Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors
Abstract
:1. Introduction
2. Materials and Methods
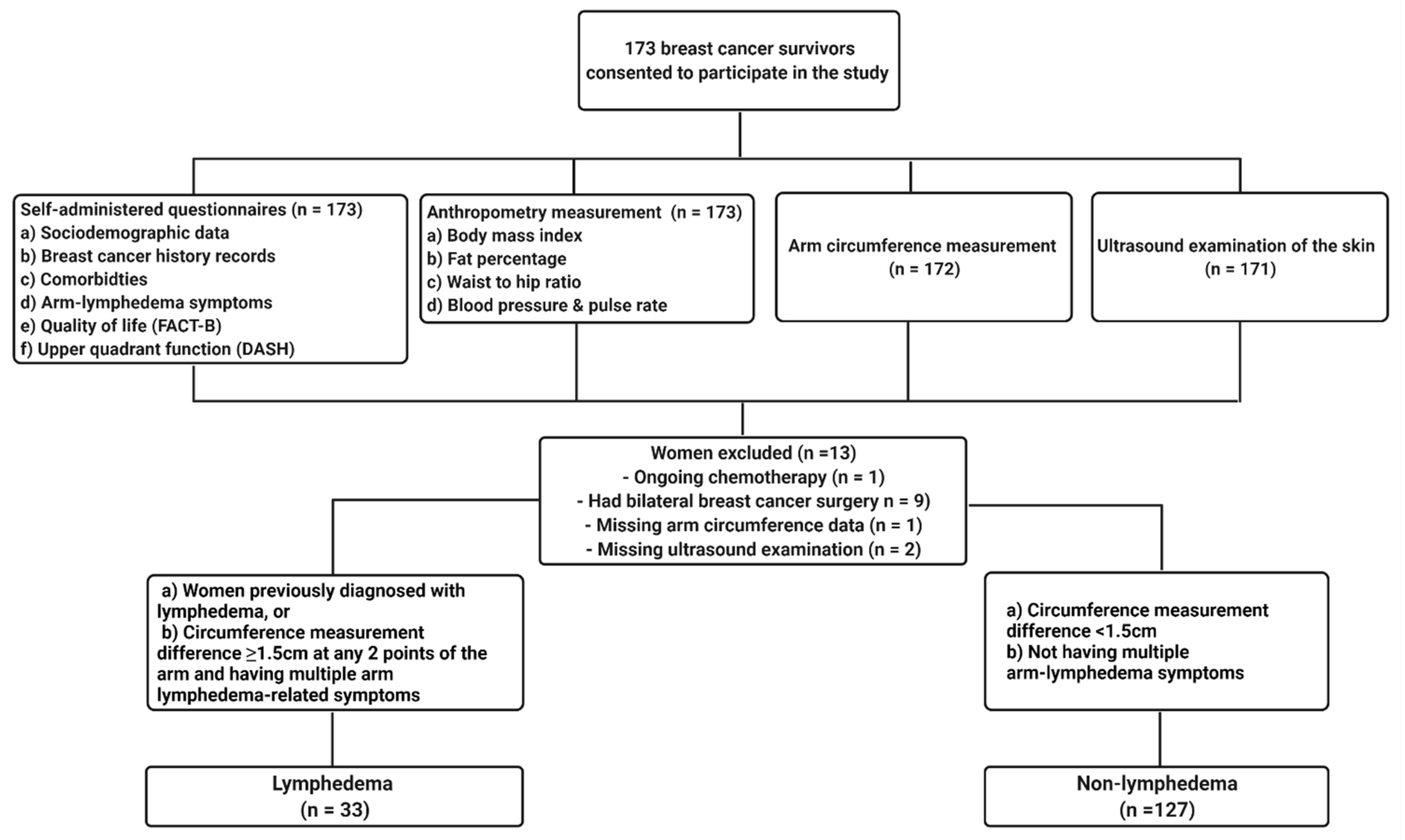
2.1. Study Design and Participants
2.2. Data Variables
2.2.1. Anthropometrical Measurement and Questionnaires
2.2.2. Arm Lymphedema Assessment
Self-Reporting of Diagnosis or Symptoms
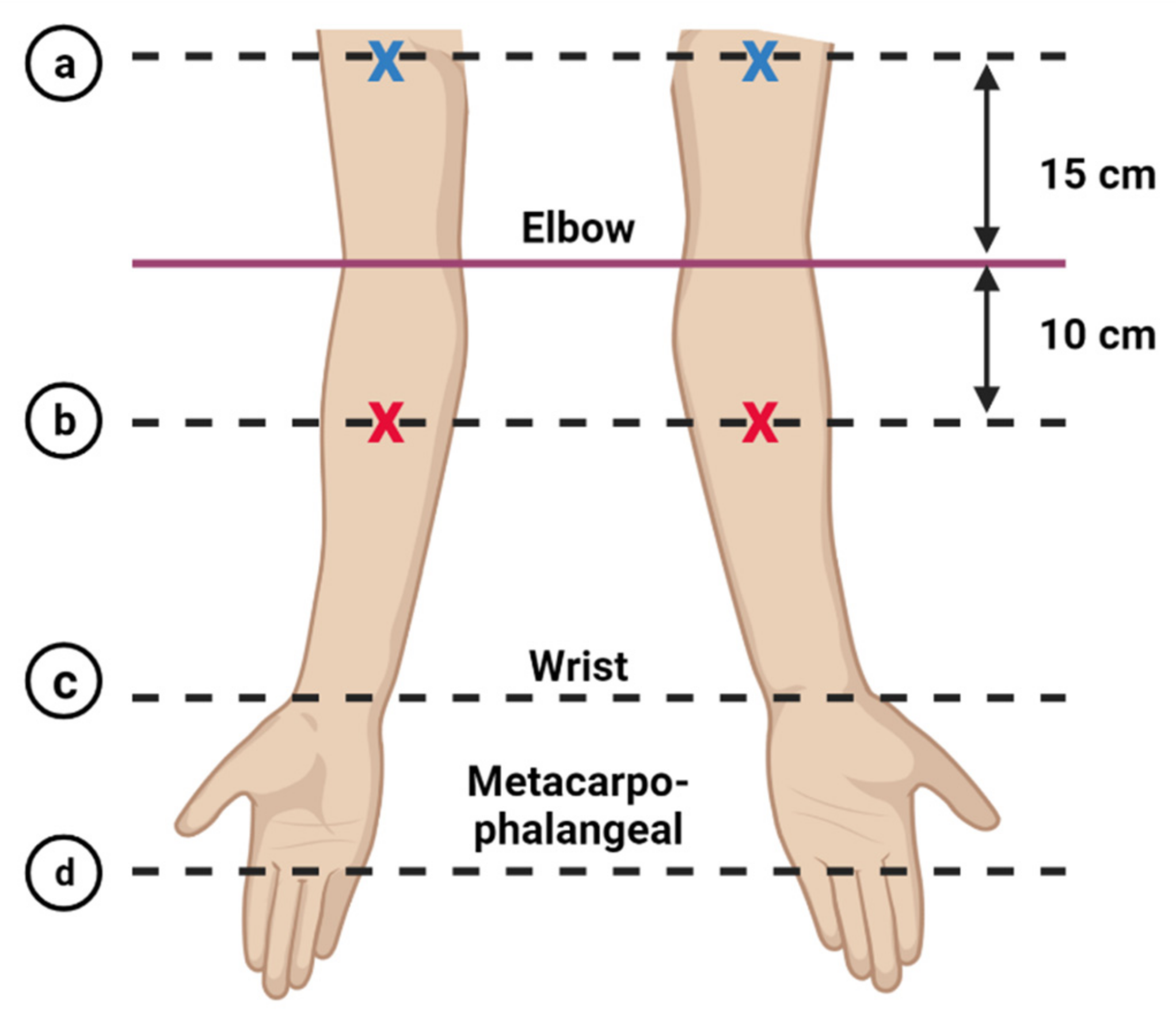
Arm Circumference Measurement
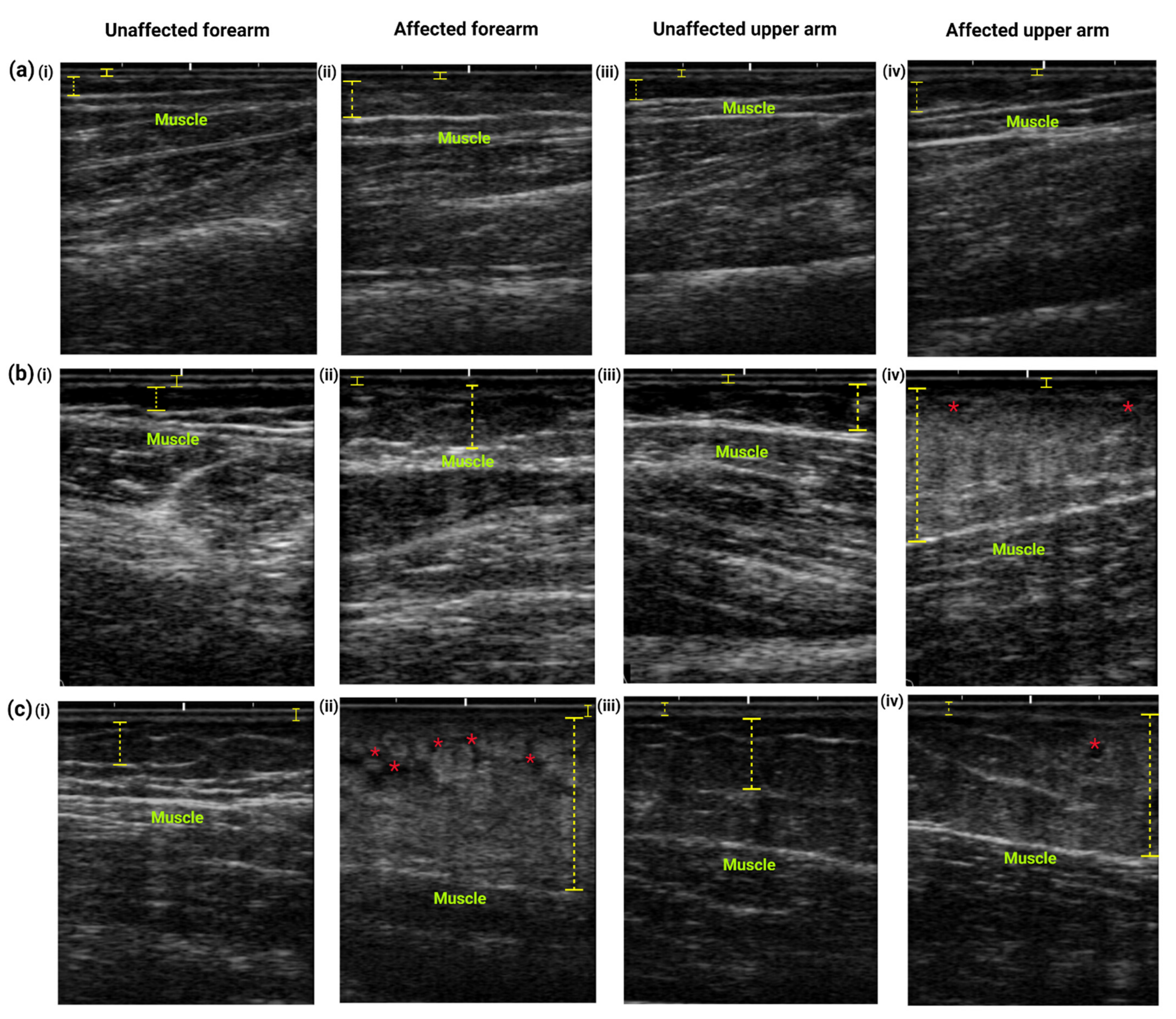
Ultrasonographic Examination
2.3. Statistical Analysis
3. Results
3.1. Demographic, QoL and Upper Extremities Disability Analysis
3.2. Treatment-Related, Modifiable Factors and Arm Symptoms Associated with Lymphedema
3.3. Objective Assessment of Breast Cancer Survivors with Lymphedema
3.3.1. Arm Circumference Measurement
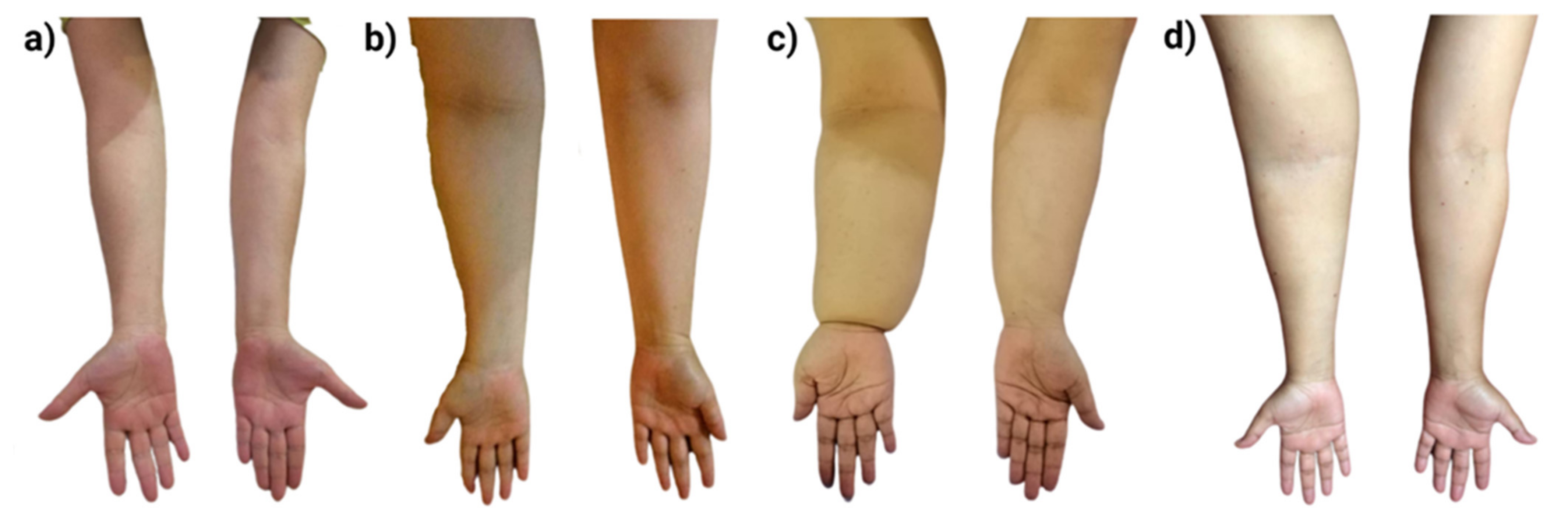
3.3.2. Ultrasound Examination Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Azizah, A.M.; Saleha, I.T.N.; Hashimah, A.N.; Asmah, Z.A.; Mastulu, W. Malaysian National Cancer Registry Report 2007–2011; National Cancer Institute: Putrajaya, Malaysia, 2016; p. 228. [Google Scholar]
- National Cancer Institute (NCI). Malaysian Study on Cancer Survival (MySCan); Ministry of Health Malaysia: Putrajaya, Malaysia, 2018; p. 72.
- Bodai, B. Breast Cancer Survivorship: A Comprehensive Review of Long-Term Medical Issues and Lifestyle Recommendations. Perm. J. 2019, 19, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Cidón, E.U.; Perea, C.; López-Lara, F. Life after Breast Cancer: Dealing with Lymphoedema. Clin. Med. Insights Oncol. 2011, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Nadia, A. Methods to improve rehabilitation of patients following breast cancer surgery: A review of systematic reviews. Breast Cancer Targets Ther. 2015, 7, 81–98. [Google Scholar] [CrossRef] [Green Version]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Miaskowski, C.; Dodd, M.; Paul, S.M.; West, C.; Hamolsky, D.; Abrams, G.; Cooper, B.A.; Elboim, C.; Neuhaus, J.; Schmidt, B.L.; et al. Lymphatic and Angiogenic Candidate Genes Predict the Development of Secondary Lymphedema following Breast Cancer Surgery. PLoS ONE 2013, 8, e60164. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Ms, T.L.H.; Habermann, E.; Cheville, A.L.; Boughey, J.C. Breast Cancer-Related Lymphedema Risk is Related to Multidisciplinary Treatment and Not Surgery Alone: Results from a Large Cohort Study. Ann. Surg. Oncol. 2017, 24, 2972–2980. [Google Scholar] [CrossRef]
- Kilbreath, S.; Refshauge, K.; Beith, J.; Ward, L.; Ung, O.; Dylke, E.; French, J.; Yee, J.; Koelmeyer, L.; Gaitatzis, K. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast 2016, 28, 29–36. [Google Scholar] [CrossRef]
- Rupp, J.; Hadamitzky, C.; Henkenberens, C.; Christiansen, H.; Steinmann, D.; Bruns, F. Frequency and risk factors for arm lymphedema after multimodal breast-conserving treatment of nodal positive breast Cancer—A long-term observation. Radiat. Oncol. 2019, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Levenhagen, K.; Davies, C.; Perdomo, M.; Ryans, K.; Gilchrist, L. Diagnosis of Upper Quadrant Lymphedema Secondary to Cancer: Clinical Practice Guideline from the Oncology Section of the American Physical Therapy Association. Phys. Ther. 2017, 97, 729–745. [Google Scholar] [CrossRef] [Green Version]
- Iyer, D.; Jannaway, M.; Yang, Y.; Scallan, J.P. Lymphatic Valves and Lymph Flow in Cancer-Related Lymphedema. Cancers 2020, 12, 2297. [Google Scholar] [CrossRef]
