Abstract
Infectious diseases are an existential health threat, potentiated by emerging and re-emerging viruses and increasing bacterial antibiotic resistance. Targeted treatment of infectious diseases requires precision diagnostics, especially in cases where broad-range therapeutics such as antibiotics fail. There is thus an increasing need for new approaches to develop sensitive and specific in vitro diagnostic (IVD) tests. Basic science and translational research are needed to identify key microbial molecules as diagnostic targets, to identify relevant host counterparts, and to use this knowledge in developing or improving IVD. In this regard, an overlooked feature is the capacity of pathogens to adhere specifically to host cells and tissues. The molecular entities relevant for pathogen–surface interaction are the so-called adhesins. Adhesins vary from protein compounds to (poly-)saccharides or lipid structures that interact with eukaryotic host cell matrix molecules and receptors. Such interactions co-define the specificity and sensitivity of a diagnostic test. Currently, adhesin-receptor binding is typically used in the pre-analytical phase of IVD tests, focusing on pathogen enrichment. Further exploration of adhesin–ligand interaction, supported by present high-throughput “omics” technologies, might stimulate a new generation of broadly applicable pathogen detection and characterization tools. This review describes recent results of novel structure-defining technologies allowing for detailed molecular analysis of adhesins, their receptors and complexes. Since the host ligands evolve slowly, the corresponding adhesin interaction is under selective pressure to maintain a constant receptor binding domain. IVD should exploit such conserved binding sites and, in particular, use the human ligand to enrich the pathogen. We provide an inventory of methods based on adhesion factors and pathogen attachment mechanisms, which can also be of relevance to currently emerging pathogens, including SARS-CoV-2, the causative agent of COVID-19.
1. Introduction
Infectious diseases, particularly those caused by potentially lethal viruses and antibiotic-resistant bacteria, were one of the key issues on the agenda of the G7-Summit in Germany in 2015 (https://www.g7uk.org/new-international-approach-to-combat-emerging-health-threats-as-crucial-g7-health-talks-begin/, accessed on 21 June 2021) (see Table 1 for a review of the currently most relevant antibiotic resistant microorganisms). After the summit, every major health authority, including the World Health Organization (WHO), confirmed that the (re-)emergence of infectious diseases in general and the decreasing efficacy of antimicrobials are major medical concerns, as antimicrobial therapies are starting to fail (Table 1) and deadly viruses cause serious global outbreaks []. Moreover, it is estimated that around 30% of bacteria responsible for hospital associated infections are antibiotic resistant, with the number of infections being about nine million each year in Europe alone []. Clinical misdiagnosis can lead to antibiotic resistance when proper diagnostic methods are not used and patients are prescribed unnecessary treatments []. Thus, novel diagnostic tests are urgently needed to prevent (re-)emerging infections and to better treat infections by clinically relevant antibiotic resistant pathogens. Therefore, continued academic and industrial investment in new in vitro diagnostic (IVD) tests is urgently required to tackle antimicrobial resistance (AMR), (multi)drug-resistance (MDR) or even pan-resistance (PDR) [,,].

Table 1.
WHO priority bacterial pathogens and their clinically relevant antibiotic resistance phenotypes which render treatment problems (adapted from www.who.int (accessed on 21 June 2021) and []). ESBL: extended spectrum beta-lactamase.
Clearly, prior to infection, pathogens colonize their host organisms via adhesion: the binding of microbial molecules—adhesins—to specific host counterparts. Consequently, adhesins can be used to specifically enrich pathogens. Below, we explore this concept from a variety of viewpoints.
1.1. Microbial Adhesion, Colonization and Host Infection
The severity of an infectious disease depends on the level of invasiveness and the extent of host cell and tissue damage caused by the pathogen involved []. A varying degree of virulence can be observed across different pathogens and among different strains of a single pathogen []. Virulence is a complicated concept in microbial pathogenesis since it depends not only on the infectious agent but also on host cell susceptibility []. Adhesion is at the heart of virulence: it plays the initial and decisive role in colonization and subsequent infection (Figure 1) [,,,]. Bacterial, viral and parasitic pathogens use adhesins to bind to individual host cells and establish interactions with host molecules, thereby initiating colonization. The exact nature of the interaction between pathogen surface molecules and cell receptors defines the cellular or tissue specificity. Such interactions can invoke mechanisms of immune evasion, as adhesion can directly result in the modulation of the host immune response []. Finally, not all adhesins have yet been identified, let alone characterized; and, in general, the precise role of adhesins in tissue tropism needs further study. Different bacterial species may target similar or different host ligands using different types of adhesins. At the same time, an individual bacterial species typically harbors multiple adhesion systems with different molecular targets. Understanding and exploiting the molecular basis of pathogen adhesion and the resulting adhesion behavior of whole cells will lead to new formats of diagnostic testing. We propose that cross-disciplinary and translational research is needed to improve our fundamental understanding of adhesion biology, and to translate this knowledge into novel detection strategies based on host–pathogen interaction [].
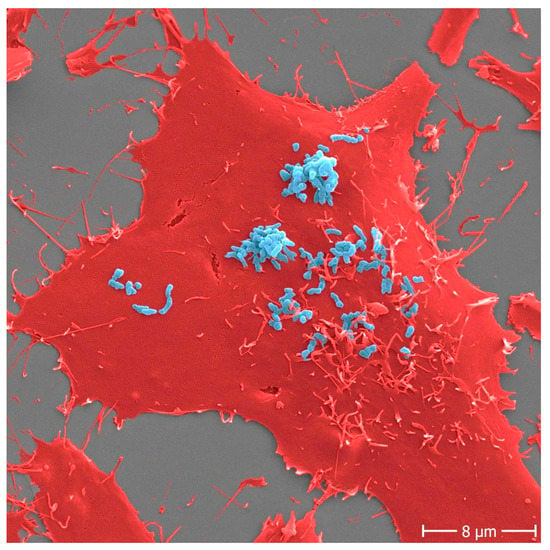
Figure 1.
Adhesion of Bartonella henselae to human cells. B. henselae (strain Marseille) bacteria (light blue) in an early stage infection process (30 min) to human HeLa-229 cells (red). Adhesion to host cells is mediated by specific interactions between B. henselae surface proteins and components of the host extracellular matrix including molecules such as fibronectin or collagen. Scale bar: 8 μm.
1.2. Current State of Infectious Disease Diagnostics
The core technologies used by the routine microbiology laboratory are still mostly microscopy- and culture-based. Immunological tests detecting pathogen-specific antigens and antibodies are also routinely used in diagnostics. Furthermore, over the past years, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) and molecular (nucleic acid-targeting) testing have been introduced successfully in the routine clinical microbiology laboratory (for some recent reviews see [,]).
The main technologies in routine high-throughput laboratories are relatively slow, usually taking at least one overnight incubation. They are limited in terms of sensitivity and specificity, suggesting that there is room for improvement [,]. Cultivation methodologies are still internationally accepted as the Gold Standard (Figure 2). Nonetheless these approaches may profit from new, adhesin-based technology to reduce turn-around time and improve test qualities. Actual development of innovative clinical diagnostics requires careful consideration of many parameters, including sensitivity, specificity, cost, shelf-life, robustness, simplicity and user-friendliness (Figure 2 and Figure 3). To validate their quality, new tests must be carefully compared with those from existing diagnostics platforms.
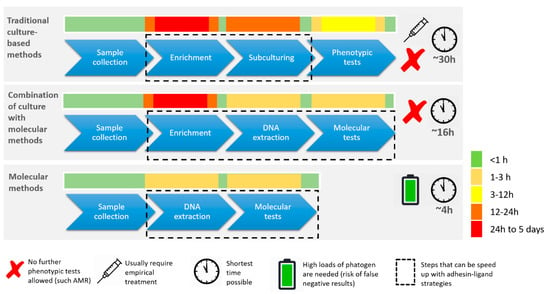
Figure 2.
Comparison of culture-based versus molecular diagnostics in routine clinical microbiology. Innovative clinical diagnostics with inclusion of molecular methodology requires careful consideration of many important test parameters among which sensitivity, specificity, cost, shelf life, robustness, simplicity, and user-friendliness. Although molecular techniques are faster, there is frequently a mandatory need for cultivation throughout the diagnostic process. Viable cells are often required for storage, downstream AST or simply for reproducibility testing at a later stage.
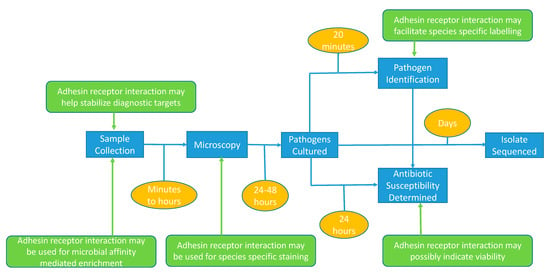
Figure 3.
Diagnostic workflow and technologies (blue boxes) used for the routine detection of pathogens. Current timing is indicated in orange boxes whereas the possible impact of adhesion-based assays is indicated in green.
1.3. Mandatory Improvement of IVD
Development of better diagnostic tools requires an integrated and interdisciplinary research environment drawing on engineering, biomedical sciences, and product development activities in both academia and the IVD industry []. Sharing knowledge, expertise, as well as financial and technical resources, is key to global improvement of the diagnostic field. Innovative start-ups, global players in the clinical diagnostics industry, leading academic institutions, and health care institutions should jointly provide expertise in the domain of medical diagnostics, intellectual property development, marketing, business development and sales. Medical institutions, but also the many (independent) biobanks, should provide access to relevant patient samples (clinical and controls) for verification and validation of new tests []. Comprehensive outreach, science communication efforts, and stakeholder engagement are essential to optimize the use of adequate diagnostics.
An important recent example of the benefits of such an integrated approach was provided during the COVID-19 pandemic. All parties involved generated a huge portfolio of diagnostic tests, immunological and molecular, many of which were rapidly authorized for emergency use (Emergency Use Authorization, EUA) by the US Food and Drug Administration (FDA) [,]. The availability of such approved tests allowed for the implementation of high-quality molecular tests during the first wave of the pandemic. Examples are (semi)-quantitative multiplex PCR tests, rapid lateral flow antigen and antibody tests, and most recently the exploitation of next-generation DNA sequencing technologies [,]. It has to be noted that this also introduced problems concerning the required capacities of several of these tests, and rapidity of introduction was not always compatible with test quality. Hence, a portion of the immunological tests in particular had to be withdrawn from the market upon accumulation of diagnostic data.
