The Natural History of Uterine Venous Plexus Thrombosis
Abstract
:1. Introduction
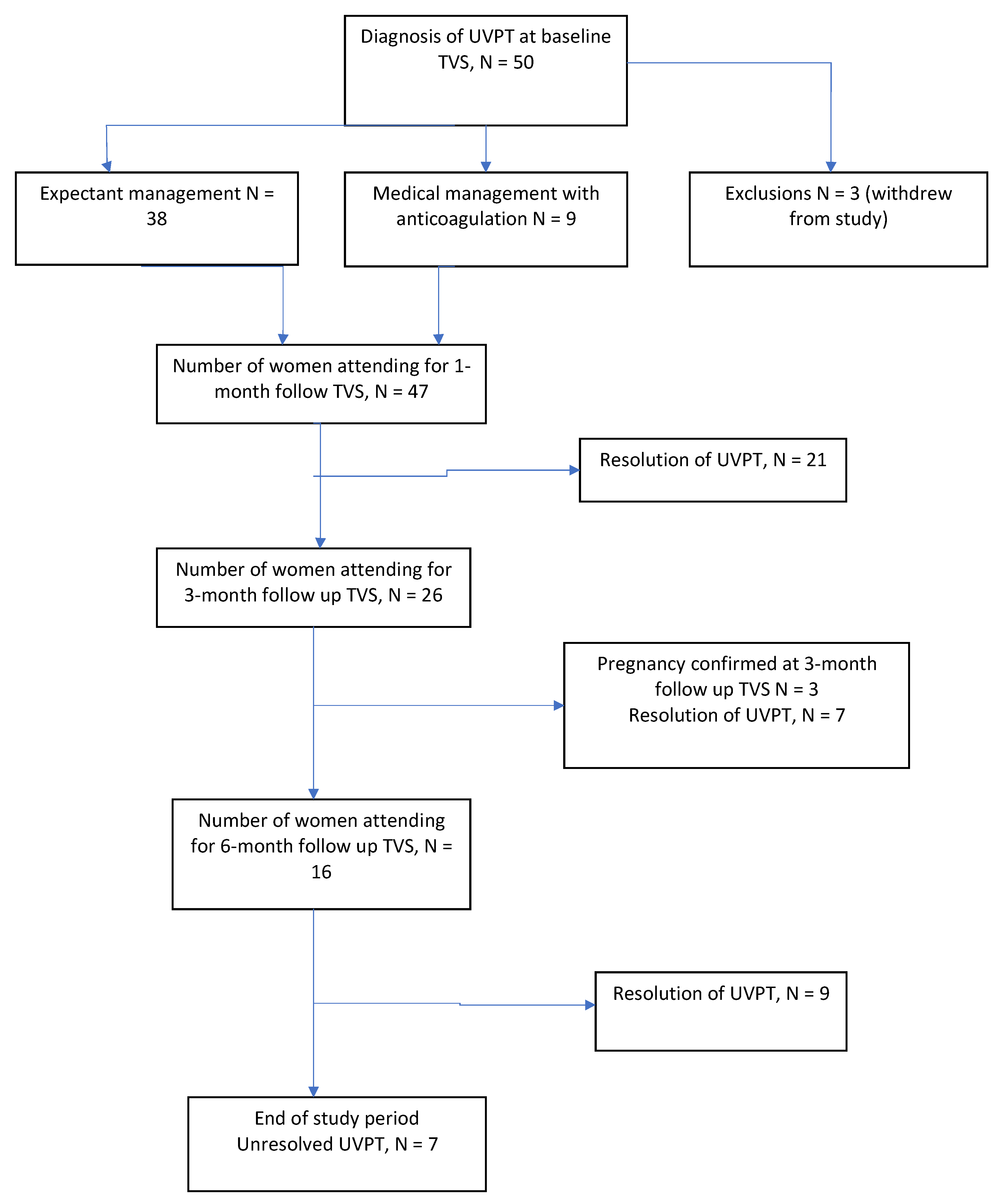
2. Methods
Statistical Analysis
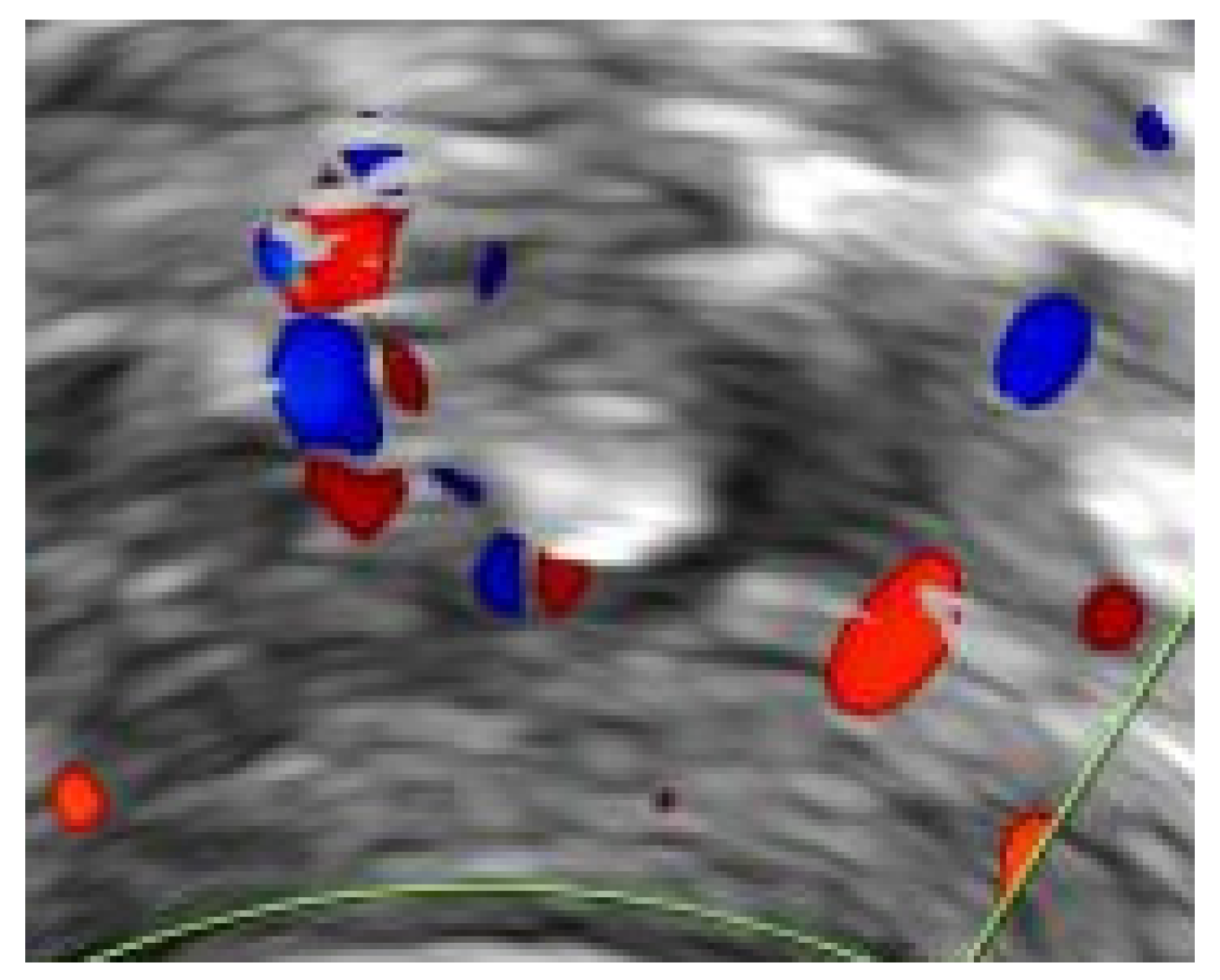
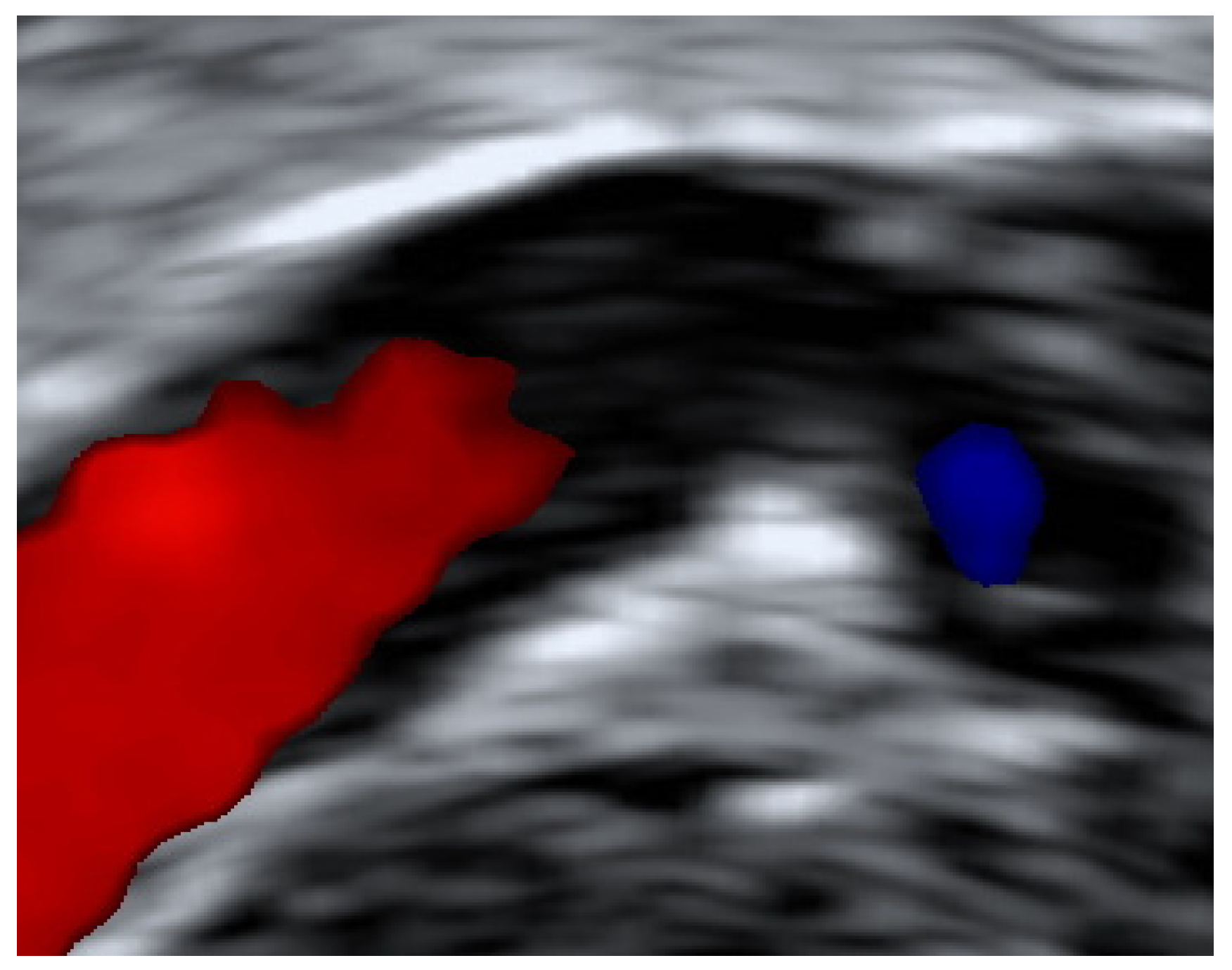
3. Results
3.1. Patients
3.2. Management
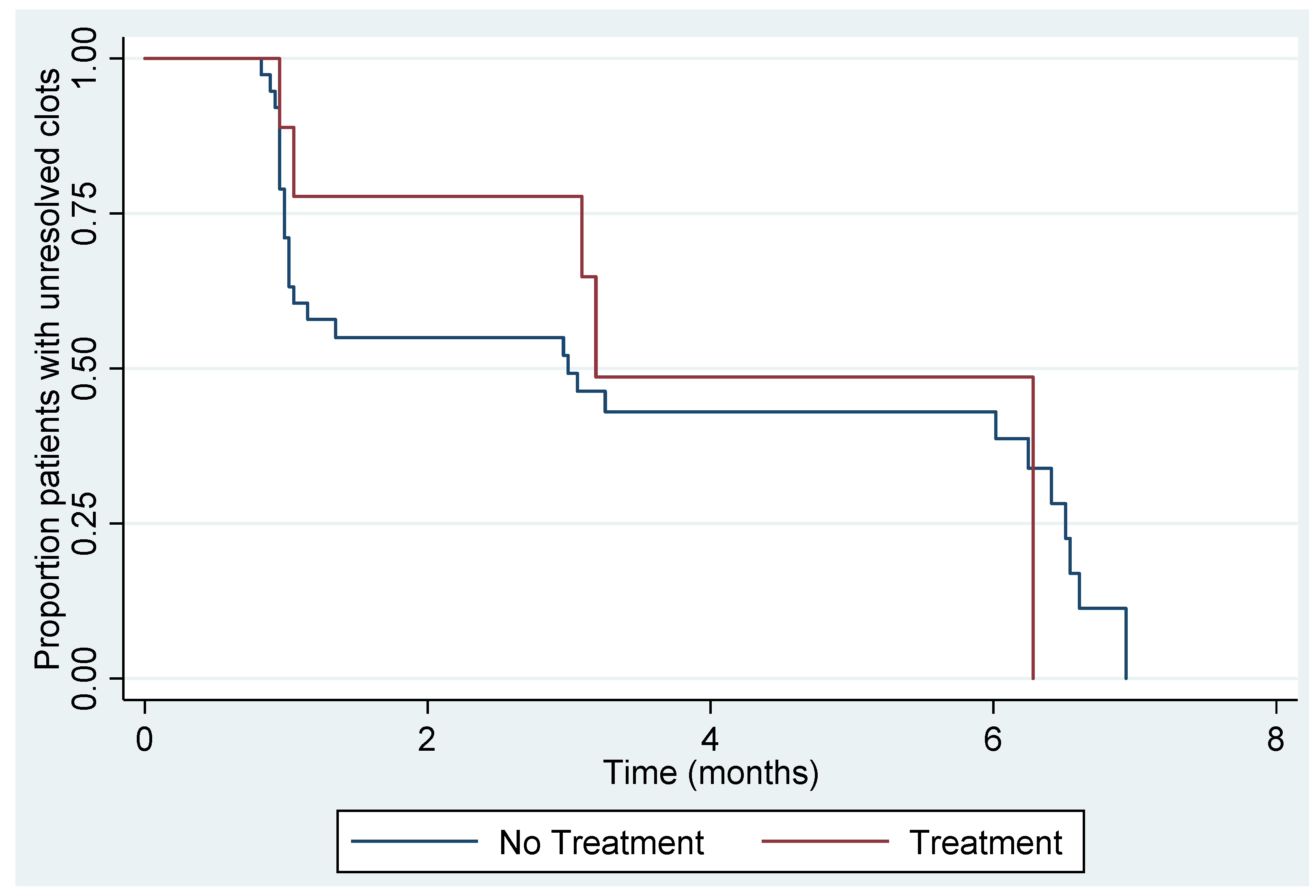
3.3. Resolution of UVPT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masuda, E.M.; Kessler, D.M.; Kistner, R.L.; Eklof, B.; Sato, D.T. The natural history of calf vein thrombosis: Lysis of thrombi and development of reflux. J. Vasc. Surg. 1998, 28, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Amin, T.N.; Cohen, H.; Wong, M.; Goodhart, V.; Pointer, S.-L.; Jurkovic, D. The prevalence of uterine venous plexus thrombosis. J. Thromb Haemost. 2020, 18, 2557–2565. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Dejani, S.; Shapiro, I.; Tal, J.; Paz, B.; Levitan, Z.; Aharoni, A.; Toubi, A.; Schliamser, L.; Bar-Meir, E.; et al. Diagnosis of pregnancy associated uterine venous plexus thrombosis on the basis of transvaginal sonography. J. Ultrasound Med. 2003, 22, 287–293. [Google Scholar] [CrossRef]
- Mavrelos, D.; Cohen, H.; Pateman, K.; Hoo, W.; Foo, X.; Jurkovic, D. Diagnosis of uterine vein thrombosis on transvaginal ultrasound. Ultrasound Obstet. Gynecol. 