- Warren, L.E.; Miller, C.L.; Horick, N.; Skolny, M.N.; Jammallo, L.S.; Sadek, B.T.; Shenouda, M.N.; O’Toole, J.A.; MacDonald, S.M.; Specht, M.C.; et al. The Impact of Radiation Therapy on the Risk of Lymphedema After Treatment for Breast Cancer: A Prospective Cohort Study. Int. J. Radiat. Oncol. 2014, 88, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Tsai, R.J.; Dennis, L.K.; Lynch, C.F.; Snetselaar, L.G.; Zamba, G.K.D.; Scott-Conner, C. The Risk of Developing Arm Lymphedema Among Breast Cancer Survivors: A Meta-Analysis of Treatment Factors. Ann. Surg. Oncol. 2009, 16, 1959–1972. [Google Scholar] [CrossRef]
- Crosby, M.A.; Card, A.; Liu, J.; Lindstrom, W.A.; Chang, D.W. Immediate Breast Reconstruction and Lymphedema Incidence. Plast. Reconstr. Surg. 2012, 129, 789e–795e. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H.; Dietrich, M.S.; Stewart, B.R.; Armer, J.M. Body mass index and breast cancer treatment-related lymphedema. Support. Care Cancer 2011, 19, 853–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Liu, F.-H.; Shen, P.-P.; Hu, Y.; Liu, X.-Q.; Xu, Y.-Y.; Pen, Q.-L.; Wang, B.; Zhu, Y.-Q.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hidding, J.T.; Beurskens, C.H.G.; Van Der Wees, P.J.; Bos, W.C.A.M.; Der Sanden, M.W.G.N.-V.; Van Laarhoven, H.W.M. Changes in volume and incidence of lymphedema during and after treatment with docetaxel, doxorubicin, and cyclophosphamide (TAC) in patients with breast cancer. Support. Care Cancer 2017, 26, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-Q.; Xie, Y.-H.; Liu, F.-H.; Guo, Q.; Shen, P.-P.; Tian, Y. Systemic analysis on risk factors for breast cancer related lymphedema. Asian Pac. J. Cancer Prev. 2014, 15, 6535–6541. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland. Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
- He, L.; Qu, H.; Wu, Q.; Song, Y. Lymphedema in survivors of breast cancer (Review). Oncol. Lett. 2020, 19, 2085–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gündüz, N.E.; Dilek, B.; Şahin, E.; Ellidokuz, H.; Akalın, E. Diagnostic Contribution of Ultrasonography in Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2021. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.Y.; Lee, W.H.; Lim, J.-Y.; Lee, J. Diagnostic Accuracy of Clinical Measures Considering Segmental Tissue Composition and Volume Changes of Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2018, 16, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leray, H.; Malloizel-Delaunay, J.; Lusque, A.; Chantalat, E.; Bouglon, L.; Chollet, C.; Chaput, B.; Garmy-Susini, B.; Yannoutsos, A.; Vaysse, C. Body Mass Index as a Major Risk Factor for Severe Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2020, 18, 510–516. [Google Scholar] [CrossRef]
- Penn, I.-W.; Chang, Y.-C.; Chuang, E.; Chen, C.-M.; Chung, C.-F.; Kuo, C.-Y.; Chuang, T.-Y. Risk factors and prediction model for persistent breast-cancer-related lymphedema: A 5-year cohort study. Support. Care Cancer 2019, 27, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.; James, W.P.T.; Barzi, F.; Patel, J.V.; Lear, S.A.; Suriyawongpaisal, P.; Janus, E.; Caterson, I.; Zimmet, P.; Prabhakaran, D.; et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes. Rev. 2008, 9, 53–61. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.E.; Bonomi, A.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Yusof, K.M.; Mahmud, R.; Abdullah, M.; Avery-Kiejda, K.A.; Rosli, R. Cross-Cultural Adaptation of the Functional Assessment of Cancer Therapy-Breast (FACT-B) in Malaysian Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2021, 22, 1055–1061. [Google Scholar] [CrossRef]