From a purely technological perspective there are a number of competing approaches that will significantly influence the use of adhesion in clinical microbiological diagnostics. Three of these technologies need a brief assessment. First, PCR and related nucleic acid amplification technologies are sensitive, increasingly cheap, can be deployed widely and essentially detect all microbial species and resistance genes. This technology can be used directly on clinical specimens and it strongly depends on the number of pathogen cells available whether adhesion-based enrichment of such pathogens is required or not. Second, next generation (genome) sequencing (NGS) has become faster and more cost-effective over recent decades, and this is likely to continue. Its performance will soon equal or better that of amplification testing. This suggests that NGS will be routinely used in the microbiology lab. Finally, there is an increase in the rapid availability of high-affinity specific binding reagents other than functional adhesins, such as (monoclonal) antibodies, adhirons and aptamers [,]. These have the advantage that they share a basic molecular structure, rendering them suitable for “plug and play” diagnostic applications using the same platforms. If a good diagnostic platform has also been developed, essentially all binding reagents can be applied. Adhesion based assays will have to compete with tests based upon the three concepts mentioned above.
In the following sections, we describe the major interactions between microbial adhesins and host ligands or receptors. We try to define what further structural biology studies are needed, and how these might be useful in the development of novel diagnostic tests.
2. Microbial Adhesin–Receptor Pairs
The initial interaction between pathogens and their hosts is defined at the molecular level by the selective interaction of pathogen adhesins with their host receptors. This specific interplay can be exploited in various stages of the classical microbiological diagnostic workflow. To do so, we must extend our understanding of the basic principles of pathogen adhesion so we can apply adhesion assays in the initial capture and enrichment of (complete or parts of) pathogens []. All microbial adhesion molecules are surface exposed structures, but their expression may depend on physiological parameters such as environmental temperature, growth stage or availability of nutrients [,]. Understanding precisely how microbial adhesins interact with their host receptors poses challenges because the receptor may, for instance, be part of a structurally complex cellular membrane []. Site-directed mutagenesis and adhesion assays with whole cells or purified adhesins have shed light on basic aspects of the binding interactions [].
The emergence of SARS-CoV-2 has made it clear that not all adhesins have been identified yet. New ones can be detected using nucleic acid sequencing strategies and searches for new structures that are homologous to known adhesins. Otherwise, proteomic research can be applied to detect cellular surface proteins that provide adhesive characteristics. Random knock-out mutagenesis can also generate cells deficient in adhesion and reverse genetics then allows the functional analysis of the genes and proteins involved. Biophysical technologies can be used to define adhesin structures (see sections below).
2.1. Viral Adhesion Processes
Viruses, with relatively small genomes, have a limited repertoire of adhesin structures per individual viral lineage, although an individual adhesin structure is repeated frequently on a single virion. It has to be noted that viruses that are becoming endemic or pandemic exist, even in single hosts, as a species swarm with differing receptor affinities. Recent examples include the SARS-CoV-2 variants such as the B.1.1.7 (α-variant, UK), B.1.351 (β-variant, South Africa), P.1 (γ-variant, Brazilian) and B.617.2 (δ-variant, India) variants of concern []. The viral surface is normally quite homogenous allowing for fewer possible receptor specificities []. Viral interactions with glycan-based receptors are frequent but typically have affinities in the mM range, while interactions with protein receptors are usually of higher affinity [,,,,]. For example, human coronavirus NL63 (HCoV-NL63) uses heparan sulfate proteoglycans (HSPGs) as the initial host receptor. The membrane protein (M) of HCoV-NL63 mediates this attachment to HSPGs and is not spike (S) protein-dependent. It was recently shown that the M protein is also an important player during the early stages of HCoV-NL63 infection, thereby identifying a new adhesin for this virus []. Both fungi and viruses exploit a variety of immune modulators to achieve host colonization [,]. Recent examples of human receptors relevant for SARS-CoV-2 adhesion are described in Box 1. The emergence of new viruses will undoubtedly lead to the identification of new viral adhesins.
Box 1. Adhesion of SARS-CoV-2, the causative agent of COVID-19.
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The precise mechanisms of disease are still incompletely understood [] and replicating virus particles can be observed in a variety of host tissues. Nonetheless, two key host receptors have been identified: angiotensin-converting enzyme 2 (ACE2) [,] and liver/lymph node-specific intracellular adhesion molecule-3 grabbing non-integrin (L-SIGN) [,]. The crystal structure for ACE2 complexed with the receptor binding domain (RBD) of the SARS-CoV-2 spike protein was solved [,]. The binary complex showed clear conservation as compared to similar complexes for the original SARS-CoV-1 virus. This hints at functional conservation of the process of ACE2 binding, but also at the possibility of immunological cross-influences between SARS-CoV-1 and SARS-CoV-2 antibodies. Other forms of structure-based strategies would be the use of the RBD as a subunit vaccine [] or as a target for inhibition by possible compounds. Neuropilin recognizes a furin cleavage on the SARS-CoV-2 spike protein and is a key target in the development of therapeutics against COVID-19. Recently, X-ray structure-based studies of the neuropilin complexed to the S fragment of the spike protein indicated potentially important design opportunities for therapeutic compounds [,]. In addition, virtual drug screening and actual high-throughput screening of compound libraries has been exploited for the key SARS-CoV-2 protease MPRO, which led to the successful identification of potential antiviral drugs []. Despite a wide variety of new tests [], formats based on anti-adhesive strategies have not yet been developed for this priority pathogen.
2.2. Modes of Bacterial Adhesion
The nature of the bacterial adhesion molecule varies from distinct organelles such as flagellae or fimbriae to surface exposed, cell wall- or cell membrane-attached proteins, lipids, and sugar (poly- or oligo-saccharide) moieties []. Adhesins, especially proteinaceous ones, are often repetitive in primary structure, either by repeating similar domains within a protein chain or by polymerizing subunits into long fibrous structures [,]. Most bacterial adhesins tend to bind to host structures that are also often structurally repetitive and ubiquitously distributed, including extracellular matrix (ECM) components such as collagen, fibronectin, or glycoprotein receptors that harbor repeating carbohydrate units.
In certain bacterial adhesins, such as the trimeric autotransporter adhesins (TAAs) [], the individual binding affinities can be very low (0.1–0.5 M). In this context, binding of pathogens to host cell surfaces is accomplished by avidity, like ‘Velcro’ on a shoe: the three-dimensional arrangement of multiple weak binding sites leads to tight and hence effective binding [,,]. TAAs are can be divided into three domains; a membrane anchored β-barrel domain, a stalk domain and a head domain [,,]. The head domain, once assembled, then adheres to the host ECM via, for example, collagen, vitronectin or fibronectin []. Recent work showed that different adhesins bind differently to ECM components and that binding is dramatically influenced by shear forces [,,,]. In general, improving our understanding of adhesin–receptor interaction requires more detailed insights into their structural aspects [].
2.3. Adhesion Diversity and Evolution
Surface exposed adhesion domains are external moieties and exposed parts of the proteins may be subject to strong environmental selection and possible natural adaptation [,]. Hence, the evolution of pathogens is critically linked to the variation in adhesins and their receptor affinities [], potentially allowing for the colonization of novel hosts. Evolutionary changes in adhesins and ligands can be easily identified by NGS combined with quantitative MS-based proteomics, a combination referred to as proteo-genomics [,,,]. For instance, conserved peptide sequences (conserved at least within the same species) can be used to perform quantification of species–specific peptides in complex (patient-derived) samples [,,]. The combination of these two methods thus facilitates the correlation of genotypes with adhesion-related phenotypes. There is a continued need for the characterization of additional adhesin–ligand pairs to define conservation of the adhesin or ligand between microbial species.
3. Structural Analysis of Adhesin–Ligand Pairs
There are few structures of bacterial adhesins complexed with their ligands, even though there are many of virus-receptor complexes [,]. This may be due to the low-affinity/high-avidity binding of bacterial adhesins, leading to many different complexes and frequent non-specific aggregation. This is problematic because (a) it makes it hard to define the biologically relevant interactions and (b) structural techniques, even cryo-electron microscopy, depend on having a small number (<10) of different conformations and complexes in a single experiment. The modular repetitive structure of some bacterial adhesins and of their host receptors (collagen, laminin, fibronectin, etc.) hampers the determination of their structure and specific interactions by standard methods. Nonetheless, we believe that structural investigations will contribute to the design of better adhesin constructs. These could in return serve as diagnostic tools, as vaccine components, and potentially to develop anti-adhesive drugs.
Technological and Methodological Developments
NMR spectroscopy, X-ray crystallography, cryo-electron microscopy (cryoEM; see [,] for reviews), and mass spectrometry (MS) can be used to characterize the individual adhesin binding domains or their receptors in molecular detail. CryoEM and, to a lesser extent, X-ray crystallography are the best methods for higher-order assemblies. Furthermore, cross-linking MS (XL–MS) and hydrogen-deuterium exchange MS (HDX–MS) are being increasingly used to determine structural constraints between interacting proteins, protein complexes, and their binding interfaces [,,,,]. Such constraints can facilitate binding optimization, improvement of ligand design, and thus improved capture for pre-analytical diagnostic steps [,,,,,,].
Nano-biosensors for miniaturized detection of adhesion, (cryo)EM for visualization of adhesion and identification of molecular partners, X-ray crystallography for the definition of global adhesin structure and more generic tools like NMR for local information on binding partners, and advanced bioinformatics all play essential roles in the further optimization of structure determination and translational applications [,,]. Integrative methods for structure determination of adhesin complexes and for defining their clinical relevance have been shown to be useful [,,,,]. For example, structure analysis of Yersinia enterocolitica YadA helped to further the understanding of interleukin-1 expression by epithelial cells []. The structure of the Escherichia coli immunoglobulin binding proteins (Eibs) showed how they are involved in entero–hemorrhagic pathogenicity [,]. Another innovative tool that was developed for use in molecular recognition applications was the use of “adhirons” to help stabilize complexes and to gain structural information (e.g., []). Adhirons are non-antibody scaffold binding proteins []. Well-characterized adhirons display low-nanomolar affinity and high specificity for defined proteins and selectively recognize their target molecules.
