Abstract
Backgrounds: The purpose of this paper is to investigate the prognostic value of fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) parameters in patients treated with concurrent chemoradiotherapy (CCRT) for locally advanced cervical cancer (LACC). Methods: Studies that met the following criteria were retrieved from PubMed and Embase: patients treated with CCRT for LACC; FDG PET/CT scans performed before CCRT treatment; and a detected relationship between the parameters of FDG PET/CT and the prognosis of patients. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were used to estimate the overall survival (OS) or event-free survival (EFS). Results: In total, 14 eligible studies with 1313 patients were included in this meta-analysis. Patients with a high maximum standardized uptake value (SUVmax) have a shorter OS than those with a low SUVmax (HR = 2.582, 95% = CI 1.936–3.443, p < 0.001). Primary tumor SUVmax values (HR = 1.938, 95% CI = 1.203–3.054, p = 0.004) were significantly correlated with EFS, with a relatively high heterogeneity (I2 = 84% and I2 = 69.4%, respectively). Based on the limited data, the combined HR for EFS with the highest primary tumor total lesion glycolysis (TLG) and metabolic tumor volume (MTV) was 1.843 (95% CI = 1.100–3.086, p = 0.02) and 2.06 (95% CI = 1.21–3.51, p = 0.007), respectively. Besides, the combined HR for OS with the highest nodal SUVmax was 2.095 (95% CI = 2.027–2.166, p < 0.001). Conclusion: A high primary SUVmax has a significant correlation with the OS and EFS of patients treated with CCRT for LACC and may therefore serve as a prognostic predictor. Due to the limited data, to explore the correlation between survival and TLG, MTV, and nodal SUVmax, further large-scale prospective studies are needed.
1. Introduction
Cervical cancer is a common gynecological malignancy, with around 569,847 new cases and 311,365 deaths annually worldwide, and a significant proportion of patients are diagnosed at a locally advanced stage [1]. For locally advanced cervical cancer (LACC), concurrent chemoradiotherapy (CCRT), using cisplatin-based chemotherapy in association with pelvic external-beam radiotherapy and subsequent brachytherapy, is considered the standard treatment [2]. While the contribution of CCRT toward an improvement in survival outcomes and reduction in recurrence has been confirmed, the complete clinical response of these patients is 70–90%, and about one-third of patients experience recurrence [3,4].
The accurate prediction of recurrence is of great significance. Several traditional clinical and pathological factors are identified as poor prognostic factors, which include the advanced International Federation of Gynecology and Obstetrics (FIGO) stage, presence of lymph node metastasis, parametrial invasion, histological tumor type, and a large tumor diameter [5,6,7]. However, there are still no satisfactory parameters sufficient to predict prognosis accurately.
Computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) are the most commonly used imaging modalities for CC. Compared to CT or MRI, FDG PET/CT could show metabolic information on tumors and more accurately assess lymph node involvement, distant metastasis, and recurrent disease [8]. Therefore, FDG PET/CT has been widely used in staging, therapeutic strategies, and the treatment response assessment of patients with CC [9].
With the technology developed, imaging has provided the potential for prognostic biomarkers [10]. Except for the aforementioned roles, quantitative parameters derived from FDG-PET/CT, including the maximum standardized uptake value (SUVmax), average SUV (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), have recently been proposed as prognostic biomarkers for patients with LACC who are treated with CCRT. However, the results of some studies show some differences. For example, Im et al. detected that patients with a high SUVmax measurement in tumor tissue had a higher risk of recurrence or progression than those with a low SUVmax (hazard ratio [HR] = 2.14, 95%CI = 1.08–4.22, p = 0.029) [11], but Chong et al. drew the opposite conclusion (HR = 0.673, 95%CI = 0.5–4.0, p = 0.412) [12]. Therefore, we performed this meta-analysis to synthetize the relevance of FDG PET/CT parameters as prognostic biomarkers for patients with LACC who are treated with CCRT.
2. Methods
2.1. Literature Search and Selection Criteria
Embase and PubMed (from inception to December 2020) were systematically searched using the appropriate terminology, as described in Table S1. Besides, the reference lists of the articles reviewed as full texts were also searched manually. The inclusion criteria in the meta-analysis were as follows: patients treated with CCRT for LACC; FDG PET/CT scans performed before or during CCRT treatment; and a detected relationship between the parameters of FDG PET/CT and the prognosis of patients. Studies meeting the following criteria were excluded: publication type other than original research articles (e.g., review articles, conference abstracts, or editorial), patients treated with surgery at any point in the treatment, patients treated with chemotherapy or radiotherapy alone, and patients lacking important information for analysis (e.g., articles reporting HR, using Cox proportional hazards modeling with parameters as a continuous variable). Two authors conducted the searches and screening independently. Any discrepancies were resolved by consensus with another reviewer.