- Al-Husuny, A.; Rampal, I.; Zakarian, J.B. Validation of a Malay version of disability of arm, shoulder and hand questionnaire. In Proceedings of the Asian Pacific Conference on Public Health, Kuala Lumpur, Malaysia, 14–15 November 2011. [Google Scholar]
- McDuff, S.G.; Mina, A.; Brunelle, C.L.; Salama, L.; Warren, L.E.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int. J. Radiat. Oncol. 2019, 103, 62–70. [Google Scholar] [CrossRef]
- Yoo, J.-N.; Cheong, Y.-S.; Min, Y.-S.; Lee, S.-W.; Park, H.Y.; Jung, T.-D. Validity of Quantitative Lymphoscintigraphy as a Lymphedema Assessment Tool for Patients With Breast Cancer. Ann. Rehabil. Med. 2015, 39, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nami, H.; Jung, C.Y.; Sun, H.J.; Dong, K.H.; Yeol, C.G. Usefulness of ultrasound examination in evaluation of breast cancer-related lymphedema. J. Korean Acad. Rehabil. Med. 2011, 35, 101–109. [Google Scholar]
- Kabak, V.Y.; Gursen, C.; Aytar, A.; Akbayrak, T.; Duger, T. Physical activity level, exercise behavior, barriers, and preferences of patients with breast cancer–related lymphedema. Support. Care Cancer 2021, 29, 3593–3602. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Tjalma, W.A.A.; Thomis, S.; De Groef, A.; Dams, L.; Van Der Gucht, E.; Devoogdt, N. Physical activity level and age contribute to functioning problems in patients with breast cancer-related lymphedema: A multicentre cross-sectional study. Support. Care Cancer 2020, 28, 5717–5731. [Google Scholar] [CrossRef]
- Cornelissen, A.J.; Kool, M.; Keuter, X.H.; Heuts, E.M.; De Grzymala, A.A.P.; Van Der Hulst, R.R.; Qiu, S.S. Quality of Life Questionnaires in Breast Cancer-Related Lymphedema Patients: Review of the Literature. Lymphat. Res. Biol. 2018, 16, 134–139. [Google Scholar] [CrossRef]
- AhmedKathryn, R.L.; Schmitz, K.H.; Prizment, A.E.; Folsom, A.R. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res. Treat. 2011, 130, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Botter, B.; Heuts, E.M.; Voogd, A.C.; Von Meyenfeldt, M.F.; Van Der Hulst, R.R.; Penha, T.R.L. Quality of Life in Patients with Breast Cancer–Related Lymphedema and Reconstructive Breast Surgery. J. Reconstr. Microsurg. 2016, 32, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Pusic, A.L.; Cemal, Y.; Albornoz, C.; Klassen, A.; Cano, S.; Sulimanoff, I.; Hernandez, M.; Massey, M.; Cordeiro, P.; Morrow, M.; et al. Quality of life among breast cancer patients with lymphedema: A systematic review of patient-reported outcome instruments and outcomes. J. Cancer Surviv. 2012, 7, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafa, S.; Zarrati, M.; Malakootinejad, M.; Totmaj, A.S.; Zayeri, F.; Salehi, M.; Sanati, V.; Haghighat, S. Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. Breast 2020, 54, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Consultation, W.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Swaroop, M.N.; Horick, N.; Skolny, M.N.; Miller, C.L.; Jammallo, L.S.; Brunelle, C.; O’Toole, J.A.; Salama, L.; Specht, M.C.; et al. Impact of Ipsilateral Blood Draws, Injections, Blood Pressure Measurements, and Air Travel on the Risk of Lymphedema for Patients Treated for Breast Cancer. J. Clin. Oncol. 2016, 34, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, N.; Oliver, G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab. 2017, 26, 598–609. [Google Scholar] [CrossRef] [Green Version]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO Expert Consultation: Geneva, Switzerland, 2008. [Google Scholar]
