Abstract
The cobas® EGFR Test provides a semiquantitative index (SQI) that reflects the proportion of mutated versus wild-type copies of the EGFR gene in plasma. The significance of SQI as an indirect measure of the variant allele frequency (VAF) or mutated copies/mL remains unclear. The aim of this study was to evaluate the correlation of SQI with the VAF and the number of mutated copies/mL obtained by a digital droplet PCR (ddPCR) test in NSCLC samples. The study included 118 plasma samples from a retrospective cohort of 25 stage IV adenocarcinoma patients with EGFR exon 19 deletions (Ex19Del), obtained before and during tyrosine kinase inhibitor (TKI) treatment. Both SQI and VAF and SQI and mutated copies/mL showed the same significant correlation (r2 = 0.79, p < 0.00001) across the whole study cohort. We found better correlation in samples collected at the baseline between SQI and VAF (r2 = 0.94, p < 0.00001) and SQI and mutated copies/mL (r2 = 0.97, p < 0.00001) compared to samples collected during TKI treatment: r2 = 0.76; p < 0.00001 for SQI and VAF and r2 = 0.75; p < 0.00001 for SQI and mutated copies/mL. The study indicates that SQI is a robust quantitative indirect measure of VAF and the number of mutated copies/mL in plasma from patients with an EGFR Ex19Del mutation. Further studies are desirable to assess the SQI cut-off values related to the clinical status of the patient.
1. Introduction
Molecular characterization of non-small cell lung cancer (NSCLC) has allowed a better classification of NSCLC tumors, permitting the introduction of personalized therapies that reduce toxicity and increase survival rates [1]. Recurrent genetic drivers in NSCLCs are mutations in the epidermal growth factor receptor (EGFR) gene. These mutations are present in 10 to 30% of patients [2], with deletions in exon 19 (Ex19Del) being the most common EGFR mutation [3]. EGFR tyrosine kinase inhibitors (TKIs) are currently the standard-of-care in first line therapy for patients with mutant EGFR NSCLC, with new generations of TKIs developed for patients with acquired resistance to the first and second generation TKIs [4]. This enhances the need for simplified strategies to measure the patient’s molecular response to TKI treatment to enable further treatment optimization and improved outcomes.
Tumor tissue analysis remains the gold standard to molecularly stratify advanced NSCLC patients to select optimal TKI treatment, but cell-free DNA (cfDNA) analysis is now recommended when the tissue biopsy is not available or insufficient [5]. Moreover, cfDNA from plasma is more informative than tissue analysis since it captures the heterogeneity of the tumor [6]. The analysis of cfDNA to molecularly stratify advanced NSCLC patients has proven useful in the clinical setting by our group, among others [7]. Beyond the qualitative information provided by such testing, the quantitative information of the variant allele frequency (VAF) has been proposed as a biomarker for monitoring treatment response [8,9], for minimal residual disease assessment (MRD) [10], and for relapse prediction in NSCLC patients [11].
Distinct approaches for cfDNA analysis exist such as real-time PCR (RT-PCR), beads, emulsion, amplification, and magnetics (BEAMing), droplet digital PCR (ddPCR), and next generation sequencing (NGS). These techniques differ both in terms of the sensitivity they achieve and their complexity [12]. Another technique is the commercial assay cobas® EGFR Mutation Test v2 (cobas® EGFR Test, Hoffman-La Roche Ltd., Basel, Switzerland). This test was approved by the FDA in 2016 as the first test for the identification of NSCLC patients harboring EGFR mutations in cfDNA from plasma [13] for TKI treatment selection. The cobas® EGFR Test is a RT-PCR based method that interrogates 42 mutations located in exons 18, 19, 20, and 21 of the EGFR gene [14] and provides a semiquantitative index (SQI) for the EGFR mutation, which reflects the trend for the proportion of mutated versus wild-type copies of the EGFR gene in the cfDNA. However, the cobas® EGFR Test is not approved as a quantitative test [15].
Robust and sensitive techniques are needed to translate liquid biopsy strategies to the clinical setting, since the threshold level of mutation corresponding to therapeutic implications is extremely low (VAF < 0.1%) [11,16]. The cobas® EGFR Test has a detection limit of around 0.1–1% of the VAF [17]. In comparison, ddPCR techniques have a higher VAF sensitivity with a detection limit of 0.01–0.1% while also quantifying the absolute number of EGFR mutated copies (copies/mL) [18].
The correlation between SQI provided by the cobas® EGFR Test and the number of EGFR mutated copies/mL and VAF has previously been explored with contradictory results. One study observed that SQI reflects the VAF of the EGFR mutant allele obtained by NGS methods and that dynamic changes in SQI reflect the tumor progression [19]. In contrast, Macías et al. [20] did not find a significant correlation between SQI and the number of EGFR mutated copies/mL obtained by ddPCR, concluding that the parameters are not interchangeable. One external quality assurance (EQA) program found a good correlation between SQI and VAF for EGFR Ex19Del, but showed low reproducibility of SQI when VAF was <1% [21]. Other studies have not found steady correlations between SQI and VAF for different EGFR mutations [22,23]. The reproducibility and correlation of SQI values to VAF and mutated copies/mL need to be validated in real patient material before using the data for the interpretation of clinical samples [24]. Further studies are therefore necessary to elucidate the value of SQI as a measure of VAF in EGFR mutations.