4. Microbial Adhesion and Future High-Throughput Diagnostic Microbiology Technology
Exploiting the adhesion capacity of pathogens for in vitro diagnostics is a relatively new concept [,], but adhesion-based principles can be applied at various stages of the diagnostic process: for specific staining of bacteria, for enhanced species identification, for antimicrobial susceptibility testing, or ultimately maybe even for in vivo therapy (Figure 3). Therefore, prerequisites for further translation into clinical practice are bottom-up research in adhesion from a clinical research perspective, the definition of molecular structure–function relationships, and the design and development of new diagnostic tests and devices [].
4.1. Adhesin-Based Sample Processing in Microbial Diagnostics
Bacterial or viral detection and identification is a complicated multi-step process starting with the collection of clinical samples of diverse origin (blood, sputum, saliva, feces, tears, biopsies, etc.) and consistency (purity, presence of contaminating and possibly test-inhibitory host factors, other microbial species, etc.). Several IVD development projects aim to optimize existing diagnostic tests or to develop novel, specific, and preferably ‘point-of-care’ (PoC) diagnostic tools []. We envisage the ability of enriching pathogens from complex samples (e.g., fecal specimens, sputa, or urine samples) to a level where they are free of contaminants and relatively easy to detect by classical tests (Figure 4). This approach is useful in settings with significant sample heterogeneity and contamination, where low numbers of pathogens are present, and where classical clinical microbiology is prone to fail resulting in false-negatives. For instance, E. coli O157:H7 has been successfully enriched from contaminated water samples []; and it was demonstrated that cell wall binding domains derived from bacteriophage proteins could be used to enrich Listeria monocytogenes cells []. Pathogen adhesion capacity can be integrated into the pre-analytical sample handling before the actual detection assays to create a unified high-throughput device or protocol.
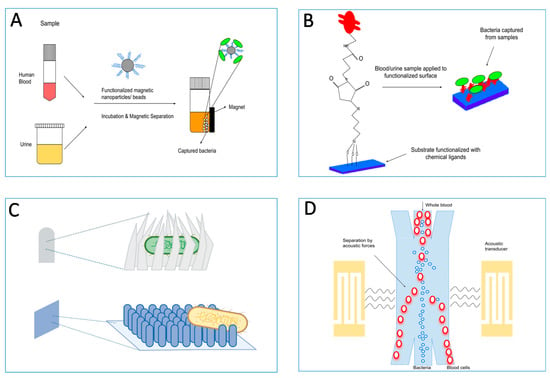
Figure 4.
Adhesin–ligand interactions can be used to enrich pathogens from complex mixtures (e.g., fecal specimens, sputa, or urine samples) to a level where they would be clean and relatively easily detectable by classical tests. (A) Most common pre-enrichment methods make use of functionalized magnetic particles such as nanoparticles or beads, which bind to pathogens present in biological samples and are afterwards separated magnetically. (B) Surfaces functionalized with chemical cross-linkers and affinity ligands are used to directly capture bacteria with high specificity from biological samples. (C) Various types of nano-topographies such as prickly or nano-patterned surfaces, or nano-claws are used to capture bacteria. They are used alone or in combination with capture ligands. (D) Separation of bacteria from blood cells using surface acoustic waves in a microfluidic device. Other separation techniques such as viscoelastic separation are also used in microfluidic devices.
In many cases the sample needs to be pre-treated in order to prepare it for the actual diagnostic process. Unfortunately, uniform processing methods for samples of diverse origin are rare (for a review see [] and references therein). Despite the numerous examples listed in Table 2, the development of methods for working with variable sample types and requirements of novel adhesion-based pre-analytical steps for clinical diagnostics are still in their infancy. Further developmental efforts are needed to translate research on adhesion and pathogen capture into diagnostic tests for detection of infections or colonization (e.g., with MDR pathogens).

Table 2.
Review of pre-analytical target enrichment methods using adhesion receptor interactions to detect infection. Note that samples mostly consisted of artificially spiked materials. Hence, most of the tests target bacteria. The test costs could not be compared, as data were frequently missing. (LPS: Lipo-Poly-Saccharide; LTA: Lipo-Teichoic Acid).
Progress has been made with certain receptors and bacterial ligands, however. Mannose binding lectin is a host receptor capable of signaling or sensing pathogens exposing mannose at their outer cell surface, and can thus be used to capture a variety of microbial species [,]. If mannose binding lectin is attached to a solid surface, mannose-presenting pathogens can be captured on the surface []. This approach allows highly sensitive detection of pathogens from a clinical sample at the capturing surface and is applicable in a variety of downstream classical and molecular diagnostic methods. It has been successfully applied in sepsis testing in experimental animals, where an extra-corporal blood-cleansing device was developed to detect pathogens circulating in their blood [].
A second example concerns protein A (SpA), a surface protein expressed by Staphylococcus aureus and other species of coagulase positive staphylococci []. SpA has high affinity for the Fc region of IgG antibodies. When immobilized to a solid support, SpA can be used to affinity purify Langerhans cells expressing receptors for the Fc portion of IgG (Fc-IgG), thus generating clean specimens that are well suited for various formats of immune detection []. Binding via the Fc part supports the proper presentation of the antigen-binding sites of not only natural but also monoclonal antibodies [,]. SpA has proven to be an important biotechnological tool not only in the development of immune tests, but also for the purification and concentration of a variety of human and animal antibodies []. However, recent work has shown that SpA does not bind all antibodies uniformly well, an issue that must be kept in mind when developing SpA-mediated protocols []. With a similar approach, a 50 amino acid residue termed SAP peptide has been derived from M protein, one of the major virulence factors of Streptococcus pyogenes. This can be used to enrich IgA from biological mixtures [].
4.2. Target Enrichment Technology
The development of diagnostic tools requires detailed testing in artificially spiked and “real-life” samples from clinical, environmental, and industrial origins. Institutional or commercially available pathogen strain collections should be used to define the natural variation in the adhesins and the effect of such variation on adhesion efficiency and, hence, test quality []. This could even work for an unknown pathogen if the adhesin in question were reasonably well-conserved. The SpA of a S. non-aureus strain or a new Coronavirus spike protein are significant examples. The question in novel sample enrichment approaches is whether there is a need for test devices that can capture most if not all clinically relevant pathogens or whether sequential testing would be a better option. In any case, new technologies should preferably allow the direct enrichment of pathogens from low-titer samples.
5. Clinical–Diagnostic Application of Pathogen Adhesion Tests
High content proteomics [], electrochemical biosensors [,], lanthanide-based fluorescent up-conversion particle assays for detection of adhesin–ligand binding [], and In Situ Hybridization (ISH) using peptide nucleic acid (PNA) probes and nano-biosensors [] are only four examples of complex experimental technologies to identify adhesin–receptor interactions, all of which can be translated into new IVD test formats. Such tests can be used in translational research to simplify and accelerate pathogen identification and/or characterization processes (Table 2), which lists tests that are in use summarizing the core competencies and technologies used in these tests.
Currently used physical test platforms range from microfluidic biosensors and nanowires to more classical Raman spectroscopy-, electrochemical- or PCR-based equipment. Furthermore, enrichment platforms frequently utilize nanorods or -wires when straightforward analytical signal detection is required (pathogen present or absent as the final test result). When, after the initial adhesion test, follow-up testing is required in a more preparative manner, magnetic beads are by far the most common approach. The entities to be detected can vary from intact cells, through simple enzymes, to the products of nucleic acid amplification reactions. Of note, nucleic acids rarely play a role in microbial adhesion though biofilms contain relatively large amounts of these molecules and are thought to function as adhesins under biofilm conditions []. Still, in diagnosis, nucleic acids are usually employed because they efficiently hybridize to other nucleic acids. These concepts are beyond the scope of the current review.
The most commonly used procedures at the level of detection are coloration, fluorescence measurement, and detection of amplified nucleic acid. The most frequent model organisms used are E. coli and S. aureus, representatives of Gram-negative and Gram-positive pathogens, respectively. All methods described generate results in 7 min to 3 h, show high sensitivity (to about ten colony-forming units at their most sensitive) and are of great quality; still, their implementation into routine use is sparse. Table 2 and references therein summarize studies that have successfully demonstrated the relevance of adhesion for improved microbiological testing.
5.1. Biosensor-Based Pathogen Detection
Biosensors, analytical devices that detect and quantify biomolecules or cells, are composed of three elements: the bioreceptor (allowing binding of the analyte), a transducer (translating the signal into analytical data), and the display set-up []. Their advantages are miniaturization, rapidity, mass-production, low cost, high specificity, and automation. This is especially true for electrochemical biosensors where screen-printing of electrodes (SPE) has allowed further miniaturization []. Biosensors can be integrated into microfluidic platforms allowing efficient, rapid, portable testing, and permitting reduced volumes of analyte and waste []. The basic biosensor consists of a bioreceptor molecule attached to a transducer surface. Upon analyte binding, a recordable change at the transducer surface is measured, usually electrochemical, optical, or mechanical. Different types of bio-receptors are employed for pathogen detection, where antibodies are currently the Gold standard []. Other biosensor receptors include, for instance, lectins and bacteriophages or subunits thereof []. Most biosensors rely on antibodies or DNA, but the use of adhesins and ECM proteins as receptors for biomolecules or pathogens is increasing.
There is promising biosensor-mediated research for different microbial pathogens. E. coli strain ORN178 can be detected through the binding of type-1 fimbriae to α-D-mannose by attaching the sugar to a nanomechanical cantilever in the biosensor []. Upon binding, a change in the resonance frequency of the cantilever is generated. To quantify the adhered bacteria, standard curves displaying the resonance frequencies of the cantilever against bacterial numbers were developed. Biosensors have been developed to study the binding between E. coli and ECM proteins in the presence of polysaccharides [] in a model system, using surface plasmon resonance (SPR) for the inhibition of collagen- and laminin-mediated E. coli binding using poly-sulfated polysaccharides. In this work, the binding of pathogenic E. coli O157:H7 was also evaluated. SPR and electrochemical impedance spectroscopy (EIS) have been used for rapid detection of E. coli through lectin binding using concanavalin A immobilized as a self-assembled monolayer onto a gold electrode surface. These biosensors could be used for screening bacterial load in water samples [].
