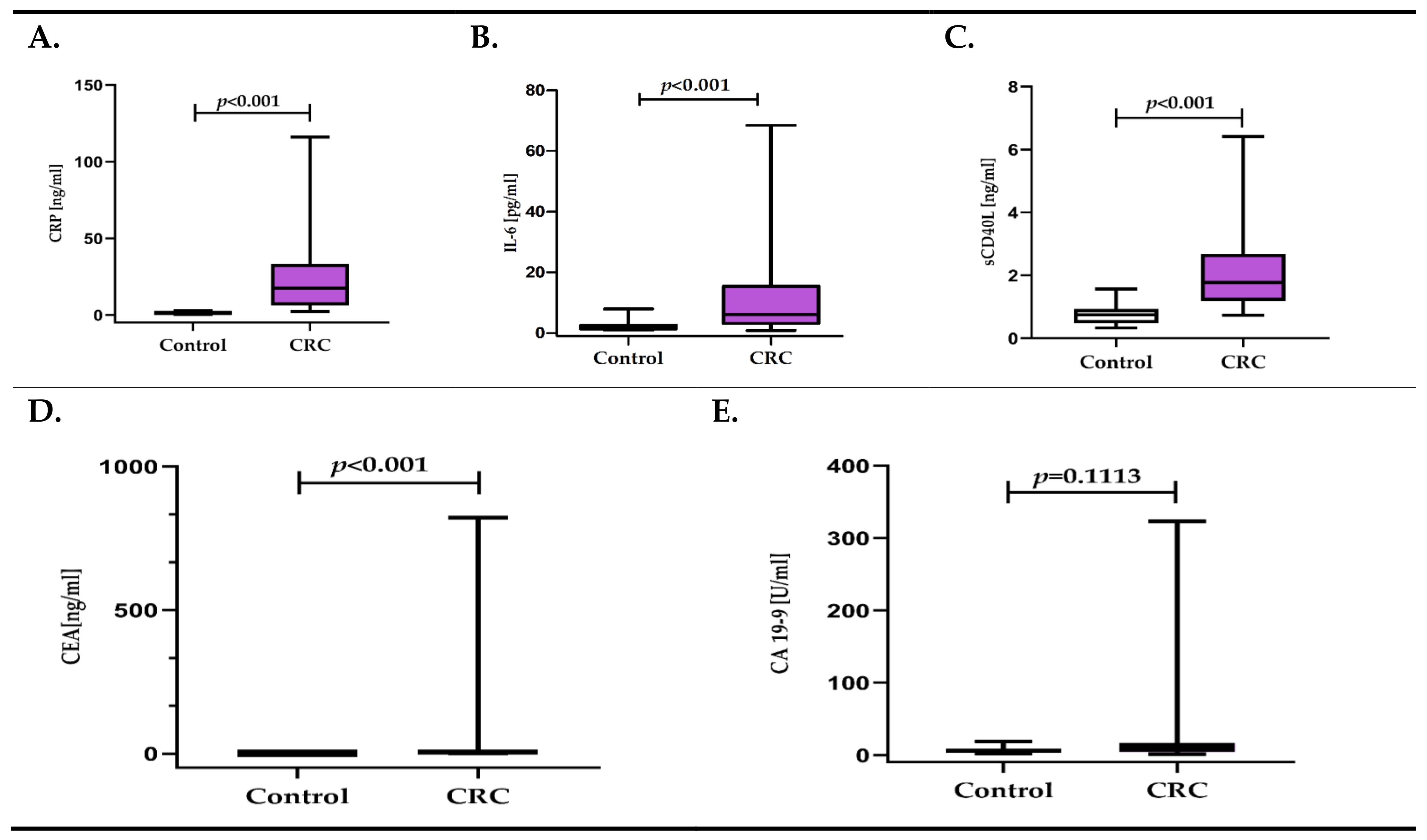
3.1. Inflammatory Response Biomarkers (CRP, IL-6, sCD40-L) and Cancer Biomarkers (CEA, CA 19-9) in CRC Patients versus Control Group
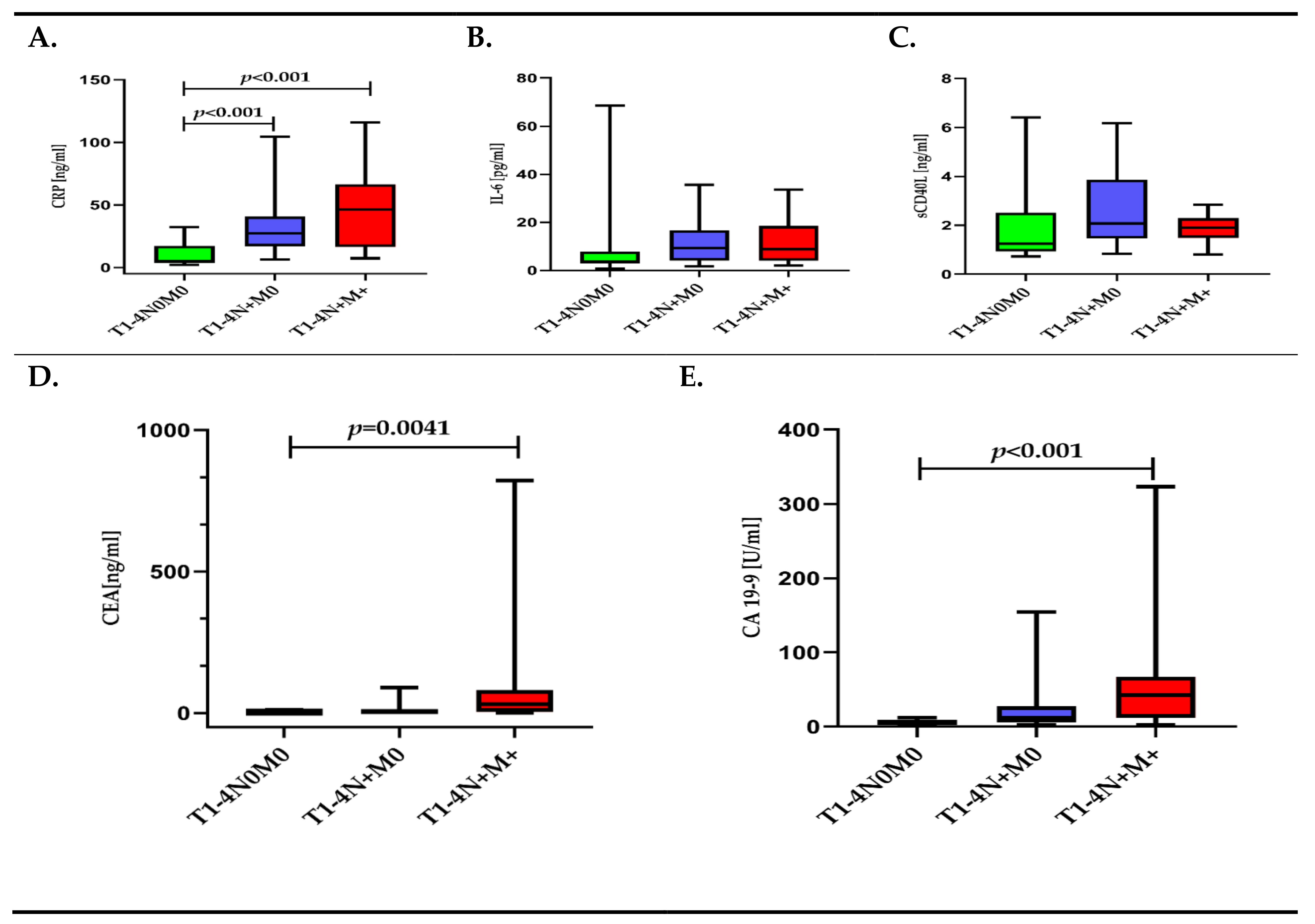
3.2. Inflammatory Response Biomarkers (CRP, IL-6, sCD40-L) and Cancer Biomarkers (CEA, CA 19-9) Depending on TNM Classification
3.3. Inflammatory Response Biomarkers (CRP, IL-6, sCD40-L) and Cancer Biomarkers (CEA, CA 19-9) Depending on the Presence of Metastasis
3.4. Inflammatory Response Biomarkers (CRP, IL-6, sCD40-L) and Cancer Biomarkers (CEA, CA 19-9) Depending on WHO Grade
3.5. Univariate and Multivariate Linear Regression Analysis for CRP
3.6. Univariate and Multivariate Linear Regression Analysis for IL-6
3.7. Univariate and Multivariate Linear Regression Analysis for sCD40L
3.8. Diagnostic Utility of Inflammatory Response Biomarkers (CRP, IL-6, sCD40L) and Cancer Biomarkers (CEA, CA 19-9)
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, P.H.; Pan, Y.P.; Fan, C.W.; Tseng, W.K.; Huang, J.S.; Wu, T.H.; Chou, W.C.; Wang, C.H.; Yeh, K.Y. Pretreatment serum interleukin-1β, interleukin-6, and tumor necrosis factor-α levels predict the progression of colorectal cancer. Cancer Med. 2016, 5, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Nasioulas, G.A.; Kosmidis, P. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737. [Google Scholar] [PubMed]
- Wang, C.-S.; Sun, C.-F. C-reactive protein and malignancy: Clinico-pathological association and therapeutic implication. Chang. Gung. Med. J. 2009, 32, 471–482. [Google Scholar] [PubMed]
- Pathak, S.; Nunes, Q.M.; Daniels, I.R.; Smart, N.J. Is C-reactive protein useful in prognostication for colorectal cancer? A systematic review. Color. Dis. 2014, 16, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Miki, C.; Konishi, N.; Ojima, E.; Hatada, T.; Inoue, Y.; Kusunoki, M. C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig. Dis. Sci. 2004, 49, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Matsumata, T.; Sugimachi, K. Preoperative Elevation of Serum C-Reactive Protein Is Related to Impaired Immunity in Patients With Colorectal Cancer. Am. J. Clin. Oncol. Cancer Clin. Trial. 2000, 23, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, K.; Ebrahim, S.; Lawlor, D.A. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J. Epidemiol. Community Health 2007, 61, 824–832. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Naugler, W.E.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef]