In this study, we evaluated the SQI parameter from the cobas® EGFR Test as a measure for the number of mutated copies/mL and for the VAF of mutant EGFR allele in plasma in advanced NSCLC patients harboring an Ex19Del of EGFR gene.
2. Materials and Methods
2.1. Patients and Plasma Samples
The study included 118 cfDNA samples from plasma belonging to 25 stage IV adenocarcinoma patients harboring an Ex19Del of EGFR gene. The samples were collected at Hospital Clínic of Barcelona between June 2017 and May 2019. A sample was collected at the baseline before TKI treatment in 12 patients, and in the remaining 13 patients, all samples were collected exclusively over the clinical follow-up during TKI treatment. Clinical data such as gender, age, tumor histology, molecular status in tumor, and disease stage were obtained from the patients’ medical records.
The patients belong to a prospective observational study approved by the Hospital Clínic Ethics Committee (approval registration number HCB/2016/0889). The study was conducted in accordance with the precepts of the Code of Ethics of The World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all patients.
2.2. Extraction of cfDNA from Plasma Samples
Peripheral whole blood was collected from each subject in a 5 mL EDTA-K2 tube. After 15 to 20 min at rest in an upright position at room temperature, samples were centrifuged at 1600× g for 10 min to collect 2 mL of plasma, which was transferred to a sterile tube. After a second centrifugation at 16,000× g for 10 min, plasma samples were stored at −20 C. The entire procedure was completed within three hours of blood extraction. The cfDNA was isolated using the cobas® cfDNA Sample EGFR Preparation Kit as per the manufacturer’s instructions.
The cfDNA quantification and quality assessment were performed using a Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) with the Qubit® dsDNA HS Assay Kit, and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) with the Agilent High Sensitivity DNA Assay respectively.
2.3. Molecular Characterization of EGFR in cfDNA from Plasma
Analysis of EGFR mutations in cfDNA was first assessed with the cobas® EGFR Test (Hoffman-La Roche Ltd., Basel, Switzerland), following the manufacturer’s recommendations, which provided a SQI value along with the detected EGFR mutation. The limit of detection for EGFR Ex19Del declared by the manufacturer is 75 mutated copies/mL.
A second analysis of the EGFR mutations was then performed using a ddPCRTM EGFR exon 19 Deletion Screening Kit Assay (ddPCRTM EGFR Test, Bio-Rad Laboratories, Hercules, CA, USA). The ddPCRTM EGFR Test detects 15 deletion mutations in exon 19 of the EGFR gene. The ddPCR was carried out in a reaction volume of 20 µL on a QX200TM Droplet DigitalTM PCR System (Bio-Rad). The 20 µL PCR mix contained 10 µL of 2× ddPCR Supermix for probes (No dUTP), 1 µL of 20× of the corresponding probe, 1 µL of ECOR1 enzyme, 1 µL of water, and 7 µL of plasma cfDNA. Droplets were generated by the QX200TM droplet generator and PCR was performed using a C1000TM Touch thermal cycler (Bio-Rad). Reading and analysis was executed using QuantasoftTM software (Bio-Rad). The results were reported as wild-type and the number of mutated copies/mL of plasma and the VAF of the EGFR mutation was then calculated as ((mutated copies/mL)/(total copies/mL)) × 100. The criteria to determine if the assay worked properly were: number of accepted droplets ≥13,000, good separation between the different clusters, and at least 50 copies/reaction. The limit of detection reported by the manufacturer is 0.5% of VAF.
2.4. Statistical Analysis
The agreement between the two methods was measured by calculating the Kappa coefficient. Spearman rank correlation was used to analyze the associations between SQI and VAF and SQI and the number of mutated copies/mL. Results were considered statistically significant with a p-value < 0.05. All statistical analysis was performed using STATA/IC software version 13.1 (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. Study Cohort
The 25 stage IV adenocarcinoma patients consisted of 18 females and seven males, harboring a deletion in exon 19 of the EGFR gene. We selected the patients included in the study based on a positive result obtained by the initial cobas® EGFR Test in the first sample tested.The mean age at first sample collection was 68.2 ± 13.6 years old. Overall, we analyzed 118 plasma samples ranging from 1 to 20 (Median = 3) per patient. A plasma sample was collected at baseline before TKI treatment in 12 patients (N = 12 samples), and only during follow-up in 13 patients (N = 85 samples). Nine of the 12 patients analyzed at the baseline also had at least one sample analyzed in follow-up (N = 21). The molecular status of EGFR in tumor tissue was available in 60% (15/25) of patients. In this subset of patients, five different EGFR mutations were found: four deletions and one insertion (Table 1).