Legionella collagen-like (Lcl) adhesin binds ECM components and mediates bacterial binding to host cells. Lcl has been used to detect glycosaminoglycans (GAGs) via SPR and EIS onto gold electrodes and gold screen-printed electrodes []. The Lcl proteins were immobilized and exposed to different GAGs (Figure 5). Both SPR and EIS could detect high-affinity binding of GAGs. This shows that both techniques can be used for the diagnosis of L. pneumophila lung infection. In addition, haemagglutinin (HA), a homo-trimeric glycoprotein expressed on the surface of the influenza virus, shows high affinity towards sialic acid-terminated trisaccharides of epidermal cell membranes. Researchers developed both a mechanical and an electrochemical biosensor for the detection of the human influenza virus based on this interaction []. Binding of 2,6-sialyllactose to HA could be detected in a label-free manner via impedance using a Quartz Crystal Microbalance (QCM).
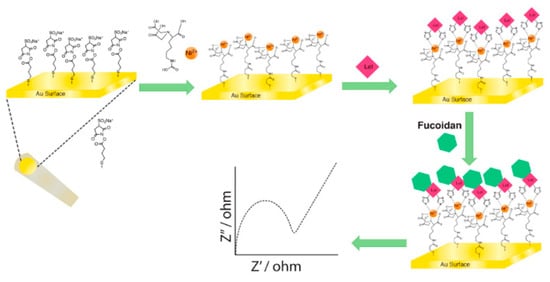
Figure 5.
Illustrative representation of Lcl immobilization and fucoidan detection using EIS. A gold electrode was chemically modified after which nickel was electrostatically bound to the surface. This facilitated the binding of Legionella proteins, which were detected by electrochemical impedance spectroscopy (EIS). Reproduced with permission from [].
5.2. Next Generation Test Formats—Bioactive Surfaces and Materials
There is still an urgent need both for improved insight into how to capture and enrich pathogens from complex clinical samples for downstream analytical diagnostics, and for the design and study of anti-adhesive compounds (to help prevent non-specific binding) []. For instance, do tests exploiting multiple adhesins for target enrichment perform better than assays using a single adhesin or not? What volume of clinical sample is sufficient or needed for adequate diagnostics? Complex clinical specimens (sputa, fecal material, blood) will be much more difficult to work with than, for instance, simple ones such as infected urine with relatively high numbers of pathogens present per unit of volume. In many cases it is also not yet known what the duration of the capture step should be, nor what the costs of an assay are going to be. For IVD manufacturers, knowing the answers to such questions is essential in the decision-making process preceding the development of an adhesion assay.
Current diagnostics for bacterial and viral pathogens are typically unspecific and include cultivations of uncertain sensitivity, as pathogen concentrations as low as one viable count per mL can be indicative of infection []. Enrichment directly from a patient sample can speed up pathogen diagnostics by many hours and increase sensitivity, but may render downstream analysis of testing for, e.g., antibiotic resistance somewhat complex []. Specific ligands (e.g., peptides, peptide nucleic acids, glycans, and aptamers) that bind to ECM, membrane components, or capsids of (a) given pathogen(s) can be immobilized onto the surface of materials used for fabrication of diagnostic devices, such as peptide or nucleic acid arrays, microbeads, membranes, and even electrodes using a paper format. Such bioactive surfaces provide sufficiently high and well-controlled binding capacities for ligands, intact cells, and cellular extracts. The surface characteristics prevent denaturation of the immobilized ligands, allow for convenient and efficient immobilization techniques, and are preferably reversible to allow regeneration. Such surfaces should prevent non-specific interactions, i.e., be anti-adhesive or anti-fouling or even prevent infection, which is particularly relevant for intra-corporeal devices []. A recurring problem is the significant fraction of bacteria that is accidentally lost by non-specific binding in miniaturized devices and microfluidics due to their high surface area to volume ratios. Thus, antifouling properties are of greater importance []. Furthermore, fouling by non-specific biomolecules can also hinder sensitivity and selectivity and result in false-positive or -negative readings []. Improved materials can be obtained by physico–chemical modification of the surface [], grafting [,], coating [], surface topography modification [] and surfactant adsorption []. At present, the development of novel multi-functional materials to capture pathogens from biological samples combining grafting with plasma or UV treatments will help integrate the pre-enrichment methods into full diagnostics workflows [,,]. Overall, this approach can lead to fast, specific, and efficient pathogen trapping strategies, reduced sample processing times, and better sensitivity for downstream detection techniques. The inverse process, anti-adhesion, can be used to develop “clean” materials (see Box 2).
Box 2. Anti-Adhesion.
The development of anti-adhesive materials with minimal fouling, based on ‘grafting-from’ approaches, may be useful to inhibit adhesion. The development of ‘anti-ligands’ to inhibit adhesion provides additional therapeutic approaches. Pilicides, which inhibit the first steps in biofilm formation for E. coli, constitute a special category of such anti-ligands [,], and these are being considered as alternative therapeutic approaches in, for instance, bacterial urinary tract infections []. The target of pilicides is the biofilm, but some also show surprising anti-pilin activity, thereby tackling infections from two fundamentally different angles []. Their diagnostic value is limited at this stage although detection of pilicide activity could indicate early stage biofilm formation. Altogether this could provide important tools for the prevention of (nosocomial) infections in general. Simultaneous detection of multiple pathogens may thus be a distinct possibility. This will facilitate new formats for syndrome testing, where all possible pathogens involved in a certain type of infection can be detected at the same time and ruled in or out simultaneously (e.g., gastro–intestinal or respiratory infections) [,,]. This has clear benefits both in terms of cost, morbidity, and even mortality, as the faster the correct pathogen(s) are identified, the faster the correct treatment can be given.
6. Conclusions
The IVD workflows show the overall state of readiness for innovation in clinical microbiology (Figure 3 and Figure 4). Expansion of the existing portfolio of IVD tests is a must, and innovative adhesion-based assays have been proposed. Some of these are already accepted for routine diagnostic use, but there are still many procedures in development that require additional validation, verification and, in the end, user acceptance as valuable IVD tools. Acceptance of such tests will improve overall public health status by helping control the spread of pathogens and allowing for personalized treatment. We believe that the application of integrative approaches, including bioinformatics, quantitative and structural proteomics and structure modeling, will improve the understanding of the pathogenesis of infectious diseases in general []. Understanding the sequential and structural determinants of adhesion will further drive the translational aspects, leading to the design of novel tests. New technologies, as described here, will play a key role in facilitating this essential phase of test design [,,,]. Cost effective scale-up and application of adhesins to commercially relevant sensor-activated testing systems needs to be implemented in the developmental cycles exploited by commercial companies.
COVID-19 has, at last, made the general public aware of the global impact of infectious diseases and the need for and relevance of rapid diagnostics []. We must seize the moment: this current appreciation of the value of infectious disease testing should be used to push for affordable, high-quality, rapid diagnostic tests for all infectious diseases to be available worldwide. We believe that adhesion research will contribute to this, since it defines fundamental new processes that allow identification and enrichment of new binding partner molecules. Such molecules can then be implemented in IVD using the continuously expanding experimental toolbox to allow sensitive detection of molecular binding events. The combination of accelerated detection and identification of microbial adhesins and the technological capabilities defines a prosperous future for this field of in vitro diagnostics.
Author Contributions
Conceptualization, A.v.B. and D.L.; validation, all other authors; writing—original draft preparation, A.v.B. and D.L.; writing—review and editing, all authors; writing, final review, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation program in a project named Viral and Bacterial Adhesin Network Training (ViBrANT) under Marie Skłodowska-Curie Grant Agreement No. 765042. A.G. also acknowledges support from the BBSRC (grant number BB/M021610/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors gratefully thank Jürgen Berger and Katharina Hipp (both Max Planck-Institute for Developmental Biology, Tübingen, Germany) for the electron microscopy displayed in Figure 1.