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer 2014, 110, 469–478. [Google Scholar] [CrossRef]
- Coward, J.; Kulbe, H.; Chakravarty, P.; Leader, D.; Vassileva, V.; Leinster, D.A.; Thompson, R.; Schioppa, T.; Nemeth, J.; Vermeulen, J.; et al. Interleukin-6 as a Therapeutic Target in Human Ovarian Cancer. Clin. Cancer Res. 2011, 17, 6083–6096. [Google Scholar] [CrossRef]
- Santer, F.; Malinowska, K.; Culig, Z.; Cavarretta, I.T. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr. Relat. Cancer 2010, 17, 241–253. [Google Scholar] [CrossRef]
- Tsui, K.-H.; Wang, S.-W.; Chung, L.-C.; Feng, T.-H.; Lee, T.-Y.; Chang, P.-L.; Juang, H.-H. Mechanisms by Which Interleu-kin-6 Attenuates Cell Invasion and Tumorigenesis in Human Bladder Carcinoma Cells. Biomed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Kim, D.-K.; Oh, S.Y.; Kwon, H.-C.; Lee, S.A.; Kwon, K.; Kim, B.G.; Kim, S.-G.; Kim, S.-H.; Jang, J.S.; Kim, M.C.; et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer 2009, 9, 155. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, M. Autocrine IL-6 Signaling: A Key Event in Tumorigenesis? Cancer Cell 2008, 13, 7–9. [Google Scholar] [CrossRef]
- Johnson, C.; Han, Y.; Hughart, N.; McCarra, J.; Alpini, G.; Meng, F. Interleukin-6 and its recepA tor, key players in hepato-biliary inflammation and cancer. Transl. Gastrointest. Cancer 2012, 1, 58–70. [Google Scholar]
- Ara, T.; DeClerck, Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer 2010, 46, 1223–1231. [Google Scholar] [CrossRef]
- Rossi, J.-F.; Lu, Z.-Y.; Jourdan, M.; Klein, B. Interleukin-6 as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1248–1257. [Google Scholar] [CrossRef]
- Kamińska, J.; Koper, O.M.; Dymicka-Piekarska, V.; Motybel-Iwańczuk, E.; Ołdziej, A.; Kemona, H. Serum soluble CD40L concentration depending on the stage of multiple myeloma and its correlation with selected angiogenic cytokines. Pol. Arch. Intern. Med. 2016, 126, 321–329. [Google Scholar] [CrossRef][Green Version]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Schönbeck, U.; Mach, F.; Sukhova, G.K.; Murphy, C.; Bonnefoy, J.