Table 1.
Characteristics of lung cancer patients.
The cobas® EGFR Test and the ddPCRTM EGFR Test differ in which exon 19 deletions are evaluated. Thirteen mutations are common to both tests, while the cobas® EGFR Test includes a further 16 mutations not included in the ddPCRTM EGFR Test. The ddPCR test also analyzes two mutations not included in the cobas® EGFR Test. Based on the characterization of tumor tissue, the five EGFR mutations could be detected by the cobas® EGFR Test while two patients harbored an EGFR Ex19Del included in the cobas® EGFR Test but not included in the ddPCRTM EGFR exon 19 deletion screening kit. However, the ddPCR analysis resulted in positive droplets in samples belonging to these patients. In the patient who harbored the c.2337_2255insT EGFR mutation, positive droplets were observed in both samples analyzed. In the other patient, with the c.2240_2251del12 EGFR mutation, positive droplets were found in two out of four samples analyzed.
3.2. Agreement between Cobas® EGFR Test and the ddPCRTM EGFR Test
First, we evaluated the agreement between the cobas® EGFR Test and the ddPCR Test and found discrepancies in the positive results obtained by each method. A total of 72.0% (85/118) of samples presented an EGFR Ex19Del positive result by at least one method. We detected a positive result by the cobas® EGFR Test in 76 samples but in 26.3% (20/76) of these, the EGFR mutation was not detected by ddPCR. In all of these cases, the SQI value reported was below 12. On the other hand, we found a positive Ex19Del EGFR result by ddPCR in 65 samples, of which the EGFR mutation was not detected by the cobas® EGFR Test in 13.8% (9/65) of cases. In all these samples, the number of mutated copies was <75 copies/mL. All samples that had a positive result by the ddPCRTM EGFR Test in which the mutation was not detected by the cobas® EGFR Test were obtained during follow-up under TKI treatment.
Next, we analyzed the samples regarding Ex19Del EGFR status depending on TKI treatment status. In the subset of samples obtained at the baseline before TKI treatment, an Ex19Del EGFR mutation was detected in 100% (12/12) of patients by the cobas® EGFR Test and in 75.0% (9/12) by the ddPCRTM EGFR Test. In samples drawn after the TKI treatment was initiated, a mutation was found in 60.4% (64/106) of samples by the cobas® EGFR Test and in 52.8% (56/106) by ddPCR.
The agreement between both methods for samples at the baseline before TKI treatment was 75%, and for the follow-up samples, it was 75.5% with a Kappa coefficient of 0.50 (p < 0.00001). The overall agreement between all samples was 75.4% with a Kappa coefficient of 0.49 (p < 0.00001).
The median SQI of Ex19Del EGFR mutation was significantly lower in those samples where there was no agreement between the two methods (8.3 vs. 12.1, p < 0.00001) as well as VAF (0.2 vs. 0.8, p = 0.002) and the number of mutated copies/mL (11.7 vs. 49.0, p = 0.02).
We also evaluated the agreement between the cobas® EGFR Test and the ddPCRTM EGFR Test in the subset of samples where tissue analysis was available (N = 15 patients, 79 samples) and obtained concordant results to those of the whole study cohort. The percentage of samples that presented a positive result by at least one method was slightly higher (73.4%, 58/79), and the discrepancies between methods were slightly lower (21.2% for the mutations detected by the cobas® EGFR Test and not by ddPCR, and 12.8% the other way around).
An Ex19Del EGFR mutation was detected in 100% (6/6) of patients by the cobas® EGFR Test and in 66.7% (4/6) by the ddPCRTM EGFR Test in samples obtained before TKI treatment and in 63.0% (46/73) of samples by the cobas® EGFR Test and in 58.9% (43/73) by ddPCR in samples drawn after the TKI treatment was initiated. The agreement for samples at the baseline before TKI treatment was 66.7% and for follow-up samples 79.5% with a Kappa coefficient of 0.57 (p < 0.00001). The overall agreement between all samples was 78.5% with a Kappa coefficient of 0.54 (p < 0.00001).
As in the whole study cohort, the median SQI of Ex19Del EGFR mutation was significantly lower in those samples where there was no agreement between the two methods (9.0 vs. 12.1, p < 0.001). However, VAF (0.3 vs. 0.7, p = 0.07) and the number of mutated copies/mL (17.5 vs. 49.4, p = 0.11) were also lower in samples with no agreement, but these differences were not significant.