Conflicts of Interest
A.v.B., M.G. and R.D. are employees of bioMérieux, a company designing, developing, and marketing tests in the domain of infectious diseases. The company was not involved in the design of the current study and the opinions expressed are those of the authors and may be different from formal company opinions and policies. Carina Almeida (Biomode, Braga, Portugal) and Ian Eastwood (Eluceda, UK) also have commercial affiliations. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Markwell, A.; Mitchell, R.; Wright, A.L.; Brown, A.F. Clinical and ethical challenges for emergency departments during communicable disease outbreaks: Can lessons from Ebola Virus Disease be applied to the COVID-19 pandemic? Emerg. Med. Australas. 2020, 32, 520–524. [Google Scholar] [CrossRef]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Zumla, A.; Memish, Z.A.; Maeurer, M.; Bates, M.; Mwaba, P.; Al-Tawfiq, J.A.; Denning, D.W.; Hayden, F.G.; Hui, D.S. Emerging novel and antimicrobial-resistant respiratory tract infections: New drug development and therapeutic options. Lancet. Infect. Dis. 2014, 14, 1136–1149. [Google Scholar] [CrossRef]
- Sloots, T.P.; Nissen, M.D.; Ginn, A.N.; Iredell, J.R. Rapid identification of pathogens using molecular techniques. Pathology 2015, 47, 191–198. [Google Scholar] [CrossRef]
- Pinsky, B.A.; Hayden, R.T. Cost-Effective Respiratory Virus Testing. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kashimoto, T.; Kado, T.; Akeda, Y.; Yoshioka, K.; Kodama, T.; Yamamoto, M.; Okamura, M.; Kakuda, T.; Ueno, S. Chemotactic invasion in deep soft tissue by Vibrio vulnificus is essential for the progression of necrotic lesions. Virulence 2020, 11, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Phukan, U.J. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med. Microbiol. Immunol. 2019, 208, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19’s Morbidity and Mortality. Int. J. Environ. Res. Public. Health. 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Arciola, C.R.; Pietrocola, G. Fibronectin and Its Role in Human Infective Diseases. Cells 2019, 8, 1516. [Google Scholar] [CrossRef]
- Duell, B.L.; Su, Y.C.; Riesbeck, K. Host-pathogen interactions of nontypeable Haemophilus influenzae: From commensal to pathogen. FEBS Lett. 2016, 590, 3840–3853. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Pietrocola, G.; Arciola, C.R.; Rindi, S.; Montanaro, L.; Speziale, P. Streptococcus agalactiae Non-Pilus, Cell Wall-Anchored Proteins: Involvement in Colonization and Pathogenesis and Potential as Vaccine Candidates. Front. Immunol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Receptor mimicry as novel therapeutic treatment for biothreat agents. Bioeng. Bugs. 2010, 1, 17–30. [Google Scholar] [CrossRef]
- Miller, M.B.; Atrzadeh, F.; Burnham, C.A.; Cavalieri, S.; Dunn, J.; Jones, S.; Mathews, C.; McNult, P.; Meduri, J.; Newhouse, C.; et al. Clinical Utility of Advanced Microbiology Testing Tools. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Dubourg, G.; Lamy, B.; Ruimy, R. Rapid phenotypic methods to improve the diagnosis of bacterial bloodstream infections: Meeting the challenge to reduce the time to result. Clin. Microbiol. Infect. 2018, 24, 935–943. [Google Scholar] [CrossRef]
- Vandenberg, O.; Durand, G.; Hallin, M.; Diefenbach, A.; Gant, V.; Murray, P.; Kozlakidis, Z.; van Belkum, A. Consolidation of Clinical Microbiology Laboratories and Introduction of Transformative Technologies. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus. Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Li, R.; Tan, P.; Zhang, R.; Li, J. mNGS in clinical microbiology laboratories: On the road to maturity. Crit. Rev. Microbiol. 2019, 45, 668–685. [Google Scholar] [CrossRef]
- van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; St George, K.; Rhoads, D.D.; Butler-Wu, S.M.; Dharmarha, V.; McNult, P.; Miller, M.B. Understanding, Verifying, and Implementing Emergency Use Authorization Molecular Diagnostics for the Detection of SARS-CoV-2 RNA. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-Free Biosensors for Laboratory-Based Diagnostics of Infections: Current Achievements and New Trends. Biosensors 2020, 10, 11. [Google Scholar] [CrossRef]
- Bedford, R.; Tiede, C.; Hughes, R.; Curd, A.; McPherson, M.J.; Peckham, M.; Tomlinson, D.C. Alternative reagents to antibodies in imaging applications. Biophys. Rev. 2017, 9, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, X.; Fu, X.; Li, Z.; Huang, Y.; Liang, C. Advances in Aptamer-Based Biomarker Discovery. Front. Cell. Dev. Biol. 2021, 9, 659760. [Google Scholar] [CrossRef]
- Sharon, N.; Ofek, I. Identification of receptors for bacterial lectins by blotting techniques. Methods Enzymol. 1995, 253, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kitts, G.; Giglio, K.M.; Zamorano-Sánchez, D.; Park, J.H.; Townsley, L.; Cooley, R.B.; Wucher, B.R.; Klose, K.E.; Nadell, C.D.; Yildiz, F.H.; et al. A Conserved Regulatory Circuit Controls Large Adhesins in Vibrio cholerae. MBio 2019, 10. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Xia, A.; Zhang, R.; Huang, Y.; Yang, S.; Ni, L.; Jin, F. Carbon Starvation Induces the Expression of PprB-Regulated Genes in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Sequeira, S.; Kavanaugh, D.; MacKenzie, D.A.; Šuligoj, T.; Walpole, S.; Leclaire, C.; Gunning, A.P.; Latousakis, D.; Willats, W.G.T.; Angulo, J.; et al. Structural basis for the role of serine-rich repeat proteins from Lactobacillus reuteri in gut microbe-host interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E2706–E2715. [Google Scholar] [CrossRef] [PubMed]
- Loukachevitch, L.V.; Bensing, B.A.; Yu, H.; Zeng, J.; Chen, X.; Sullam, P.M.; Iverson, T.M. Structures of the Streptococcus sanguinis SrpA Binding Region with Human Sialoglycans Suggest Features of the Physiological Ligand. Biochemistry 2016, 55, 5927–5937. [Google Scholar] [CrossRef]
- Peacock, T.P.; Penrice-Randal, R.; Hiscox, J.A.; Barclay, W.S. SARS-CoV-2 one year on: Evidence for ongoing viral adaptation. J. Gen. Virol. 2021, 102. [Google Scholar] [CrossRef]
- Sherman, M.B.; Smith, H.Q.; Smith, T.J. The Dynamic Life of Virus Capsids. Viruses 2020, 12, 618. [Google Scholar] [CrossRef]
- Neu, U.; Bauer, J.; Stehle, T. Viruses and sialic acids: Rules of engagement. Curr. Opin. Struct. Biol. 2011, 21, 610–618. [Google Scholar] [CrossRef]
- Stroh, L.J.; Stehle, T. Glycan Engagement by Viruses: Receptor Switches and Specificity. Annu. Rev. Virol. 2014, 1, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Bohorova, N.; Bohorov, O.; Goodman, C.; Kim, D.; Pauly, M.H.; Velasco, J.; Whaley, K.J.; Piedra, P.A.; Gilbert, B.E.; et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc. Natl. Acad. Sci. USA 2014, 111, 5992–5997. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Okamoto, M.; Nakakita, S.; Suzuki, A.; Saito, M.; Tamaki, R.; Lupisan, S.; Roy, C.N.; Hiramatsu, H.; Sugawara, K.E.; et al. Antigenic and receptor binding properties of enterovirus 68. J. Virol. 2014, 88, 2374–2384. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Goda, T.; Matsumoto, A.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Direct and label-free influenza virus detection based on multisite binding to sialic acid receptors. Biosens. Bioelectron. 2017, 92, 234–240. [Google Scholar] [CrossRef]
- Naskalska, A.; Dabrowska, A.; Szczepański, A.; Milewska, A.; Jasik, K.P.; Pyrc, K. Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Serrano-Gómez, D.; Domínguez-Soto, A.; Ancochea, J.; Jimenez-Heffernan, J.A.; Leal, J.A.; Corbí, A.L. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J. Immunol. 2004, 173, 5635–5643. [Google Scholar] [CrossRef]
- Wang, Q.C.; Feng, Z.H.; Nie, Q.H.; Zhou, Y.X. DC-SIGN: Binding receptors for hepatitis C virus. Chin. Med. J. 2004, 117, 1395–1400. [Google Scholar] [PubMed]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Jeffers, S.A.; Tusell, S.M.; Gillim-Ross, L.; Hemmila, E.M.; Achenbach, J.E.; Babcock, G.J.; Thomas, W.D., Jr.; Thackray, L.B.; Young, M.D.; Mason, R.J.; et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 15748–15753. [Google Scholar] [CrossRef]
- Amraie, R.; Napoleon, M.A.; Yin, W.; Berrigan, J.; Suder, E.; Zhao, G.; Olejnik, J.; Gummuluru, S.; Muhlberger, E.; Chitalia, V.; et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ke, B.; Feng, Q.; Yang, D.; Lian, Q.; Li, Z.; Lu, L.; Ke, C.; Liu, Z.; Liao, G. Construction and immunogenic studies of a mFc fusion receptor binding domain (RBD) of spike protein as a subunit vaccine against SARS-CoV-2 infection. Chem. Commun. 2020, 56, 8683–8686. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Viela, F.; Mathelié-Guinlet, M.; Viljoen, A.; Dufrêne, Y.F. What makes bacterial pathogens so sticky? Mol. Microbiol. 2020, 113, 683–690. [Google Scholar] [CrossRef]