-Y.; Fabunmi, R.P.; Libby, P. Regulation of Matrix Metallo-proteinase Expression in Human Vascular Smooth Muscle Cells by T Lymphocytes. Circ. Res. 1997, 81, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. Molecular biology of Hodgkin lymphoma. Hematology 2009, 2009, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bereznaya, N.M.; Chekhun, V.F. Expression of CD40 and CD40L on tumor cells: The role of their interaction and new ap-proach to immunotherapy. Exp. Oncol. 2007, 29, 2–12. [Google Scholar] [PubMed]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef]
- Wang, L.-C.S.; Thomsen, L.; Sutherland, R.; Reddy, C.B.; Tijono, S.M.; Chen, C.-J.J.; Angel, C.E.; Dunbar, P.R.; Ching, L.-M. Neutrophil Influx and Chemokine Production during the Early Phases of the Antitumor Response to the Vascular Disrupting Agent DMXAA (ASA404). Neoplasia 2009, 11, 793–803. [Google Scholar] [CrossRef]
- Tada, N.; Tsuno, N.H.; Kawai, K.; Murono, K.; Nirei, T.; Ishihara, S.; Sunami, E.; Kitayama, J.; Watanabe, T. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncol. Rep. 2013, 31, 463–471. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Korniluk, A.; Gryko, M.; Siergiejko, E.; Kemona, H. Potential Role of Soluble CD40 Ligand as In-flammatory Biomarker in Colorectal Cancer Patients. Int. J. Biol. Markers 2014, 29, 261–267. [Google Scholar] [CrossRef]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017; ISBN 9788578110796. [Google Scholar]
- Gómez, A.C.-P. Interleukins and colon cancer. Rev. Española Enfermedades Dig. 2005, 97, 613–618. [Google Scholar] [CrossRef]
- Malicki, S.; Winiarski, M.; Matlok, M.; Kostarczyk, W.; Guzdek, A.; Konturek, P.C. IL-6 and IL-8 responses of colorectal cancer in vivo and in vitro cancer cells subjected to simvastatin. J. Physiol. Pharmacol. 2009, 60, 141–146. [Google Scholar]
- Liu, H.; Shen, J.; Lu, K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 239–244. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Kemona, H.; Guzinska-Ustymowicz, K. Does surgery affect certain mediators of thrombocytopoiesis in patients with colorectal cancer? Hepatogastroenterology 2007, 54, 1407–1411. [Google Scholar]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Sato, M.; Takeyama, H. Preoperative Serum Interleukin-6 Is a Po-tential Prognostic Factor for Colorectal Cancer, including Stage II Patients. Gastroenterol. Res. Pract. 2016, 2016, 1–8. [Google Scholar]