3.3. The SQI from Cobas® EGFR Test Correlates with the VAF and the Number of Mutated Copies/mL from ddPCRTM EGFR Test
In patients with an Ex19Del mutation detected by the cobas® EGFR Test, the median SQI value was 11.1 (IQR = 4.3), ranging from 6.0 to 22.5. We observed higher SQI values for samples at the baseline before TKI treatment than for those taken during follow-up under TKI treatment (Median = 12.4 vs. 11.0, p = 0.19). In patients with an Ex19Del mutation detected by ddPCR, the median VAF was 0.62 (IQR = 2.5), ranging from 0.04 to 39.3; and the median mutated copies/mL was 34.0 (IQR = 123.3), ranging from 9.2 to 42798. Samples obtained at the baseline before TKI treatment presented higher VAF values (median = 1.6 vs. 0.4, p = 0.02) and mutated copies/mL (Median = 109.5 vs. 25.4, p = 0.03) than the samples collected during follow-up.
We evaluated the correlation between the SQI and the VAF value in the whole set of samples and found a statistically significant correlation between both parameters (r2 = 0.79, p < 0.00001). Next, we evaluated the samples based on TKI treatment. Samples collected before TKI treatment showed a better correlation (r2 = 0.94, p < 0.00001) than the samples obtained after TKI treatment (r2 = 0.76, p < 0.00001).
We obtained similar results comparing SQI to the number mutated copies/mL. For the whole study cohort, we found a statistically significant correlation between SQI and the mutated copies/mL (r2 = 0.79, p < 0.00001). A higher correlation was detected for samples before TKI treatment (r2 = 0.97, p < 0.00001) than for samples obtained after TKI treatment (r2 = 0.75, p < 0.00001) (Figure 1).

Figure 1.
Correlation analysis between (a) SQI and VAF in all samples, (b) SQI and VAF in samples at the baseline before TKI treatment, (c) SQI and VAF in the samples after TKI treatment, (d) SQI and ln (mutated copies/mL) in all samples, (e) SQI and ln (mutated copies/mL) in samples at the baseline before TKI treatment, and (f) SQI and ln (mutated copies/mL) in samples after TKI treatment. Mutated copies/mL were transformed to their natural logarithm to ease graphical representation.
In the subset of patients in which tissue analysis was available, the correlations for SQI and VAF for both the whole set of samples and for samples from patients under TKI treatment were practically the same as for the whole study cohort (r2 = 0.78, p < 0.00001 and r2 = 0.8., p < 0.00001, respectively). However, in samples collected before TKI treatment was initiated, the correlation was better in patients where the tissue result was known (r2 = 0.99, p = 0.0003). The same trend was observed for the SQI and mutated copies/mL, where the correlation for the whole set of samples was r2 = 0.75 (p < 0.00001), for samples before TKI treatment it was r2 = 0.99 (p = 0.0003), and for samples obtained after TKI treatment, it was r2 = 0.72 (p < 0.00001).
3.4. Example of Correlation between SQI and VAF and SQI and Mutated Copies/mL in a Patient with Longitudinal Follow-Up
One patient included in the study had a long follow-up with frequent monthly cfDNA sample analysis (ID15). She was monitored with the cobas® EGFR Test every month from three months after initial diagnosis (sample 1) until follow-up was lost. At the time of the first sample included in this study, the patient had already been under TKI treatment for three months. The agreement between the result of the cobas® EGFR Test and the ddPCRTM EGFR Test was 85.0% (17/20). Two out of the three discordant results corresponded to two samples that tested positive for the ddPCR method, but not for the cobas® EGFR Test. These two samples showed low VAF values (0.23% and 0.15%) and 23.4 and 10.4 mutated copies/mL. The sample that tested positive for the cobas® EGFR Test, but not for the ddPCR method had a SQI value of 9.0. The correlation between the SQIs and VAF and SQI and mutated copies/mL was r2 = 0.79 (p < 0.00001) (Figure 2a). When representing the SQI value and the natural log (ln) of the mutated copies/mL throughout the chronological monitoring process of this patient, we can see how both SQI and the mutated copies/mL were zero or very low during the first 13 monitoring analyses. However, in sample 14, a small amount of mutated copies/mL was detected. In sample 15, the SQI appeared and in sample 16, both parameters reached their highest level thus far. In sample 17, the resistance mutation T790M was detected along with the sensitizing mutation Ex19Del. Between sample 17 and sample 18, treatment with Osimertinib, a third generation TKI, began, and both SQI and mutated copies/mL began to decline until sample 20, when the resistance mutation T790M disappeared and SQI and mutated copies/mL returned to low levels (Figure 2b).