- Card, A.; Crosby, M.A.; Liu, J.; Lindstrom, W.A.; Lucci, A.; Chang, D.W. Reduced Incidence of Breast Cancer–Related Lymphedema following Mastectomy and Breast Reconstruction versus Mastectomy Alone. Plast. Reconstr. Surg. 2012, 130, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Chiang, Y.-J.; Griffin, K.D.; DeSnyder, S.M.; Smith, B.D.; Schaverien, M.V.; Woodward, W.; Cormier, J.N. Radiation therapy targets and the risk of breast cancer-related lymphedema: A systematic review and network meta-analysis. Breast Cancer Res. Treat. 2017, 162, 201–215. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.W.; Lee, S.U.; Lee, N.K.; Jung, S.-Y.; Kim, T.H.; Lee, E.S.; Kang, H.-S.; Shin, K.H. A Model to Estimate the Risk of Breast Cancer-Related Lymphedema: Combinations of Treatment-Related Factors of the Number of Dissected Axillary Nodes, Adjuvant Chemotherapy, and Radiation Therapy. Int. J. Radiat. Oncol. 2013, 86, 498–503. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.-P.; Liu, A.-N.; Wang, D.-B.; Yang, Y.-J.; Duan, Y.-Q.; Zhang, Q.-N. A Scoring System to Predict Arm Lymphedema Risk for Individual Chinese Breast Cancer Patients. Breast Care 2016, 11, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togawa, K.; Ma, H.; Sullivan-Halley, J.; Neuhouser, M.L.; Imayama, I.; Baumgartner, K.B.; Smith, A.W.; Alfano, C.M.; McTiernan, A.; Ballard-Barbash, R.; et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: A prospective cohort study. Breast Cancer Res. 2014, 16, 414. [Google Scholar] [CrossRef] [Green Version]
- Meijer, E.; Bouta, E.M.; Mendonca, C.; Skolny, M.N.; Salama, L.W.; Taghian, A.G.; Padera, T.P. A retrospective analysis of commonly prescribed medications and the risk of developing breast cancer related lymphedema. Clin. Res. Trials 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Asdourian, M.S.; Skolny, M.N.; Brunelle, C.E.; Seward, C.; Salama, L.; Taghian, A.G. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016, 17, e392–e405. [Google Scholar] [CrossRef]
- Lopez-De-Andres, A.; Jimenez-Trujillo, I.; Hernandez-Barrera, V.; de Miguel-Díez, J.; Mendez-Bailon, M.; de Miguel-Yanes, J.M.; Perez-Farinos, N.; Salinero-Fort, M.A.; Del Barrio, J.L.; Romero-Maroto, M.; et al. Association of type 2 diabetes with in-hospital complications among women undergoing breast cancer surgical procedures. A retrospective study using the Spanish National Hospital Discharge Database, 2013–2014. BMJ Open 2017, 7, e017676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashing, K.T.; Lai, L.; Meyers, E.; Serrano, M.; George, M. Exploring the association between diabetes and breast cancer morbidity: Considerations for quality care improvements among Latinas. Int. J. Qual. Health Care 2020, 32, 120–125. [Google Scholar] [CrossRef]
- Rifkin, W.J.; Kantar, R.S.; Cammarata, M.J.; Wilson, S.C.; Diaz-Siso, J.R.; Golas, A.R.; Levine, J.P.; Ceradini, D.J. Impact of Diabetes on 30-Day Complications in Mastectomy and Implant-Based Breast Reconstruction. J. Surg. Res. 2019, 235, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kilbreath, S.L.; Refshauge, K.M.; Beith, J.; Ward, L.; Lee, M.; Simpson, J.M.; Hansen, R. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: A randomized controlled trial. Breast Cancer Res. Treat. 