- Galizia, G.; Orditura, M.; Romano, C.; Lieto, E.; Castellano, P.; Pelosio, L.; Imperatore, V.; Catalano, G.; Pignatelli, C.; De Vita, F. Prognostic Significance of Circulating IL-10 and IL-6 Serum Levels in Colon Cancer Patients Undergoing Surgery. Clin. Immunol. 2002, 102, 169–178. [Google Scholar] [CrossRef]
- Benson, A.B.; Bekaii-Saab, T.; Chan, E. Metastatic colon cancer, version 3.2013: Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2013, 11, 141–152. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Matowicka-Karna, J.; Gryko, M.; Kemona-Chętnik, I.; Kemona, H. Relationship between soluble P-selectin and inflammatory factors (interleukin-6 and C-reactive protein) in colorectal cancer. Thromb. Res. 2007, 120, 585–590. [Google Scholar] [CrossRef]
- Danese, S.; Sans, M.; Fiocchi, C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut 2004, 53, 1035–1043. [Google Scholar] [CrossRef]
- Korniluk, A.; Kemona, H.; Dymicka-Piekarska, V. Multifunctional CD40L: Pro- and anti-neoplastic activity. Tumor Biol. 2014, 35, 9447–9457. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.-C.; Pang, X.-Q.; Hua, C.; Li, L.; Zhang, X.-G. Expression of CD40 and CD40L in Gastric Cancer Tissue and Its Clinical Significance. Int. J. Mol. Sci. 2009, 10, 3900–3917. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Qian, K.; Qi, C. Role of vascular endothelial growth factor receptor in the pro-proliferation activity of CD40-CD40L in AGS gastric cancer cells. Asian Biomed 2010, 4, 797–802. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Jochems, C.; Talaie, T.; Anderson, A.; Jales, A.; Tsang, K.Y.; Madan, R.A.; Gulley, J.L.; Schlom, J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012, 120, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Büning, C.; Krüger, K.; Sieber, T.; Schoeler, D.; Schriever, F. Increased expression of CD40 ligand on activated T cells of pa-tients with colon cancer. Clin. Cancer Res. 2002, 8, 1147–1151. [Google Scholar] [PubMed]