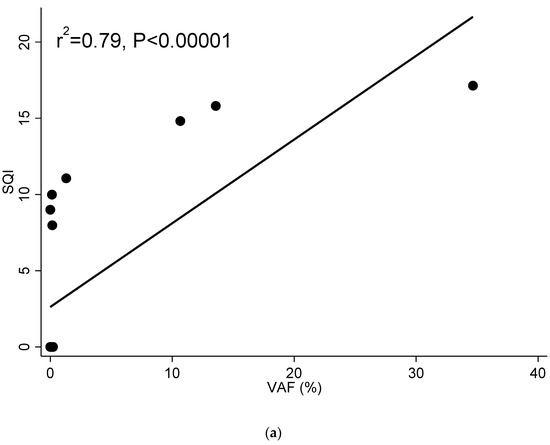
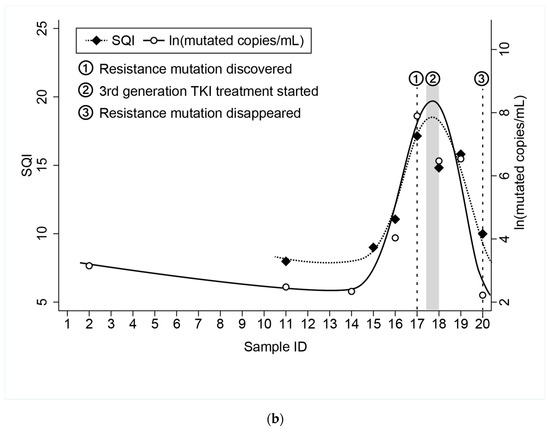
Figure 2.
(a) Correlation between SQI and VAF in patient ID15. (b) Chronological monitoring of SQI and ln(mutated copies/mL) in patient ID 15. Samples in chronological order with one month between each sample. Guides to the eye are included to indicate the trend in the values as resistance mutation was found and treatment adjusted.
4. Discussion
In this study, we compared the qualitative and quantitative performance of the cobas® EGFR Test and the ddPCRTM EGFR Test for the detection of deletions in exon 19 in the EGFR gene in 118 samples from 25 NSCLC patients. The overall agreement between both tests was 79%, which was slightly lower compared to other qualitative studies [25]. The lower number of probes in the ddPCRTM EGFR Test compared to the cobas® EGFR Test as well as technical factors such as the differential performance of these probes in samples with low mutated copies/mL might have affected our results.
Serial molecular analysis of cfDNA from plasma during treatment may have potential clinical utility, but the periodicity of longitudinal blood sampling is not yet established [26]. In our study, for follow-up samples during treatment, we obtained several negative results using one method that gave positive results in the other using the Cobas® EGFR and the ddPCRTM EGFR Test, regardless of their analytical sensitivity. The false negative results seen for the cobas® EGFR Test, involved samples with less than 75 mutated copies/mL, which is the limit of detection declared by the manufacturer, and agrees with other published papers [24]. In the case of the false negative results seen with the ddPCR method, the EQA study [21] has shown that SQI values below 12 correlate to VAFs below 0.5%, which is the limit of detection reported by the manufacturer for this method. In our study, all samples that gave a false negative result on the ddPCR method had a SQI value below 12. This emphasizes the need for higher sensitivity methods like ddPCR to test samples collected during TKI treatment, where the response to the treatment results in a decrease in the tumor burden and consequently lower levels of mutation.
There is no consensus on how to act in front of a negative result during monitoring analysis. Clinical guidelines recommend a tissue biopsy to assess the resistance mutation T790M if plasma testing is negative due to the high risk of false negative results [26,27], but no such recommendations have been set for sensitizing mutations. One way to approach this issue would be to reanalyze the negative sample through an alternative method where possible. Another approach would be to repeat the blood extraction and plasma analysis in a defined period, following the example of serum tumor marker analysis, in which retesting is recommended at 3–4 weeks, or at least a period longer than the tumor marker’s plasma half-life, which is 15–20 days for most of them [28,29], or, as recommended for radiological diagnosis, every 6–12 weeks for advanced NSCLC [30] or 6–12 months for stage III NSCLC [31]. As liquid biopsy is rapidly gaining more relevance in clinical practice, the need for standardized protocols and algorithms regarding its usefulness as a treatment monitoring tool has become an important issue that must soon be addressed by clinical guidelines.
Both the cobas® EGFR Test [24,32] and ddPCR tests [33,34] have proven useful in routine clinical practice to molecularly stratify NSCLC patients. Fast and robust tests like the cobas® EGFR Test are useful as an initial approach for molecular characterization. However, in terms of quantitative outcomes, the significance of the SQI value and its role as a reliable measure of VAF remains unclear. This is a crucial point when the cobas® EGFR Test in cfDNA is used for quantitative rather than qualitative purposes such as for evaluating minimal residual disease or for monitoring TKI treatment response. The EQA study showed that the SQI value presents high imprecision among different EGFR mutations, especially at low VAF values [21]. In a previous study, we observed a lack of correlation between the SQI value with the cfDNA concentration or the stage of the disease, and a moderate reproducibility that differed between distinct mutations [7]. However, in this study, we focused on the most recurrent EGFR alterations, and found a strong correlation between the SQI value and VAF and SQI and the number of mutated copies/mL, indicating that the SQI accurately reflects the VAF kinetics in NSCLC patients harboring deletions in exon 19 in EGFR. The correlation was stronger in samples collected before the initiation of TKI treatment compared to samples obtained during treatment, most probably because the circulating tumor DNA (ctDNA) levels significantly decreased with TKI treatment [35]. Notably, our study and the EQA study found a similar correlation between SQI and the VAF for deletions in exon 19. However, the EQA study was carried out with reference standards spiked to normal human plasma and the correlation was assessed only as part of a sensitivity study evaluation, while our results were obtained from samples collected in a real clinical setting, which enhances the translational meaning of our work, since the results were validated under the standardized conditions of the clinical laboratory. Therefore, based on our results, the SQI value is a robust quantitative measure that has the potential to provide useful information of the mutation load of the tumor. We saw in the longitudinal example shown in the study that the SQI and the mutated copies/mL showed similar kinetics in the patient’s follow-up, with low or undetectable values during treatment, higher levels when resistance was acquired, and again a descent in both parameters as the patient responded to new treatment. However, while cut-off values for VAF to predict progression-free survival were calculated both for activating EGFR (act-EGFR) mutations and for the ratio T790M/act-EGFR [8], further studies are needed to calculate SQI cut-offs with clinical relevance for the management of NSCLC patients, especially to detect TKI response failure or MDR.