2013, 42, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Shiomi, H.; Morimoto, T.; Yoneda, T.; Yamada, C.; Makiyama, T.; Kato, T.; Saito, N.; Shizuta, S.; Ono, K.; et al. Asymptomatic Lower Extremity Deep Vein Thrombosis-Clinical Characteristics, Management Strategies, and Long-Term Outcomes. Circ. J. 2017, 81, 1936–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, T.N.; Wong, M.; Pointer, S.; Goodhart, V.; Bean, E.; Jurkovic, D. Reference ranges for uterine vein dimensions in non-pregnant women with normal pelvic organs. Ultrasound Obstet. Gynecol. 2019, 54, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Killewich, L.A.; Macko, R.F.; Cox, K.; Franklin, D.R.; Benjamin, M.E.; Lilly, M.P.; Flinn, W.R. Regression of proximal deep venous thrombosis is associated with fibrinolytic enhancement. J. Vasc. Surg. 1997, 26, 861–868. [Google Scholar] [CrossRef] [Green Version]
- O’Shaughnessy, A.M.; Fitzgerald, D.E. Determining the stage of organisation and natural history of venous thrombosis using computer analysis. Int. Angiol. 2000, 19, 220–227. [Google Scholar] [PubMed]
- Zhao, L.; Prior, S.J.; Kampmann, M.; Sorkin, J.D.; Caldwell, K.; Braganza, M.; McEvoy, S.; Lal, B.K. Measurement of thrombus resolution using three-dimensional ultrasound assessment of deep vein thrombosis volume. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 140–147. [Google Scholar] [CrossRef]