- Georgopoulos, N.T.; Merrick, A.; Scott, N.; Selby, P.J.; Melcher, A.; Trejdosiewicz, L.K. CD40-mediated death and cytokine secretion in colorectal cancer: A potential target for inflammatory tumour cell killing. Int. J. Cancer 2007, 121, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Mineo, T.C.; Basili, S.; Martini, F.; Mariotti, S.; Aloe, S.; Del Monte, G.; Ambrogi, V.; Spila, A.; Palmirotta, R.; et al. Soluble CD40 Ligand Plasma Levels in Lung Cancer. Clin. Cancer Res. 2004, 10, 610–614. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Canna, K.; McArdle, C.S. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. BJS 2003, 90, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liddle, F.J.; Mahajan, S.; Frank, D.A. IL-6-induced survival of colorectal carcinoma cells is inhibited by butyrate through down-regulation of the IL-6 receptor. Carcinogenesis 2004, 25, 2247–2255. [Google Scholar] [CrossRef]
- Becker, C.; Fantini, M.C.; Schramm, C.; Lehr, H.A.; Wirtz, S.; Nikolaev, A.; Burg, J.; Strand, S.; Kiesslich, R.; Huber, S.; et al. TGF-β Suppresses Tumor Progression in Colon Cancer by Inhibition of IL-6 trans-Signaling. Immunity 2004, 21, 491–501. [Google Scholar] [CrossRef]
- Koper-Lenkiewicz, O.M.; Kamińska, J.; Milewska, A.; Sawicki, K.; Jadeszko, M.; Mariak, Z.; Reszeć, J.; Dymicka-Piekarska, V.; Matowicka-Karna, J. Serum and cerebrospinal fluid Neudesin concentration and Neudesin Quotient as potential circu-lating biomarkers of a primary brain tumor. BMC Cancer 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Yamamoto, M.; Saito, H.; Uejima, C.; Tanio, A.; Takaya, S.; Sakamoto, T.; Honjo, S.; Maeta, Y.; Ashida, K.; Fujiwara, Y. Prognostic value of the combination of pre- and postoperative C-reactive protein in colorectal cancer patients. Surg. Today 2018, 48, 986–993. [Google Scholar] [CrossRef]
- Song, M.; Mehta, R.S.; Wu, K.; Fuchs, C.S.; Ogino, S.; Giovannucci, E.L.; Chan, A.T. Plasma Inflammatory Markers and Risk of Advanced Colorectal Adenoma in Women. Cancer Prev. Res. 2015, 9, 27–34. [Google Scholar] [CrossRef]
- Nikiteas, N.I.; Tzanakis, N.; Gazouli, M.; Rallis, G.; Daniilidis, K.; Theodoropoulos, G. Serum IL-6, TNFα and CRP levels in Greek colorectal cancer patients: Prognostics implications. World J. Gastroenterol. 2005, 11, 1639–1643. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K. Antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase be markers of colorectal cancer advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]



| Variable | Group A | Group B | Group C |
|---|---|---|---|
| T1-4N0M0 | T1-4N+M0 | T1-4N+M+ | |
| n = 25 | n = 16 | n = 14 | |