- Kwon, H.; Squire, C.J.; Young, P.G.; Baker, E.N. Autocatalytically generated Thr-Gln ester bond cross-links stabilize the repetitive Ig-domain shaft of a bacterial cell surface adhesin. Proc. Natl. Acad. Sci. USA 2014, 111, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.; Ottoni, C.; Leo, J.C.; Gulla, S.; Linke, D. The repeat structure of two paralogous genes, Yersinia ruckeri invasin (yrInv) and a “Y. ruckeri invasin-like molecule”, (yrIlm) sheds light on the evolution of adhesive capacities of a fish pathogen. J. Struct. Biol. 2018, 201, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Riess, T.; Autenrieth, I.B.; Lupas, A.; Kempf, V.A. Trimeric autotransporter adhesins: Variable structure, common function. Trends Microbiol. 2006, 14, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.F.; Kaiser, P.O.; Linke, D.; Schwarz, H.; Riess, T.; Schäfer, A.; Eble, J.A.; Kempf, V.A. Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect. Immun. 2011, 79, 2544–2553. [Google Scholar] [CrossRef][Green Version]
- Weidensdorfer, M.; Chae, J.I.; Makobe, C.; Stahl, J.; Averhoff, B.; Müller, V.; Schürmann, C.; Brandes, R.P.; Wilharm, G.; Ballhorn, W.; et al. Analysis of Endothelial Adherence of Bartonella henselae and Acinetobacter baumannii Using a Dynamic Human Ex Vivo Infection Model. Infect. Immun. 2015, 84, 711–722. [Google Scholar] [CrossRef]
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V Secretion Systems: An Overview of Passenger Domain Functions. Front. Microbiol. 2019, 10, 1163. [Google Scholar] [CrossRef]
- Koiwai, K.; Hartmann, M.D.; Linke, D.; Lupas, A.N.; Hori, K. Structural Basis for Toughness and Flexibility in the C-terminal Passenger Domain of an Acinetobacter Trimeric Autotransporter Adhesin. J. Biol. Chem. 2016, 291, 3705–3724. [Google Scholar] [CrossRef]
- Bassler, J.; Hernandez Alvarez, B.; Hartmann, M.D.; Lupas, A.N. A domain dictionary of trimeric autotransporter adhesins. Int. J. Med. Microbiol. 2015, 305, 265–275. [Google Scholar] [CrossRef]
- Shin, P.K.; Pawar, P.; Konstantopoulos, K.; Ross, J.M. Characteristics of new Staphylococcus aureus-RBC adhesion mechanism independent of fibrinogen and IgG under hydrodynamic shear conditions. Am. J. Physiol. Cell. Physiol. 2005, 289, C727–C734. [Google Scholar] [CrossRef]
- Reichhardt, C.; Wong, C.; Passos da Silva, D.; Wozniak, D.J.; Parsek, M.R. CdrA Interactions within the Pseudomonas aeruginosa Biofilm Matrix Safeguard It from Proteolysis and Promote Cellular Packing. MBio 2018, 9. [Google Scholar] [CrossRef]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Stones, D.H.; Krachler, A.M. Fatal attraction: How bacterial adhesins affect host signaling and what we can learn from them. Int. J. Mol. Sci. 2015, 16, 2626–2640. [Google Scholar] [CrossRef]
- Adrian, J.; Bonsignore, P.; Hammer, S.; Frickey, T.; Hauck, C.R. Adaptation to Host-Specific Bacterial Pathogens Drives Rapid Evolution of a Human Innate Immune Receptor. Curr. Biol. 2019, 29, 616–630.e5. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, T.D.; Annis, D.S.; O’Neill, M.B.; Bohr, L.L.; Smith, T.M.; Poinar, H.N.; Mosher, D.F.; Pepperell, C.S. Adaptation in a Fibronectin Binding Autolysin of Staphylococcus saprophyticus. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Markus, B.; Carstens Marques de Sousa, K.; Marcos-Hadad, E.; Mugasimangalam, R.C.; Nachum-Biala, Y.; Hawlena, H.; Covo, S.; Harrus, S. Prophage-Driven Genomic Structural Changes Promote Bartonella Vertical Evolution. Genome. Biol. Evol. 2018, 10, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, A.; Harris, S.R.; Röltgen, K.; Dangy, J.P.; Hauser, J.; Kingsley, R.A.; Connor, T.R.; Sie, A.; Hodgson, A.; Dougan, G.; et al. Emergence of a new epidemic Neisseria meningitidis serogroup A Clone in the African meningitis belt: High-resolution picture of genomic changes that mediate immune evasion. MBio 2014, 5, e01974-14. [Google Scholar] [CrossRef]
- Diderrich, R.; Kock, M.; Maestre-Reyna, M.; Keller, P.; Steuber, H.; Rupp, S.; Essen, L.O.; Mösch, H.U. Structural Hot Spots Determine Functional Diversity of the Candida glabrata Epithelial Adhesin Family. J. Biol. Chem. 2015, 290, 19597–19613. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Tchesnokova, V.; McVeigh, A.; Kisiela, D.I.; Dori, K.; Navarro, A.; Sokurenko, E.V.; Savarino, S.J. Adaptive evolution of class 5 fimbrial genes in enterotoxigenic Escherichia coli and its functional consequences. J. Biol. Chem. 2012, 287, 6150–6158. [Google Scholar] [CrossRef]
- Lorenz, S.C.; Monday, S.R.; Hoffmann, M.; Fischer, M.; Kase, J.A. Plasmids from Shiga Toxin-Producing Escherichia coli Strains with Rare Enterohemolysin Gene (ehxA) Subtypes Reveal Pathogenicity Potential and Display a Novel Evolutionary Path. Appl. Environ. Microbiol. 2016, 82, 6367–6377. [Google Scholar] [CrossRef]
- Sjöholm, K.; Kilsgård, O.; Teleman, J.; Happonen, L.; Malmström, L.; Malmström, J. Targeted Proteomics and Absolute Protein Quantification for the Construction of a Stoichiometric Host-Pathogen Surface Density Model. Mol. Cell. Proteom. 2017, 16, S29–S41. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Malmström, L.; Aebersold, R.; Malmström, J. Proteome-wide selected reaction monitoring assays for the human pathogen Streptococcus pyogenes. Nat. Commun. 2012, 3, 1301. [Google Scholar] [CrossRef]
- Karlsson, C.A.Q.; Järnum, S.; Winstedt, L.; Kjellman, C.; Björck, L.; Linder, A.; Malmström, J.A. Streptococcus pyogenes Infection and the Human Proteome with a Special Focus on the Immunoglobulin G-cleaving Enzyme IdeS. Mol. Cell. Proteom. 2018, 17, 1097–1111. [Google Scholar] [CrossRef]
- Lawson, C.L.; Chiu, W. Comparing cryo-EM structures. J. Struct. Biol. 2018, 204, 523–526. [Google Scholar] [CrossRef]
- Earl, L.A.; Falconieri, V.; Subramaniam, S. Microbiology catches the cryo-EM bug. Curr. Opin. Microbiol. 2018, 43, 199–207. [Google Scholar] [CrossRef]
- Hauri, S.; Khakzad, H.; Happonen, L.; Teleman, J.; Malmström, J.; Malmström, L. Rapid determination of quaternary protein structures in complex biological samples. Nat. Commun. 2019, 10, 192. [Google Scholar] [CrossRef]
- Bastos, V.A.; Gomes-Neto, F.; Rocha, S.L.G.; Teixeira-Ferreira, A.; Perales, J.; Neves-Ferreira, A.G.C.; Valente, R.H. The interaction between the natural metalloendopeptidase inhibitor BJ46a and its target toxin jararhagin analyzed by structural mass spectrometry and molecular modeling. J. Proteom. 2020, 221, 103761. [Google Scholar] [CrossRef] [PubMed]
- Slavata, L.; Chmelik, J.; Kavan, D.; Filandrová, R.; Fiala, J.; Rosůlek, M.; Mrázek, H.; Kukačka, Z.; Vališ, K.; Man, P.; et al. MS-Based Approaches Enable the Structural Characterization of Transcription Factor/DNA Response Element Complex. Biomolecules 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Seacrist, C.D.; Kuenze, G.; Hoffmann, R.M.; Moeller, B.E.; Burke, J.E.; Meiler, J.; Blind, R.D. Integrated Structural Modeling of Full-Length LRH-1 Reveals Inter-domain Interactions Contribute to Receptor Structure and Function. Structure 2020, 28, 830–846.e9. [Google Scholar] [CrossRef]
- Zhang, M.M.; Beno, B.R.; Huang, R.Y.; Adhikari, J.; Deyanova, E.G.; Li, J.; Chen, G.; Gross, M.L. An Integrated Approach for Determining a Protein-Protein Binding Interface in Solution and an Evaluation of Hydrogen-Deuterium Exchange Kinetics for Adjudicating Candidate Docking Models. Anal. Chem. 2019, 91, 15709–15717. [Google Scholar] [CrossRef] [PubMed]
- López-Castilla, A.; Thomassin, J.L.; Bardiaux, B.; Zheng, W.; Nivaskumar, M.; Yu, X.; Nilges, M.; Egelman, E.H.; Izadi-Pruneyre, N.; Francetic, O. Structure of the calcium-dependent type 2 secretion pseudopilus. Nat. Microbiol. 2017, 2, 1686–1695. [Google Scholar] [CrossRef]
- Bardiaux, B.; de Amorim, G.C.; Luna Rico, A.; Zheng, W.; Guilvout, I.; Jollivet, C.; Nilges, M.; Egelman, E.H.; Izadi-Pruneyre, N.; Francetic, O. Structure and Assembly of the Enterohemorrhagic Escherichia. Coli. Type 4 Pilus. Structure 2019, 27, 1082–1093.e5. [Google Scholar] [CrossRef]
- Bardiaux, B.; Cordier, F.; Brier, S.; López-Castilla, A.; Izadi-Pruneyre, N.; Nilges, M. Dynamics of a type 2 secretion system pseudopilus unraveled by complementary approaches. J. Biomol. NMR 2019, 73, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Ashton, A.; Brandt, R.; Butcher, S.; Carzaniga, R.; Chiu, W.; Collinson, L.; Doux, P.; Duke, E.; Ellisman, M.H.; et al. A 3D cellular context for the macromolecular world. Nat. Struct. Mol. Biol. 2014, 21, 841–845. [Google Scholar] [CrossRef]
- He, L.; Bardiaux, B.; Ahmed, M.; Spehr, J.; König, R.; Lünsdorf, H.; Rand, U.; Lührs, T.; Ritter, C. Structure determination of helical filaments by solid-state NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2016, 113, E272–E281. [Google Scholar] [CrossRef] [PubMed]
- Ferber, M.; Kosinski, J.; Ori, A.; Rashid, U.J.; Moreno-Morcillo, M.; Simon, B.; Bouvier, G.; Batista, P.R.; Müller, C.W.; Beck, M.; et al. Automated structure modeling of large protein assemblies using crosslinks as distance restraints. Nat. Methods 2016, 13, 515–520. [Google Scholar] [CrossRef]
- Ramboarina, S.; Garnett, J.A.; Zhou, M.; Li, Y.; Peng, Z.; Taylor, J.D.; Lee, W.C.; Bodey, A.; Murray, J.W.; Alguel, Y.; et al. Structural insights into serine-rich fimbriae from Gram-positive bacteria. J. Biol. Chem. 2010, 285, 32446–32457. [Google Scholar] [CrossRef]
- Olano, J.P.; Walker, D.H. Diagnosing emerging and reemerging infectious diseases: The pivotal role of the pathologist. Arch. Pathol. Lab. Med. 2011, 135, 83–91. [Google Scholar] [CrossRef]
- Ramanan, P.; Bryson, A.L.; Binnicker, M.J.; Pritt, B.S.; Patel, R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Özenci, V.; Patel, R.; Ullberg, M.; Strålin, K. Demise of Polymerase Chain Reaction/Electrospray Ionization-Mass Spectrometry as an Infectious Diseases Diagnostic Tool. Clin. Infect. Dis. 2018, 66, 452–455. [Google Scholar] [CrossRef]