2012, 133, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Ligabue, M.B.; Campanini, I.; Veroni, P.; Cepelli, A.; Lusuardi, M.; Merlo, A. Efficacy of self-administered complex decongestive therapy on breast cancer-related lymphedema: A single-blind randomized controlled trial. Breast Cancer Res. Treat. 2019, 175, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sagen, Åse.; Kåresen, R.; Risberg, M.A. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009, 48, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.; Cheville, A.; Lewis-Grant, L.; Smith, R.; Bryan, C.J.; Williams-Smith, C.T.; Chittams, J. Weight Lifting for Women at Risk for Breast Cancer–Related Lymphedema. JAMA 2010, 304, 2699–2705. [Google Scholar] [CrossRef]
- Jeffs, E.; Wiseman, T. Randomised controlled trial to determine the benefit of daily home-based exercise in addition to self-care in the management of breast cancer-related lymphoedema: A feasibility study. Support. Care Cancer 2012, 21, 1013–1023. [Google Scholar] [CrossRef]
- Svensson, B.J.; Dylke, E.S.; Ward, L.C.; Black, D.A.; Kilbreath, S.L. Screening for breast cancer–related lymphoedema: Self-assessment of symptoms and signs. Support. Care Cancer 2019, 28, 3073–3080. [Google Scholar] [CrossRef]
- LeBlanc, M.; Stineman, M.; DeMichele, A.; Stricker, C.; Mao, J.J. Validation of QuickDASH Outcome Measure in Breast Cancer Survivors for Upper Extremity Disability. Arch. Phys. Med. Rehabil. 2014, 95, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.J.; Sim, Y.-J.; Hwang, K.H.; Kim, G.C. Causes of Shoulder Pain in Women with Breast Cancer-Related Lymphedema: A Pilot Study. Yonsei Med. J. 2011, 52, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Chrischilles, E.A.; Riley, D.; Letuchy, E.A.; Koehler, L.; Neuner, J.; Jernigan, C.; Gryzlak, B.; Segal, N.; McDowell, B.; Smith, B.; et al. Upper extremity disability and quality of life after breast cancer treatment in the Greater Plains Collaborative clinical research network. Breast Cancer Res. Treat. 2019, 175, 675–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.E.; Jang, H.J.; Seo, K.S. Quality of Life, Upper Extremity Function and the Effect of Lymphedema Treatment in Breast Cancer Related Lymphedema Patients. Ann. Rehabil. Med. 2012, 36, 240–247. [Google Scholar] [CrossRef]
- Giray, E.; Akyuz, G. Assessment of Family Caregiver Burden and Its Relationships Between Quality of Life, Arm Disability, Grip Strength, and Lymphedema Symptoms in Women with Postmastectomy Lymphedema: A Prospective Cross-Sectional Study. Eur. J. Breast Health 2019, 15, 111–118. [Google Scholar] [CrossRef]
- Hayes, S.C.; Janda, M.; Cornish, B.H.; Battistutta, D.; Newman, B.M. Lymphedema After Breast Cancer: Incidence, Risk Factors, and Effect on Upper Body Function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Dawes, D.J.; Meterissian, S.; Goldberg, M.E.; Mayo, N. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J. Rehabil. Med. 2008, 40, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Hidding, J.T.; Beurskens, C.H.; De Vries, M.T.; Der Sanden, M.W.N.-V.; Van Laarhoven, H.W.; Van Der Wees, P.J. Accuracy of a single measurement site for self-monitoring of patients with breast cancer at risk for lymphedema. Physiother. Theory Pr. 2018, 35, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Smoot, B.J.; Wong, J.; Dodd, M.J. Comparison of Diagnostic Accuracy of Clinical Measures of Breast Cancer–Related Lymphedema: Area Under the Curve. Arch. Phys. Med. Rehabil. 2011, 92, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Mellor, R.; Stanton, A.; Azarbod, P.; Sherman, M.; Levick, J.; Mortimer, P. Enhanced Cutaneous Lymphatic Network in the Forearms of Women with Postmastectomy Oedema. J. Vasc. Res. 2000, 37, 501–512. [Google Scholar] [CrossRef]