- Murphy, T.P.; Cronan, J.J. Evolution of deep venous thrombosis: A prospective evaluation with US. Radiology 1990, 177, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C. Natural history of venous thromboembolism. Circulation 2003, 107 (Suppl. 1), I22–I30. [Google Scholar] [CrossRef] [Green Version]
- Van Ramshorst, B.; van Bemmelen, P.S.; Hoeneveld, H.; Faber, J.A.; Eikelboom, B.C. Thrombus regression in deep venous thrombosis. Quantification of spontaneous thrombolysis with duplex scanning. Circulation 1992, 86, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markel, A.; Meissner, M.; Manzo, R.A.; Bergelin, R.O.; Strandness, D.E., Jr. Deep venous thrombosis: Rate of spontaneous lysis and thrombus extension. Int. Angiol. 2003, 22, 376–382. [Google Scholar]
- Sartori, M.; Lessiani, G.; Favaretto, E.; Migliaccio, L.; Iotti, M.; Giusto, L.; Ghirarduzzi, A.; Palareti, G.; Cosmi, B. Ultrasound Characteristics of Calf Deep Vein Thrombosis and Residual Vein Obstruction After Low Molecular Weight Heparin Treatment. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandoni, P.; Lensing, A.W.; Prins, M.H.; Bernardi, E.; Marchiori, A.; Bagatella, P.; Frulla, M.; Mosena, L.; Tormene, D.; Piccioli, A.; et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann. Intern. Med. 2002, 137, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.S.; Anderson, D.R.; Roger, M.; Forgie, M.; Keoron, C.; Dreyer, J.; Kovacs, G.; Mitchell, M.; Lewandowski, B.; Kovacs, M.J. Evaluation of D-dimer in the diagnosis of suspected deep vein thrombosis. N. Engl. J. Med. 2003, 349, 1227–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palareti, G.; Cosmi, B.; Lessiani, G.; Rodorigo, G.; Guazzaloca, G.; Brusi, C.; Valdre, L.; Conti, E.; Sartori, M.; Legnani, C. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: The blind, prospective CALTHRO study. Thromb. Haemost. 2010, 104, 1063–1070. [Google Scholar]
- Kuruvilla, J.; Wells, P.S.; Morrow, B.; MacKinnon, K.; Keeney, M.; Kovacs, M.J. Prospective assessment of the natural history of positive D-dimer results in persons with acute venous thromboembolism (DVT or PE). Thromb. Haemost. 2003, 89, 284–287. [Google Scholar] [CrossRef]
- Khalil, H.; Avruch, L.; Olivier, A.; Walker, M.; Rodger, M. The natural history of pelvic vein thrombosis on magnetic resonance venography after vaginal delivery. Am. J. Obstet. Gynecol. 2012, 206, 356.e1–356.e4. [Google Scholar] [CrossRef]
- Labropoulos, N.; Malgor, R.D.; Comito, M.; Gasparis, A.P.; Pappas, P.J.; Tassiopoulos, A.K. The natural history and treatment outcomes of symptomatic ovarian vein thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Macdonald, P.S.; Kahn, S.R.; Miller, N.; Obrand, D. Short-term