| Age | 71 (60–77) | 60 (54–70) | 69 (63–73) |
| Sex | |||
| male/female | 11/14 | 7/9 | 8/6 |
| Grading | |||
| G1/G2/G3 | 2/19/4 | 2/10/4 | 0/11/3 |
| Depth of tumor invasion | |||
| T1-T2 | 7 | 1 | 1 |
| T3-T4 | 18 | 15 | 13 |
| Lymph node | |||
| N0 (absent) | 25 | 0 | 0 |
| N1+2 (present) | 0 | 16 | 14 |
| Distant metastasis | |||
| M0 (absent) | 25 | 16 | 0 |
| M1 (present) | 0 | 0 | 14 |
| Tumor location | |||
| Proximal colon | 9 | 7 | 7 |
| Distal colon | 7 | 6 | 3 |
| Rectum | 9 | 3 | 4 |
| Tumor size | |||
| ≤3 cm | 14 | 1 | 4 |
| >3 cm | 11 | 15 | 10 |
| No. | Covariate | Univariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| 1 | PLT [x103/µL] | 0.003 | 1.003 (1.000–1.006) | 0.030 |
| 2 | CA 19-9 [U/mL] | 0.006 | 1.007 (1.001–1.012) | 0.019 |
| WHO 1 | base group | |||
| 3 | WHO 2 | 1.290 | 3.633 (2.144–6.151) | <0.001 |
| 4 | WHO 3 | 1.592 | 4.916 (2.838–8.516) | <0.001 |
| 5 | Metastases (T1-4N+M+) | 1.431 | 4.183 (2.679–6.531) | <0.001 |
| Distal colon tumor location | base group | |||
| 6 | Rectum tumor location | 0.601 | 1.824 (0.864–3.851) | 0.112 |
| 7 | Proximal colon tumor location | 0.777 | 2.175 (1.093–4.327) | 0.028 |
| 8 | Tumor size | 0.713 | 2.040 (1.130–3.685) | 0.019 |
| No. | Covariate | Multivariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| WHO 1 | base group | |||
| 1 | WHO 2 | 1.367 | 3.924 (2.386–6.456) | <0.001 |
| 2 | WHO 3 | 1.552 | 4.721 (2.819–7.906) | <0.001 |
| Distal colon tumor location | base group | |||
| 3 | Rectum tumor location | 0.761 | 2.139 (1.235–3.706) | 0.008 |
| 4 | Proximal colon tumor location | 0.692 | 1.998 (1.209–3.303) | 0.008 |
| No. | Covariate | Univariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| 1 | PLT [x103/µL] | 0.004 | 1.004 (1.001–1.006) | 0.008 |
| 2 | MPC [g/dL] | 0.268 | 1.307 (1.050–1.626) | 0.018 |
| 3 | sE-selectin [ng/mL] | 0.022 | 1.022 (1.005–1.039) | 0.011 |
| 4 | CRP [ng/mL] | 0.012 | 1.012 (1.003–1.022) | 0.007 |
| 5 | Metastases (T1-4N+M+) | 0.546 | 1.726 (1.054–2.828) | 0.031 |
| No. | Covariate | Multivariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| 1 | PLT [x103/µL] | 0.004 | 1.004 (1.001–1.007) | 0.005 |
| 2 | MPC [g/dL] | 0.250 | 1.284 (1.051–1.568) | 0.016 |
| No. | Covariate | Univariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| 1 | PLT [x103/µL] | 0.002 | 1.002 (1.001–1.004) | 0.002 |