When introducing a new test in a clinical laboratory, it must be verified or validated to fulfill international standard criteria [36]. For qualitative tests, it is important to calculate the standard measures of diagnostic accuracy, like sensitivity, specificity, and accuracy. False negative results can result in underdiagnosis, while false positives can result in inappropriate treatment, unnecessary tests, and anxiety for the patient [37]. It is worth noting that in our study, samples from two patients harboring EGFR mutations not available in the ddPCRTM EGFR Test resulted in positive droplets. In samples with positive results with extremely low fractions of mutant DNA, the possibility of experimental artifacts cannot be completely ruled out. Non-specific annealing of PCR primers could result in a false positive result when the concentration of the wild-type template is much higher than the mutant template [38]. One of the false positive results in our study showed a very low amount of mutated copies/mL compared to the wild-type copies/mL. In the other confirmed false positive case, the proportion of mutated copies/mL vs. wild-type copies/mL was not low, which suggests another origin of the false positive result. In these cases, it is most likely due to cross-reactivity with the probes which are designed for mutations within the same region, as has been previously described in ddPCR methods [39]. These findings illustrate the importance of technical factors such as the specificity of designed probes in ddPCR that need to be evaluated prior to their use in the clinical setting to avoid potential false positive results.
One limitation of our study is the lack of molecular results obtained by tissue biopsy in 40% of the patients. This means that we cannot confirm that the mutations present are correctly covered by the cfDNA detection kits used in the study. However, since all patients without tissue biopsy returned positive results for both cfDNA detection kits used in the study, it is fair to assume that this should not significantly affect the results or the conclusions drawn. Due to the number of available samples, we focused on exon 19 deletions. Differences in SQI among distinct EGFR mutations [7,21] mean that our results cannot be extrapolated to other less common EGFR mutations such as the p.L858R EGFR mutation.
In conclusion, this study indicates that SQI strongly correlates with VAF and to the number of mutated copies/mL in patients with exon 19 deletions in the EGFR gene, highlighting that SQI is a robust quantitative measure whose magnitude could have clinical impact as confirmed in the longitudinal study of a patient. Additional studies are needed to assess the clinical relevance of SQI cut-off values for the management of NSCLC patients.
Author Contributions
Conceptualization, J.M.G.d.A.-C., S.S.-S., N.C.-L., and J.A.P.-B.; Methodology, J.M.G.d.A.-C., S.S.-S., N.C.-L., and J.A.P.-B.; Formal analysis, J.M.G.d.A.-C. and S.S.-S.; Investigation, J.M.G.d.A.-C., S.S.-S., and J.A.P.-B.; Resources, N.R. and J.A.P.-B.; Data curation, J.M.G.d.A.-C., S.S.-S., A.A., P.J., N.R., and J.A.P.-B.; Writing—original draft preparation, J.M.G.d.A.-C. and S.S.-S.; Writing—review and editing, J.M.G.d.A.-C. and J.A.P.-B.; Visualization, J.M.G.d.A.-C. and J.A.P.-B.; Supervision, J.A.P.-B.; Project administration, J.A.P.-B.; Funding acquisition, N.R. and J.A.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Hoffman-La Roche Ltd., who supplied the reagents, cobas® cfDNA Sample Preparation Kit, and cobas® EGFR Mutation Test v2 to carry out the study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Hospital Clinic de Barcelona (approval registration number HCB/2016/0889).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the patients for their participation in the study and the study staff who were involved in collecting specimen material at the study sites. The authors would like to express our gratitude to James T. Hugall for providing language assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [Green Version]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Pao, W.; Sequist, L.V. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat. Rev. Clin. Oncol. 2014, 11, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the college of American pathologists, the international association for the study of lung cancer, and the a. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, L.A., Jr.; Bardelli, A.; Diaz, L.A.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- González de Aledo-Castillo, J.M.; Arcocha, A.; Victoria, I.; Martinez-Puchol, A.I.; Sánchez, C.; Jares, P.; Rodríguez, G.F.; Viñolas, N.; Reyes, R.; Reguart, N.; et al. Molecular characterization of advanced non-small cell lung cancer patients by cfDNA analysis: Experience from routine laboratory practice. J. Thorac. Dis. 2021, 13, 1658–1670. [Google Scholar] [CrossRef]
- Del Re, M.; Bordi, P.; Rofi, E.; Restante, G.; Valleggi, S.; Minari, R.; Crucitta, S.; Arrigoni, E.; Chella, A.; Morganti, R.; et al. The amount of activating EGFR mutations in circulating cell-free DNA is a marker to monitor osimertinib response. Br. J. Cancer 2018, 119, 1252–1258. [Google Scholar] [CrossRef] [Green Version]
- Guibert, N.; Pradines, A.; Favre, G.; Mazieres, J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef] [Green Version]
- Abbosh, C.; Birkbak, N.J.; Swanton, C. Early stage NSCLC—Challenges to implementing ctDNA-based screening and MRD detection. Nat. Rev. Clin. Oncol. 2018, 15, 577–586. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta Cytol. 2018, 63, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Roche cobas® EGFR Mutation Test v2 for in vitro diagnostic use. FDA, 2016; 1–71.