natural history of isolated gastrocnemius and soleal vein thrombosis. J. Vasc. Surg. 2003, 37, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkitt, D.P.; Latto, C.; Janvrin, S.B.; Mayou, B. Pelvis phleboliths epidemiology and postulated etiology. N. Engl. J. Med. 1977, 296, 1387–1389. [Google Scholar] [CrossRef]
- Gottlieb, R.H.; Poster, R.; Subudhi, M.K. Computed tomographic, ultrasound, and plain film appearance of phleboliths in varicoceles. J. Ultrasound Med. 1989, 8, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Baglin, T. What happens after venous thromboembolism? J. Thromb. Haemost. 2009, 7 (Suppl. 1), 287–290. [Google Scholar] [CrossRef] [PubMed]


| Demographic Risk Factors | UVPT (n = 50) |
|---|---|
| Age (years) | 44 (21–81 years) |
| Parity | |
| 0 | 9 (18%) |
| 1 | 11 (22%) |
| 2 | 11 (22%) |
| 3+ | 19 (38%) |
| BMI category | |
| Normal weight (BMI 18.5–24.9) | 26 (52%) |
| Overweight (BMI 25–29.9) | 10 (20%) |
| Obese (BMI > 30) | 14 (28%) |
| Smoking | |
| Never | 31 (62%) |
| Current/Former | 8 (16%) |
| Ethnicity | |
| White | 32 (64%) |
| Asian | 3 (6%) |
| Black | 11 (22%) |
| Middle Eastern | 2 (4%) |
| Mixed/Other | 2 (4%) |
| Menopausal status | |
| Pre-menopausal | 43 (86%) |
| Post-menopausal | 7 (14%) |
| Medical risk factors | |
| Current Hormone use (#) | 10 (20%) |
| Recent surgery (within four months) | 7 (14%) |