- Stanton, A.W.B.; Mellor, R.H.; Cook, G.; Svensson, W.E.; Peters, A.M.; Levick, J.R.; Mortimer, P.S. Impairment of Lymph Drainage in Subfascial Compartment of Forearm in Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2003, 1, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modi, S.; Stanton, A.W.B.; Mellor, R.H.; Peters, A.M.; Levick, J.R.; Mortimer, P.S. Regional Distribution of Epifascial Swelling and Epifascial Lymph Drainage Rate Constants in Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2005, 3, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Stanton, A.W.; Svensson, W.E.; Mellor, R.H.; Peters, A.M.; Levick, J.R.; Mortimer, P.S. Differences in lymph drainage between swollen and non-swollen regions in arms with breast-cancer-related lymphoedema. Clin. Sci. 2001, 101, 131–140. [Google Scholar] [CrossRef]
- Der Veen, P.; Vermeiren, K.; Von Kemp, K.; Lamote, J.; Sacre, R.; Lievens, P. A key to understanding postoperative lymphoedema: A study on the evolution and consistency of oedema of the arm using ultrasound imaging. Breast 2001, 10, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Petrek, J.A.; Senie, R.T.; Peters, M.; Rosen, P.P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001, 92, 1368–1377. [Google Scholar] [CrossRef]
- Can, A.G.; Eksioglu, E.; Bahtiyarca, Z.T.; Cakci, F.A. Assessment of Risk Factors in Patients who presented to the Outpatient Clinic for Breast Cancer-Related Lymphedema. J. Breast Health 2016, 12, 31–36. [Google Scholar] [CrossRef]
- Choi, Y.H.; Seo, K.S. Correlation among bioimpedance analysis, sonographic and circumferential measurement in assessment of breast cancer-related arm lymphedema. Lymphology 2014, 47, 123–133. [Google Scholar] [PubMed]
- Azhar, S.H.; Lim, H.Y.; Tan, B.-K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Lymphedema (n = 33) | Non-Lymphedema (n = 127) | p Value |
|---|---|---|---|
| Age a | 51.73 ± 8.15 | 50.87 ± 8.78 | 0.597 |
| Age at diagnosis (years) a | 46.42 ± 7.92 | 45.23 ± 8.35 | 0.449 |
| Years after diagnosis b | 5.30 ± 4.10 | 5.72 ± 4.40 | 0.220 |
| Body mass index (kg/m2) b | 30.03 ± 5.05 | 27.32 ± 5.49 | 0.005 ** |
| Waist-to-hip ratio a | 0.91 ± 0.06 | 0.88 ± 0.06 | 0.048 * |
| Systolic blood pressure (mm/Hg), (n = 126) | 129.72 ± 20.58 | 126.13 ± 18.14 | 0.373 |
| Diastolic blood pressure (mm/Hg), (n = 126) | 80.56 ± 12.16 | 80.48 ± 13.22 | 0.975 |
| Pulse rate (per minute), (n = 126) | 84.41 ± 14.25 | 80.56 ± 12.26 | 0.169 |
| QoL scores (FACT-B) b | 103.91 ± 21.80 | 115.49 ± 16.80 | 0.009 ** |
| Arm disability scores (DASH) b | 37.14 ± 18.90 | 20.08 ± 15.29 | <0.001 *** |
| Ethnicity c | |||
| 29 (87.8%) | 117 (92.1%) | 0.237 |
| 4 (12.1%) | 7 (5.5%) | |
| 0 (0.0%) | 3 (2.4%) | |
| Educational status d | |||
| 17 (51.5%) | 81 (63.8%) | 0.198 |
| 16 (48.5%) | 46 (36.2%) | |
| Employment status d | |||
| 7 (21.2%) | 21 (16.5%) | 0.182 |
| 4 (12.1%) | 28 (22.0%) | |
| 21 (63.6%) | 64 (50.4%) | |
| 1 (3.0%) | 14 (11.0%) | |
| 0 (0.0%) | 1 (0.1%) | |
| Monthly income (RM) d | |||
| 25 (75.8%) | 87 (68.5%) | 0.588 |
| 6 (18.2%) | 34 (26.8%) | |
| 2 (0.1%) | 6 (4.7%) | |
| Marital status d | |||
| 30 (90.9%) | 119 (93.7%) | 0.572 |
| 3 (9.1%) | 8 (6.3%) | |