- Ntzifa, A.; Kroupis, C.; Haliassos, A.; Lianidou, E. A pilot plasma-ctDNA ring trial for the Cobas® EGFR Mutation Test in clinical diagnostic laboratories. Clin. Chem. Lab. Med. 2019, 57, e97–e101. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Oh, M.S. Detection of Minimal Residual Disease Using ctDNA in Lung Cancer: Current Evidence and Future Directions. J. Thorac. Oncol. 2019, 14, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Mok, T.; Wu, Y.-L.; Lee, J.S.; Yu, C.-J.; Sriuranpong, V.; Sandoval-Tan, J.; Ladrera, G.; Thongprasert, S.; Srimuninnimit, V.; Liao, M.; et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin. Cancer Res. 2015, 21, 3196–3203. [Google Scholar] [CrossRef] [Green Version]
- Herbreteau, G.; Vallée, A.; Charpentier, S.; Normanno, N.; Hofman, P.; Denis, M.G. Circulating free tumor DNA in non-small cell lung cancer (NSCLC): Clinical application and future perspectives. J. Thorac. Dis. 2019, 11, S113. [Google Scholar] [CrossRef]
- Marchetti, A.; Palma, J.F.; Felicioni, L.; De Pas, T.M.; Chiari, R.; Del Grammastro, M.; Filice, G.; Ludovini, V.; Brandes, A.A.; Chella, A.; et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J. Thorac. Oncol. 2015, 10, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Macías, M.; Alegre, E.; Alkorta-Aranburu, G.; Patiño-García, A.; Mateos, B.; Andueza, M.P.; Gúrpide, A.; Lopez-Picazo, J.M.; Gil-Bazo, I.; Perez-Gracia, J.L.; et al. The Dynamic Use of EGFR Mutation Analysis in Cell-Free DNA as a Follow-Up Biomarker during Different Treatment Lines in Non-Small-Cell Lung Cancer Patients. Dis. Markers 2019, 2019, 7954921. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Shin, S.; Lee, K.-A. A Comparative Study for Detection of EGFR Mutations in Plasma Cell-Free DNA in Korean Clinical Diagnostic Laboratories. BioMed Res. Int. 2018, 2018, 7392419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteva-Socias, M.; Enver-Sumaya, M.; Gómez-Bellvert, C.; Guillot, M.; Azkárate, A.; Marsé, R.; Sastre, Ú.; Blasco, A.; Calabuig-Fariñas, S.; Asensio, V.J.; et al. Detection of the EGFR G719S Mutation in Non-small Cell Lung Cancer Using Droplet Digital PCR. Front. Med. 2020, 7, 594900. [Google Scholar] [CrossRef]
- So, M.-K.; Park, J.-H.; Kim, J.-W.; Jang, J.-H. Analytical Validation of a Pan-Cancer Panel for Cell-Free Assay for the Detection of EGFR Mutations. Diagnostics 2021, 11, 1022. [Google Scholar] [CrossRef]
- Keppens, C.; Palma, J.F.; Das, P.M.; Scudder, S.; Wen, W.; Normanno, N.; van Krieken, J.H.; Sacco, A.; Fenizia, F.; Gonzalez de Castro, D.; et al. Detection of EGFR Variants in Plasma: A Multilaboratory Comparison of a Real-Time PCR EGFR Mutation Test in Europe. J. Mol. Diagn. 2018, 20, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buder, A.; Setinek, U.; Hochmair, M.J.; Schwab, S.; Kirchbacher, K.; Keck, A.; Burghuber, O.C.; Pirker, R.; Filipits, M. EGFR Mutations in Cell-free Plasma DNA from Patients with Advanced Lung Adenocarcinoma: Improved Detection by Droplet Digital PCR. Target Oncol. 2019, 14, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment with Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ayala de la Peña, F.; Ortiz-Muñoz, B.; Quintanar-Verdúguez, T.; Santotoribio, J.D.; de la Cruz, S.; Trapé-Pujol, J.; Galve-Calvo, E.; Augé-Fradera, J.M.; García-Gómez, J.; González-Hernández, Á. Consensus of the Spanish society of laboratory medicine and the Spanish society of medical oncology on the methodology and criteria for evaluation of circulating tumour markers in breast cancer. Clin. Transl. Oncol. 2021, 23, 1272–1280. [Google Scholar] [CrossRef]