| Recent immobilisation/travel (within six weeks) | 3 (6%) |
| Personal history of thrombophilia (†) | 2 (4%) |
| Personal history of VTE (*) | 1 (2%) |
| Family history of DVT/PE (**) | 2 (4%) |
| Varicose veins of the legs | 13 (26%) |
| Pelvic abnormalities on ultrasound as risk factors | |
| Fibroids | 12 (24%) |
| Adenomyosis | 20 (40%) |
| Adnexal/ovarian cyst (all ipsilateral) | 3 (6%) |
| History or diagnosis of endometriosis | 3 (6%) |
| Uterine vein diameter > 95th centile for age/parity | 4 (8%) |
| Timepoint | Women at Risk (N) | Kaplan-Meier Unresolved Estimate (95% CI) |
|---|---|---|
| 0 months | 47 | - |
| 1 months | 26 | 59% (44%, 72%) |
| 3 months | 16 | 45% (30%, 59%) |
| 6 months | 7 | 15% (4% to 33%) |
| Variable | Category | Resolved n/N | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Treatment with anticoagulation | No | 28/38 | 1 | 0.64 |
| Yes | 5/9 | 0.79 (0.30, 2.09) | ||
| Initial diameter of thrombus (mm) (+) | - | - | 0.95 (0.84, 1.06) | 0.36 |
| Abnormal | No | 19/23 | 1 | 0.69 |
| thrombophilia (any) | Yes | 14/24 | 0.86 (0.41, 1.79) | |
| D-dimer level (diagnosis) (***) | - | - | 1.00 (0.92, 1.08) | 0.92 |
| Age (**) | - | - | 1.01 (0.70, 1.45) | 0.96 |
| Parity | 0 | 5/8 | 1 | 0.80 |
| 1–2 | 16/20 | 1.15 (0.42, 3.20) | ||
| 3+ | 12/19 | 0.89 (0.31, 2.56) | ||