| Number of children d | |||
| 7 (21.2%) | 12 (9.5%) | 0.023 * |
| 17 (51.6%) | 49 (38.6%) | |
| 9 (27.2%) | 66 (51.9%) | |
| Menopausal status d | |||
| 25 (75.8%) | 83 (65.6%) | 0.256 |
| 8 (24.2%) | 44 (34.6%) |
| Domain | DASH | Heaviness/Tightness | Difficulties in Finding Shirt That Fits | Pain in the Arm | Swelling of the Arm |
|---|---|---|---|---|---|
| DASH | 1 | 0.192 * | 0.388 ** | 0.309 ** | 0.316 ** |
| FACT-B | −0.646 ** | −0.179 * | −0.342 ** | −0.316 ** | −0.123 |
| Characteristics | Lymphedema (n = 33) | Non-Lymphedema (n = 127) | OR [95% CI] | p Value |
|---|---|---|---|---|
| Number of children a | ||||
| 24 (72.7%) | 61 (48.0%) | 2.89 [1.24–6.69] | 0.011 * |
| 9 (27.3%) | 66 (52.0%) | ||
| Body mass index, (kg/m2) a | ||||
| 26 (78.8%) | 75 (5915%) | 2.57 [1.04–6.38] | 0.036 * |
| 7 (21.2%) | 52 (40.9%) | ||
| Waist-to-hip ratio a | ||||
| 21 (63.6%) | 54 (42.5%) | 2.37 [1.07–5.22] | 0.030 * |
| 12 (36.4%) | 73 (57.5%) | ||
| Affected side a | ||||
| 18 (54.5% | 62 (48.8%) | 0.87 [0.72–0.85] | 0.730 |
| 15 (45.5%) | 65 (51.2%) | ||
| Breast biopsy b | ||||
| 33 (100.0%) | 121 (95.3%) | 0.79 [0.72–0.85] | 0.298 |
| 0 (0.0%) | 4 (3.1%) | ||
| 0 (0.0%) | 2 (1.6%) | ||
| Tumor stage d | ||||
| 7 (21.2%) | 28 (22.0%) | 0.96 [0.61–1.53] | 0.875 |
| 17 (51.5%) | 63 (49.7%) | ||
| 6 (18.2%) | 28 (22.0%) | ||
| 3 (9.1%) | 8 (6.3%) | ||
| Receptor status a | ||||
| 28 (84.8%) | 99 (77.9%) | 1.41 [0.45–4.48] | 0.554 |
| ||||
| 4 (12.1%) | 20 (15.7%) | ||
| 1 (3.0%) | 8 (6.4%) | ||
| Type of breast surgery a | ||||
| Lumpectomy | ||||
| 6 (18.2%) | 30 (23.6%) | 0.72 [0.27–1.90] | 0.505 |
| 27 (81.8%) | 97 (76.4%) | ||
| Mastectomy | ||||
| 23 (69.7%) | 94 (74.0%) | 0.87 [0.35–1.87] | 0.618 |
| 10 (30.3%) | 33 (26.0%) | ||
| Lumpectomy & mastectomy | ||||
| 4 (12.1%) | 3 (2.7%) | 5.70 [1.21–26.8] | 0.015 * |
| 29 (87.9%) | 124 (97.3%) | ||
| Axillary lymph nodes excision | ||||
| 33 (100.0%) | 121 (95.3%) | 0.79 [0.72–0.85] | 0.298 |
| 0 (0.0%) | 4 (3.1%) | ||
| 0 (0.0%) | 2 (1.6%) | ||
| No. of lymph nodes removed a | ||||
| 23 (69.6%) | 57 (44.8%) | 2.83 [0.98–8.12] | 0.047 * |
| 5 (15.2%) | 35 (27.6%) | ||
| 5 (15.2%) | 35 (27.6%) | ||
| Breast cancer treatment a | ||||
| Chemotherapy | ||||
| 25 (75.8%) | 105 (82.6%) | 0.66 [0.26–1.64] | 0.364 |
| 8 (24.2%) | 22 (17.2%) | ||
| Radiotherapy | ||||
| 24 (72.7%) | 94 (74.0%) | 0.94 [0.39–2.22] | 0.881 |
| 9 (27.3%) | 33 (25.9%) | ||
| Hormonal therapy | ||||
| 27 (81.8%) | 82 (64.6%) | 1.52 [0.57–4.00] | 0.399 |
| 6 (18.2%) | 43 (33.8%) | ||
| Post-surgery rehabilitation a | ||||
| 22 (69.7%) | 62 (48.8%) | 2.37 [1.05–5.39] | 0.036 * |
| 11 (30.3%) | 64 (50.4%) | ||
| 0 (0.0%) | 1 (0.8%) | ||
| Comorbidities a | ||||
| Hypertension | ||||
| 11 (33.3%) | 22 (17.3%) | 2.38 [1.01–5.62] | 0.043 * |
| 22 (66.7%) | 105 (82.7%) | ||
| Diabetes | ||||
| 7 (21.2%) | 14 (11.0%) | 2.17 [0.83–6.12] | 0.123 |
| 26 (78.8%) | 113 (89.0%) | ||
| Other (cardiovascular, lung, kidney diseases, infection) a | ||||
| 4 (12.1%) | 11 (8.7%) | 1.50 [0.44–5.04] | 0.516 |
| 28 (84.8%) | 115 (90.5%) | ||
| 1 (3.0%) | 1 (0.8%) | ||
| Additional supplement intake | ||||
| 14 (42.5%) | 53 (41.7%) | 1.06 [0.48–2.31] | 0.890 |
| 18 (54.5%) | 72 (56.7%) | ||
| 1 (3.0%) | 2 (1.6%) | ||
| History of cancer in family a | ||||
| 15 (45.5%) | 58 (45.7%) | 1.01 [0.47–2.18] | 0.982 |
| 18 (54.5%) | 69 (54.3%) | ||
| Arm morbidities symptomsa Heaviness & tightness of the chest | ||||
| 10 (30.3%) | 37 (29.1%) | 1.05 [0.45–2.41] | 0.916 |