- Gaspar Blázquez, M.J.; Trapé Pujol, J.; Augé Fradera, J.M.; Barco Sánchez, A.; Carbonell Muñoz, R.; Filella Pla, X.; Fernández Suarez, A.; González Hernández, Á.; Martínez Peinado, A.; Pérez Barrios, C.; et al. Recomendaciones para la optimización del uso de marcadores tumorales de utilización frecuente. Recomendación (2018). Rev. Lab. Clínico 2019, 12, 38–52. [Google Scholar] [CrossRef]
- de Castro, J.; Cobo, M.; Isla, D.; Puente, J.; Reguart, N.; Cabeza, B.; Gayete, A.; Sánchez, M.; Torres, M.I.; Ferreirós, J. Recommendations for radiological diagnosis and assessment of treatment response in lung cancer: A national consensus statement by the Spanish Society of Medical Radiology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2015, 17, 11–23. [Google Scholar] [CrossRef]
- Majem, M.; Hernández-Hernández, J.; Hernando-Trancho, F.; Rodríguez de Dios, N.; Sotoca, A.; Trujillo-Reyes, J.C.; Vollmer, I.; Delgado-Bolton, R.; Provencio, M. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin. Transl. Oncol. 2020, 22, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Soria-Comes, T.; Palomar-Abril, V.; Ureste, M.M.; Guerola, M.T.; Maiques, I.C.M. Real-World Data of the Correlation between EGFR Determination by Liquid Biopsy in Non-squamous Non-small Cell Lung Cancer (NSCLC) and the EGFR Profile in Tumor Biopsy. Pathol. Oncol. Res. 2020, 26, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Sacher, A.G.; Paweletz, C.; Dahlberg, S.E.; Alden, R.S.; O’Connell, A.; Feeney, N.; Mach, S.L.; Jänne, P.A.; Oxnard, G.R. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol. 2016, 2, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxnard, G.R.; Paweletz, C.P.; Kuang, Y.; Mach, S.L.; O'Connell, A.; Messineo, M.M.; Luke, J.J.; Butaney, M.; Kirschmeier, P.; Jackman, D.M.; et al. Noninvasive Detection of Response and Resistance in EGFR Mutant Lung Cancer Using Quantitative Next-Generation Genotyping of Cell-Free Plasma DNA. Clin. Cancer Res. 2014, 20, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taus, Á.; Camacho, L.; Rocha, P.; Hardy-Werbin, M.; Pijuan, L.; Piquer, G.; López, E.; Dalmases, A.; Longarón, R.; Clavé, S.; et al. Dynamics of EGFR Mutation Load in Plasma for Prediction of Treatment Response and Disease Progression in Patients with EGFR-Mutant Lung Adenocarcinoma. Clin. Lung Cancer 2018, 19, 387–394.e2. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen-de Beer, R.; Wielders, J.; Boursier, G.; Vodnik, T.; Vanstapel, F.; Huisman, W.; Vukasović, I.; Vaubourdolle, M.; Sönmez, Ç.; Linko, S.; et al. Validation and verification of examination procedures in medical laboratories: Opinion of the EFLM Working Group Accreditation and ISO/CEN standards (WG-A/ISO) on dealing with ISO 15189:2012 demands for method verification and validation. Clin. Chem. Lab. Med. 2020, 58, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, J.D. Translational genomics: The challenge of developing cancer biomarkers. Genome Res. 2012, 22, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Guo, Q.; Yu, W.; Qiao, L.; Zhao, M.; Zhang, C.; Hu, X.; Yang, G.; Xiong, L.; Lou, J. Quantification of plasma EGFR mutations in patients with lung cancers: Comparison of the performance of ARMS-Plus and droplet digital PCR. Lung Cancer 2017, 114, 31–37. [Google Scholar] [CrossRef]
- Pender, A.; Garcia-Murillas, I.; Rana, S.; Cutts, R.J.; Kelly, G.; Fenwick, K.; Kozarewa, I.; Gonzalez de Castro, D.; Bhosle, J.; O’Brien, M.; et al. Efficient Genotyping of KRAS Mutant Non-Small Cell Lung Cancer Using a Multiplexed Droplet Digital PCR Approach. PLoS ONE 2015, 10, e0139074. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).