| BMI (*) | - | - | 0.85 (0.64, 1.14) | 0.28 |
| Family history DVT | No | 32/45 | 1 | 0.76 |
| Yes | 1/2 | 0.73 (0.10, 5.45) | ||
| Menopausal status | Pre | 29/40 | 1 | 0.66 |
| Post | 4/7 | 1.27 (0.44, 3.70) | ||
| Number of clots | Single | 28/38 | 1 | 0.42 |
| Multiple | 4/5 | 0.67 (0.26, 1.76) | ||
| Vessel diameter (#) | - | 0.87 (0.72, 1.06) | 0.17 | |
| Flow velocity (#) | <5 cm/s | 24/35 | 1 | 0.94 |
| ≥5 cm/s | 9/12 | 0.97 (0.43, 2.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, T.N.; Cohen, H.; Wong, M.; Pointer, S.-L.; Aslam, N.; Jurkovic, D. The Natural History of Uterine Venous Plexus Thrombosis. Diagnostics 2021, 11, 1338. https://doi.org/10.3390/diagnostics11081338
Amin TN, Cohen H, Wong M, Pointer S-L, Aslam N, Jurkovic D. The Natural History of Uterine Venous Plexus Thrombosis. Diagnostics. 2021; 11(8):1338. https://doi.org/10.3390/diagnostics11081338
Chicago/Turabian StyleAmin, Tejal N., Hannah Cohen, Michael Wong, Sara-Louise Pointer, Naaila Aslam, and Davor Jurkovic. 2021. "The Natural History of Uterine Venous Plexus Thrombosis" Diagnostics 11, no. 8: 1338. https://doi.org/10.3390/diagnostics11081338
APA StyleAmin, T. N., Cohen, H., Wong, M., Pointer, S.-L., Aslam, N., & Jurkovic, D. (2021). The Natural History of Uterine Venous Plexus Thrombosis. Diagnostics, 11(8), 1338. https://doi.org/10.3390/diagnostics11081338