| 23 (69.7%) | 89 (70.1%) | ||
| 0 (0.0%) | 1 (0.8%) | ||
| Hardness & difficulties in finding t-shirts that fits | ||||
| 22 (66.7%) | 38 (29.9%) | 4.63 [2.05–10.49] | <0.001 ** |
| 11 (33.3%) | 88 (69.3%) | ||
| 0 (0.0%) | 1 (0.8%) | ||
| Pain at the arm | ||||
| 21 (63.7%) | 61 (48.0%) | 2.00 [0.89–4.50] | 0.089 |
| 11 (33.3%) | 64 (50.4%) | ||
| 0 (0.0%) | 2 (1.6%) | ||
| Swelling of the arm | ||||
| 31 (93.9%) | 22 (17.3%) | 73.2 [16.3–329.6] | <0.001 ** |
| 2 (6.1%) | 104 (81.9%) | ||
| 0 (0.0%) | 1 (1.8%) |
| Variable | β | S.E | Wald | p Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Higher BMI (≥25 kg/m2) | 0.898 | 0.449 | 3.300 | 0.069 | 2.45 | [0.95–6.46] |
| Lumpectomy & mastectomy | 1.763 | 0.836 | 4.489 | 0.034 | 5.83 | [1.14–29.78] |
| Hypertension | 0.882 | 0.467 | 3.572 | 0.059 | 2.41 | [0.99–6.03] |
| Post-surgery rehabilitation (<2) | 0.806 | 0.439 | 3.365 | 0.067 | 2.24 | [0.95–5.23] |
| Characteristics | Early-Onset (n = 16) | Late-Onset (n = 17) | OR [95% CI] | p Value |
|---|---|---|---|---|
| Age during recruitment a | 51.2 ± 8.6 | 52.2 ± 7.9 | 0.719 | |
| BMI, (kg/m2) a | 31.0 ± 5.1 | 29.1 ± 5.0 | 0.294 | |
| FACT-B score a | 95.9 ± 21.7 | 111.4 ± 19.4 | 0.040 * | |
| DASH score a | 42.0 ± 18.8 | 32.6 ± 18.3 | 0.153 | |
| No. of lymph nodes removed a, (n = 28) | 19.0 ± 6.5 | 12.2 ± 8.9 | 0.031 * | |
| Types of surgery b | ||||
| 2 | 4 | 0.46 [0.07–2.97] | 0.656 |
| 12 | 11 | 1.63 [0.36–7.38] | 0.708 |
| 2 | 2 | 1.07 [0.13–8.67] | 1.000 |
| Types of treatment b | ||||
| 10 | 15 | 0.22 [0.37–1.33] | 0.118 |
| 10 | 14 | 0.36 [0.07–1.78] | 0.259 |
| 12 | 15 | 0.40 [0.06–2.57] | 0.398 |
| Co-morbidities | ||||
| 6 | 5 | 1.44 [0.34–6.16] | 0.622 |
| 6 | 1 | 9.60 [1.00–91.96] | 0.039 * |
| Point of Measure (cm) | Affected Arm Mean ± SD | Unaffected Arm Mean ± SD | Mean Difference Mean ± SD | p Value |
|---|---|---|---|---|
| Metacarpo-phalangeal | 18.17 ± 1.37 | 17.73 ± 1.09 | 0.44 ± 0.96 | 0.014 * |
| Wrist | 16.52 ± 2.00 | 15.70 ± 1.24 | 0.82 ± 1.43 | 0.003 ** |
| Forearm (10 cm below epicondyle) | 25.03 ± 3.86 | 22.97 ± 2.91 | 2.07 ± 2.48 | <0.001 *** |
| Upper arm (15 cm above epicondyle) | 33.37 ± 4.32 | 32.03 ± 4.16 | 1.34 ± 1.91 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; Abdul Wahhab Ibraheem, S.; Abdullah, M.; Rosli, R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics 2021, 11, 1303. https://doi.org/10.3390/diagnostics11081303
Yusof KM, Avery-Kiejda KA, Ahmad Suhaimi S, Ahmad Zamri N, Rusli MEF, Mahmud R, Saini SM, Abdul Wahhab Ibraheem S, Abdullah M, Rosli R. Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics. 2021; 11(8):1303. https://doi.org/10.3390/diagnostics11081303
Chicago/Turabian StyleYusof, Khairunnisa’ Md, Kelly A. Avery-Kiejda, Shafinah Ahmad Suhaimi, Najwa Ahmad Zamri, Muhammad Ehsan Fitri Rusli, Rozi Mahmud, Suraini Mohd Saini, Shahad Abdul Wahhab Ibraheem, Maha Abdullah, and Rozita Rosli. 2021. "Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors" Diagnostics 11, no. 8: 1303. https://doi.org/10.3390/diagnostics11081303
APA StyleYusof, K. M., Avery-Kiejda, K. A., Ahmad Suhaimi, S., Ahmad Zamri, N., Rusli, M. E. F., Mahmud, R., Saini, S. M., Abdul Wahhab Ibraheem, S., Abdullah, M., & Rosli, R. (2021). Assessment of Potential Risk Factors and Skin Ultrasound Presentation Associated with Breast Cancer-Related Lymphedema in Long-Term Breast Cancer Survivors. Diagnostics, 11(8), 1303. https://doi.org/10.3390/diagnostics11081303