- Campos, M.; Francetic, O.; Nilges, M. Modeling pilus structures from sparse data. J. Struct. Biol. 2011, 173, 436–444. [Google Scholar] [CrossRef]
- Schmid, Y.; Grassl, G.A.; Buhler, O.T.; Skurnik, M.; Autenrieth, I.B.; Bohn, E. Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect. Immun. 2004, 72, 6780–6789. [Google Scholar] [CrossRef]
- Leo, J.C.; Goldman, A. The immunoglobulin-binding Eib proteins from Escherichia coli are receptors for IgG Fc. Mol. Immunol. 2009, 46, 1860–1866. [Google Scholar] [CrossRef]
- Leo, J.C.; Łyskowski, A.; Hattula, K.; Hartmann, M.D.; Schwarz, H.; Butcher, S.J.; Linke, D.; Lupas, A.N.; Goldman, A. The structure of E. coli IgG-binding protein D suggests a general model for bending and binding in trimeric autotransporter adhesins. Structure 2011, 19, 1021–1030. [Google Scholar] [CrossRef]
- Kearney, K.J.; Pechlivani, N.; King, R.; Tiede, C.; Phoenix, F.; Cheah, R.; Macrae, F.L.; Simmons, K.J.; Manfield, I.W.; Smith, K.A.; et al. Affimer proteins as a tool to modulate fibrinolysis, stabilize the blood clot, and reduce bleeding complications. Blood 2019, 133, 1233–1244. [Google Scholar] [CrossRef]
- Tiede, C.; Tang, A.A.; Deacon, S.E.; Mandal, U.; Nettleship, J.E.; Owen, R.L.; George, S.E.; Harrison, D.J.; Owens, R.J.; Tomlinson, D.C.; et al. Adhiron: A stable and versatile peptide display scaffold for molecular recognition applications. Protein Eng. Des. Sel. 2014, 27, 145–155. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H.; Amini, B. A fluorescence Nano-biosensors immobilization on Iron (MNPs) and gold (AuNPs) nanoparticles for detection of Shigella spp. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110113. [Google Scholar] [CrossRef]
- Govender, V.S.; Ramsugit, S.; Pillay, M. Mycobacterium tuberculosis adhesins: Potential biomarkers as anti-tuberculosis therapeutic and diagnostic targets. Microbiology 2014, 160, 1821–1831. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef]
- He, X.; Zhou, L.; He, D.; Wang, K.; Cao, J. Rapid and ultrasensitive E. coli O157:H7 quantitation by combination of ligandmagnetic nanoparticles enrichment with fluorescent nanoparticles based two-color flow cytometry. Analyst 2011, 136, 4183–4191. [Google Scholar] [CrossRef]
- Kretzer, J.W.; Lehmann, R.; Schmelcher, M.; Banz, M.; Kim, K.P.; Korn, C.; Loessner, M.J. Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells. Appl. Environ. Microbiol. 2007, 73, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Pilecky, M.; Schildberger, A.; Orth-Höller, D.; Weber, V. Pathogen enrichment from human whole blood for the diagnosis of bloodstream infection: Prospects and limitations. Diagn. Microbiol. Infect. Dis. 2019, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Auriti, C.; Prencipe, G.; Moriondo, M.; Bersani, I.; Bertaina, C.; Mondì, V.; Inglese, R. Mannose-Binding Lectin: Biologic Characteristics and Role in the Susceptibility to Infections and Ischemia-Reperfusion Related Injury in Critically Ill Neonates. J. Immunol. Res. 2017, 2017, 7045630. [Google Scholar] [CrossRef] [PubMed]
- Unione, L.; Gimeno, A.; Valverde, P.; Calloni, I.; Coelho, H.; Mirabella, S.; Poveda, A.; Arda, A.; Jimenez-Barbero, J. Glycans in Infectious Diseases. A Molecular Recognition Perspective. Curr. Med. Chem. 2017, 24, 4057–4080. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 2003, 40, 423–429. [Google Scholar] [CrossRef]
- Kangwa, M.; Yelemane, V.; Ponnurangam, A.; Fernández-Lahore, M. An engineered Staphylococcal Protein A based ligand: Production, characterization and potential application for the capture of Immunoglobulin and Fc-fusion proteins. Protein Expr. Purif. 2019, 155, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Templier, V.; Roux, A.; Roupioz, Y.; Livache, T. Ligands for label-free detection of whole bacteria on biosensors: A review. Trac-Trend. Anal. Chem. 2016, 79, 71–79. [Google Scholar] [CrossRef]
- Dunne, M.; Loessner, M.J. Modified Bacteriophage Tail Fiber Proteins for Labeling, Immobilization, Capture, and Detection of Bacteria. Methods Mol. Biol. 2019, 1918, 67–86. [Google Scholar] [CrossRef]
- Bicart-See, A.; Rottman, M.; Cartwright, M.; Seiler, B.; Gamini, N.; Rodas, M.; Penary, M.; Giordano, G.; Oswald, E.; Super, M.; et al. Rapid Isolation of Staphylococcus aureus Pathogens from Infected Clinical Samples Using Magnetic Beads Coated with Fc-Mannose Binding Lectin. PLoS ONE 2016, 11, e0156287. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Yang, Y.; Yan, R.; Zhao, Y.; Wang, Y.; Chen, A.; Shao, S.; Jiang, P.; Li, Y.Q. Bacterial species-identifiable magnetic nanosystems for early sepsis diagnosis and extracorporeal photodynamic blood disinfection. Nanoscale 2017, 10, 132–141. [Google Scholar] [CrossRef]
- Jung, S.H.; Hahn, Y.K.; Oh, S.; Kwon, S.; Um, E.; Choi, S.; Kang, J.H. Advection Flows-Enhanced Magnetic Separation for High-Throughput Bacteria Separation from Undiluted Whole Blood. Small 2018, 14, e1801731. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, Y.; Qi, P.; Sun, Y.; Zhang, D.; Yu, L. Lectin functionalized ZnO nanoarrays as a 3D nano-biointerface for bacterial detection. Talanta 2017, 167, 600–606. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhu, B.; Li, Y.; Leow, W.R.; Goh, R.; Ma, B.; Fong, E.; Tang, M.; Chen, X. A synergistic capture strategy for enhanced detection and elimination of bacteria. Angew. Chem. Int. Ed. Engl. 2014, 53, 5837–5841. [Google Scholar] [CrossRef]
- Liu, L.; Chen, S.; Xue, Z.; Zhang, Z.; Qiao, X.; Nie, Z.; Han, D.; Wang, J.; Wang, T. Bacterial capture efficiency in fluid bloodstream improved by bendable nanowires. Nat. Commun. 2018, 9, 444. [Google Scholar] [CrossRef]
- Wu, R.; Ma, Y.; Pan, J.; Lee, S.H.; Liu, J.; Zhu, H.; Gu, R.; Shea, K.J.; Pan, G. Efficient capture, rapid killing and ultrasensitive detection of bacteria by a nano-decorated multi-functional electrode sensor. Biosens. Bioelectron. 2018, 101, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sheng, E.Z.; Lu, Y.X.; Xiao, Y.; Li, Z.X.; Wang, H.S.; Dai, Z.H. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip. Biosens. Bioelectron. 2021, 181. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, Y.; Zhang, D. An integrated multifunctional photoelectrochemical platform for simultaneous capture, detection, and inactivation of pathogenic bacteria. Sensor. Actuat. B-Chem. 2018, 274, 228–234. [Google Scholar] [CrossRef]
- Dao, T.N.T.; Lee, E.Y.; Koo, B.; Jin, C.E.; Lee, T.Y.; Shin, Y. A microfluidic enrichment platform with a recombinase polymerase amplification sensor for pathogen diagnosis. Anal. Biochem. 2018, 544, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.; Petersson, K.; Augustsson, P.; Laurell, T. Acoustic impedance matched buffers enable separation of bacteria from blood cells at high cell concentrations. Sci. Rep. 2018, 8, 9156. [Google Scholar] [CrossRef]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab. Chip. 2018, 18, 923–932. [Google Scholar] [CrossRef]
- Olanrewaju, A.O.; Ng, A.; DeCorwin-Martin, P.; Robillard, A.; Juncker, D. Microfluidic Capillaric Circuit for Rapid and Facile Bacteria Detection. Anal. Chem. 2017, 89, 6846–6853. [Google Scholar] [CrossRef]
- AbdElFatah, T.; Jalali, M.; Mahshid, S. A nanosurface microfluidic device for capture and detection of bacteria. In Proceedings of the IEEE Life Sciences Conference (LSC), Montreal, QC, Canada, 28–30 October 2018; pp. 167–170. [Google Scholar]
- Qu, Y.; Wei, T.; Zhan, W.; Hu, C.; Cao, L.; Yu, Q.; Chen, H. A reusable supramolecular platform for the specific capture and release of proteins and bacteria. J. Mater. Chem B 2017, 5, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Lee, S.H.; Song, J.; Bhattacharjee, S.; Feng, J.; Hong, S.; Song, M.; Kim, W.; Lee, J.; Bang, D.; et al. Nanophotonic Cell Lysis and Polymerase Chain Reaction with Gravity-Driven Cell Enrichment for Rapid Detection of Pathogens. ACS. Nano 2019, 13, 13866–13874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.B.; Qi, P.; Zhang, D. DNA-templated fluorescent silver nanoclusters for sensitive detection of pathogenic bacteria based on MNP-DNAzyme-AChE complex. Sensor. Actuat. B-Chem. 2018, 276, 42–47. [Google Scholar] [CrossRef]
- Zeltzer, P.M.; Seeger, R.C. Microassay using radioiodinated protein A from Staphylococcus aureus for antibodies bound to cell surface antigens of adherent tumor cells. J. Immunol. Methods 1977, 17, 163–175. [Google Scholar] [CrossRef]
- Schuler, G.; Auböck, J.; Linert, J. Enrichment of epidermal Langerhans cells by immunoadsorption to Staphylococcus aureus cells. J. Immunol. 1983, 130, 2008–2010. [Google Scholar]
- Fishman, J.B.; Berg, E.A. Protein A and Protein G Purification of Antibodies. Cold. Spring. Harb. Protoc. 2019, 2019. [Google Scholar] [CrossRef]
- Rigi, G.; Ghaedmohammadi, S.; Ahmadian, G. A comprehensive review on staphylococcal protein A (SpA): Its production and applications. Biotechnol. Appl. Biochem. 2019, 66, 454–464. [Google Scholar] [CrossRef]
- Cronin, U.P.; Girardeaux, L.; O’Meara, E.; Wilkinson, M.G. Protein A-Mediated Binding of Staphylococcus spp. to Antibodies in Flow Cytometric Assays and Reduction of This Binding by Using Fc Receptor Blocking Reagent. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Sandin, C.; Linse, S.; Areschoug, T.; Woof, J.M.; Reinholdt, J.; Lindahl, G. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J. Immunol. 2002, 169, 1357–1364. [Google Scholar] [CrossRef]