| 2 | MPC [g/dL] | 0.143 | 1.153 (1.031–1.290) | 0.014 |
| WHO 1 | base group | |||
| 3 | WHO 2 | 0.395 | 1.484 (1.062–2.074) | 0.022 |
| 4 | WHO 3 | 0.147 | 1.158 (0.817–1.641) | 0.403 |
| No. | Covariate | Multivariate Linear Regression Analysis | ||
| β | eβ (95%CI) | p-Value | ||
| 1 | PLT [x103/µL] | 0.002 | 1.002 (1.001–1.004) | 0.001 |
| 2 | MPC [g/dL] | 0.132 | 1.141 (1.034–1.259) | 0.010 |
| AUC ± SE | p-Value | Youden Index | Cut-Off | Sensitivity | Specificity | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|
| A: Discriminating CRC Patients from Healthy Controls | |||||||
| CRP | 0.996 ± 0.004 | <0.001 * | 0.94 | 2.43 | 98% | 96% | 98% |
| IL-6 | 0.864 ± 0.042 | <0.001 * | 0.69 | 2.88 | 84% | 85% | 84% |
| sCD40L | 0.920 ± 0.029 | <0.001 * | 0.65 | 1.20 | 73% | 93% | 79% |
| CEA | 0.771 ± 0.054 | <0.001 * | 0.56 | 2.50 | 67% | 89% | 75% |
| B: Discriminating CRC Patients without Metastases from Patients with Lymph Node and Distant Metastases | |||||||
| CRP | 0.885 ± 0.043 | <0.001 * | 0.61 | 19.52 | 73% | 88% | 80% |
| IL-6 | 0.671 ± 0.075 | 0.0229 * | 0.41 | 4.31 | 77% | 64% | 71% |
| CEA | 0.686 ± 0.079 | 0.0179 * | 0.42 | 9.44 | 46% | 96% | 69% |
| CA 19-9 | 0.808 ± 0.064 | <0.001 * | 0.55 | 11.60 | 65% | 90% | 76% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koper-Lenkiewicz, O.M.; Dymicka-Piekarska, V.; Milewska, A.J.; Zińczuk, J.; Kamińska, J. The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location. Diagnostics 2021, 11, 1382. https://doi.org/10.3390/diagnostics11081382
Koper-Lenkiewicz OM, Dymicka-Piekarska V, Milewska AJ, Zińczuk J, Kamińska J. The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location. Diagnostics. 2021; 11(8):1382. https://doi.org/10.3390/diagnostics11081382
Chicago/Turabian StyleKoper-Lenkiewicz, Olga Martyna, Violetta Dymicka-Piekarska, Anna Justyna Milewska, Justyna Zińczuk, and Joanna Kamińska. 2021. "The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location" Diagnostics 11, no. 8: 1382. https://doi.org/10.3390/diagnostics11081382
APA StyleKoper-Lenkiewicz, O. M., Dymicka-Piekarska, V., Milewska, A. J., Zińczuk, J., & Kamińska, J. (2021). The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location. Diagnostics, 11(8), 1382. https://doi.org/10.3390/diagnostics11081382