- Gullsby, K.; Olsen, B.; Bondeson, K. Molecular Typing of Mycoplasma pneumoniae Strains in Sweden from 1996 to 2017 and the Emergence of a New P1 Cytadhesin Gene, Variant 2e. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Röst, H.L.; Rosenberger, G.; Navarro, P.; Gillet, L.; Miladinović, S.M.; Schubert, O.T.; Wolski, W.; Collins, B.C.; Malmström, J.; Malmström, L.; et al. OpenSWATH enables automated, targeted analysis of data-independent acquisition MS data. Nat. Biotechnol. 2014, 32, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Wang, K.; Li, Z.; Wang, C.; Hao, Z.; Liu, W.; Wan, J. An electrochemical biosensor based on methylene blue-loaded nanocomposites as signal-amplifying tags to detect pathogenic bacteria. Analyst 2020, 145, 4328–4334. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Zhang, Y.; Das, G.K.; Vijayaragavan, V.; Xu, Q.C.; Padmanabhan, P.; Bhakoo, K.K.; Selvan, S.T.; Tan, T.T. “Smart” theranostic lanthanide nanoprobes with simultaneous up-conversion fluorescence and tunable T1-T2 magnetic resonance imaging contrast and near-infrared activated photodynamic therapy. Nanoscale 2014, 6, 12609–12617. [Google Scholar] [CrossRef]
- Erickson, D.; Mandal, S.; Yang, A.H.; Cordovez, B. Nanobiosensors: Optofluidic, electrical and mechanical approaches to biomolecular detection at the nanoscale. Microfluid. Nanofluid. 2008, 4, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agr. Biotech. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic systems for pathogen sensing: A review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef]
- Birch, J.R.; Racher, A.J. Antibody production. Adv. Drug. Deliv. Rev. 2006, 58, 671–685. [Google Scholar] [CrossRef]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Tzen, T.R.J.; Cheng, Y.Y.R.; Saeidpourazar, R.; Aphale, S.S.; Jalili, N. Adhesin-Specific Nanomechanical Cantilever Biosensors for Detection of Microorganisms. J. Heat. Trans-T Asme. 2011, 133. [Google Scholar] [CrossRef]
- Medina, M.B. Biosensor studies of the binding of extracellular matrix components with immobilized Escherichia coli O157: H7 and inhibition by polysulfated polysaccharides. Biotechnol. Lett. 2002, 24, 77–84. [Google Scholar] [CrossRef]
- Jantra, J.; Kanatharana, P.; Asawatreratanakul, P.; Hedström, M.; Mattiasson, B.; Thavarungkul, P. Real-time label-free affinity biosensors for enumeration of total bacteria based on immobilized concanavalin A. J. Environ. Sci. Health Part A 2011, 46, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, S.; Terebiznik, M.; Guyard, C.; Kerman, K. Biosensors for the Detection of Interaction between Legionella pneumophila Collagen-Like Protein and Glycosaminoglycans. Sensors 2018, 18, 668. [Google Scholar] [CrossRef]
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Specific Recognition of Human Influenza Virus with PEDOT Bearing Sialic Acid-Terminated Trisaccharides. ACS Appl. Mater. Interfaces 2017, 9, 14162–14170. [Google Scholar] [CrossRef]
- Shimetani, N. Potential of Next-generation POCT in Infectious Disease Rapid Test. Med. Mycol. J. 2017, 58, J91–J94. [Google Scholar] [CrossRef][Green Version]
- Kretzer, J.W.; Schmelcher, M.; Loessner, M.J. Ultrasensitive and Fast Diagnostics of Viable Listeria Cells by CBD Magnetic Separation Combined with A511::luxAB Detection. Viruses 2018, 10, 626. [Google Scholar] [CrossRef]
- Saab, M.E.; Muckle, C.A.; Stryhn, H.; McClure, J.T. Comparison of culture methodology for the detection of methicillin-resistant Staphylococcus pseudintermedius in clinical specimens collected from dogs. J. Vet. Diagn. Investig. 2018, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, S.; Krieg, P.; Killinger, A.; Al-Ahmad, A.; Seidenstücker, M.; Latorre, S.H.; Bernstein, A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials 2019, 12, 3495. [Google Scholar] [CrossRef]
- Boardman, A.K.; Allison, S.; Sharon, A.; Sauer-Budge, A.F. Comparison of anti-fouling surface coatings for applications in bacteremia diagnostics. Anal. Methods 2013, 5, 273–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Andel, E.; de Bus, I.; Tijhaar, E.J.; Smulders, M.M.J.; Savelkoul, H.F.J.; Zuilhof, H. Highly Specific Binding on Antifouling Zwitterionic Polymer-Coated Microbeads as Measured by Flow Cytometry. ACS. Appl. Mater. Interfaces 2017, 9, 38211–38221. [Google Scholar] [CrossRef]
- Swar, S.; Máková, V.; Stibor, I. Effectiveness of Diverse Mesoporous Silica Nanoparticles as Potent Vehicles for the Drug L-DOPA. Materials 2019, 12, 202. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Mendonca, P.V.; Almeida, M.C.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Increasing the Antimicrobial Activity of Amphiphilic Cationic Copolymers by the Facile Synthesis of High Molecular Weight Stars by Supplemental Activator and Reducing Agent Atom Transfer Radical Polymerization. Biomacromolecules 2019, 20, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Braun, B.M.; Skelly, J.D.; Ayers, D.C.; Song, J. Significant Suppression of Staphylococcus aureus Colonization on Intramedullary Ti6Al4V Implants Surface-Grafted with Vancomycin-Bearing Polymer Brushes. ACS. Appl. Mater. Interfaces 2019, 11, 28641–28647. [Google Scholar] [CrossRef]
- Perez-Alvarez, L.; Ruiz-Rubio, L.; Azua, I.; Benito, V.; Bilbao, A.; Vilas-Vilela, J.L. Development of multiactive antibacterial multilayers of hyaluronic acid and chitosan onto poly(ethylene terephthalate). Eur. Polym. J. 2019, 112, 31–37. [Google Scholar] [CrossRef]
- Arisoy, F.D.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. Acs. Appl. Mater. Inter. 2018, 10, 20055–20063. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef]
- Wu, Z.; Willing, B.; Bjerketorp, J.; Jansson, J.K.; Hjort, K. Soft inertial microfluidics for high throughput separation of bacteria from human blood cells. Lab. Chip. 2009, 9, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Pratt, E.D.; Huang, C.; Hawkins, B.G.; Gleghorn, J.P.; Kirby, B.J. Rare Cell Capture in Microfluidic Devices. Chem. Eng. Sci. 2011, 66, 1508–1522. [Google Scholar] [CrossRef]
- Shangguan, J.; Li, Y.; He, D.; He, X.; Wang, K.; Zou, Z.; Shi, H. A combination of positive dielectrophoresis driven on-line enrichment and aptamer-fluorescent silica nanoparticle label for rapid and sensitive detection of Staphylococcus aureus. Analyst 2015, 140, 4489–4497. [Google Scholar] [CrossRef]
- Pinkner, J.S.; Remaut, H.; Buelens, F.; Miller, E.; Åberg, V.; Pemberton, N.; Hedenström, M.; Larsson, A.; Seed, P.; Waksman, G.; et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17897–17902. [Google Scholar] [CrossRef] [PubMed]
- Qvortrup, K.; Hultqvist, L.D.; Nilsson, M.; Jakobsen, T.H.; Jansen, C.U.; Uhd, J.; Andersen, J.B.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Small Molecule Anti-biofilm Agents Developed on the Basis of Mechanistic Understanding of Biofilm Formation. Front. Chem. 2019, 7, 742. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.M.; Piątek, R.J. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim. Pol. 2019, 66, 129–138. [Google Scholar] [CrossRef]
- Chorell, E.; Pinkner, J.S.; Bengtsson, C.; Banchelin, T.S.; Edvinsson, S.; Linusson, A.; Hultgren, S.J.; Almqvist, F. Mapping pilicide anti-virulence effect in Escherichia coli, a comprehensive structure-activity study. Bioorg. Med. Chem. 2012, 20, 3128–3142. [Google Scholar] [CrossRef]
- Drancourt, M.; Michel-Lepage, A.; Boyer, S.; Raoult, D. The Point-of-Care Laboratory in Clinical Microbiology. Clin. Microbiol. Rev. 2016, 29, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Guo, J.; Xiao, Y.; He, Z.; Xia, X.; Huang, Z.; Guan, H.; Ling, X.; Li, J.; Diao, B.; et al. Comparison of BioFire FilmArray gastrointestinal panel versus Luminex xTAG Gastrointestinal Pathogen Panel (xTAG GPP) for diarrheal pathogen detection in China. Int. J. Infect. Dis. 2020, 99, 414–420. [Google Scholar] [CrossRef]
- Buchan, B.W.; Windham, S.; Balada-Llasat, J.M.; Leber, A.; Harrington, A.; Relich, R.; Murphy, C.; Dien Bard, J.; Naccache, S.; Ronen, S.; et al. Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Happonen, L.; Khakzad, H.; Malmström, L.; Malmström, J. Structural proteomics, electron cryo-microscopy and structural modeling approaches in bacteria-human protein interactions. Med. Microbiol. Immunol. 2020, 209, 265–275. [Google Scholar] [CrossRef]
- Galvan, D.D.; Parekh, V.; Liu, E.; Liu, E.L.; Yu, Q. Sensitive Bacterial Detection via Dielectrophoretic-Enhanced Mass Transport Using Surface-Plasmon-Resonance Biosensors. Anal. Chem. 2018, 90, 14635–14642. [Google Scholar] [CrossRef] [PubMed]
- Prozeller, D.; Morsbach, S.; Landfester, K. Isothermal titration calorimetry as a complementary method for investigating nanoparticle-protein interactions. Nanoscale 2019, 11, 19265–19273. [Google Scholar] [CrossRef]
- Niether, D.; Wiegand, S. Thermophoresis of biological and biocompatible compounds in aqueous solution. J. Phys. Condens. Matter 2019, 31, 503003. [Google Scholar] [CrossRef] [PubMed]
- Philo, J.S. Is any measurement method optimal for all aggregate sizes and types? AAPS. J. 2006, 8, E564–E571. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes. Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).