2.2. Data Extraction
The following information was extracted using a standardized form: surname of first author, year of publication, country, design (prospective or retrospective), patients’ characteristics (including patients’ number, tumor node metastasis [TNM] staging, histology, radiotherapy technique, the schedule of systemic therapy, and follow-up period), and outcomes.
2.3. Statistical Analysis
The primary outcome, progression event-free survival (EFS), was defined as the date from initiating therapy to recurrence or metastasis. In some of the included studies, disease-free survival (DFS) or progression-free survival (PFS) was obtained as the primary outcome, but they were all redefined as EFS in this meta-analysis. The secondary outcome was overall survival (OS), defined as the date from initiating therapy until death. The effect sizes of the prognostic values of SUVmax, SUVmean, MTV, and TLG were measured in terms of hazard ratio (HR). An HR greater than 1 implied a worse survival for patients with a high SUVmax, SUVmean, MTV, or TLG. The most adjusted estimate of HR was extracted directly from each study, if provided by the authors. Otherwise, the HR estimate and its variance were extracted from Kaplan-Meier curves by the Engauge Digitizer, version 3.0 (http://digitizer.sourceforge.net, accessed date: 30 December 2020). The heterogeneity was assessed with the Q test and I2 statistic, and a p value less than 0.05 or I2 values higher than 50% indicated a significant heterogeneity. The fixed-effects model was used to estimate the cases with homogeneity, and the random-effects model was used for the cases with a significant heterogeneity. The publication bias of the studies was visually displayed by the asymmetry of an inverted funnel plot and quantitatively evaluated by Egger’s tests, with a p value less than 0.05 suggesting a significant publication bias. Sensitivity analyses were conducted to assess the stability of the meta-analysis results. All the statistical analyses were conducted using the STATA 14.0 (STATA Corporation, College Station, TX, USA) software.
3. Results
3.1. Study Selection
We picked up 822 potentially eligible articles in the electronic search, with 88 articles removed for duplication and 644 articles excluded after the reviewers read the titles and abstracts. In total, 14 eligible studies with a total of 1313 patients were included in this meta-analysis, after reviewing the full text (Figure 1).
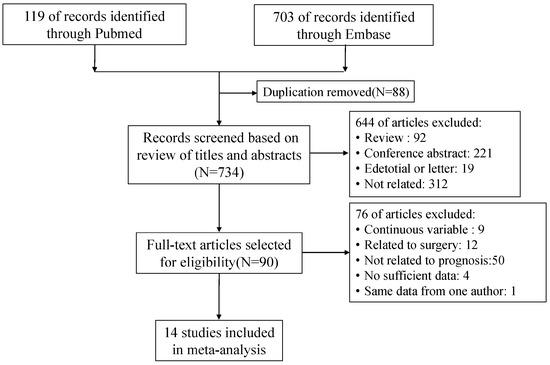
Figure 1.
Flow chart of the literature search.
3.2. Patient Characteristics and Treatment Features
The median age of the patients included in the studies was 51.5–58.0 years (range from 21 to 89 years). Only 12 of the 14 studies provided information about histology, and 89.14% patients had a squamous cell carcinoma (1076/1205) (Table 1). The largest stage subgroup was the FIGO stage ⅡB, which was the case for 64.66% (846/1313) of the patients. The median follow-up for all the patients was 22–60 months (range from 3 to 129 months) (Table 2). No information on the radiotherapy technique used was available for the three studies, and most of the patients from the other studies were treated with 3D-conformal radiotherapy. The widely adopted external beam radiotherapy regimen consisted of conventional fractionation of 1.8 Gy per fraction (8/9 studies) for a total dose of 45–50.4 Gy. The intracavitary brachytherapy regimen used in the majority of patients consisted of a fractionation of 7 Gy per fraction (6/10 studies), with four cycles (4/9 studies). The adopted concurrent chemotherapy regimen was a standard weekly 40 mg/m2 cisplatin in most patients (10/13 studies) (Table 3).

Table 1.
Basic information and patient characteristics.

Table 2.
Disease features: stage and follow-up.

Table 3.
Treatment-related features.
3.3. Primary Outcome: EFS
Nine articles on SUVmax in tumor tissue were included in the study [11,12,14,15,16,17,18,21,23]. The combined HR for EFS with the highest SUVmax was 1.938 (95% CI = 1.203–3.054, p = 0.004) (Figure S1A), which meant that the patients with a high SUVmax measurement in tumor tissue had a higher risk of recurrence or progression than those with a low SUVmax. However, a significant heterogeneity existed between the articles (I2 = 84%, p = 0.000). The publication bias was significant based on the funnel graph (Figure S1B) and Egger’s test (p = 0.015). The results obtained through the sensitivity analysis was relatively stable (Figure S1C).
Four articles on primary tumor SUVmean were included in the study [16,17,18,19]. Due to there being no significant heterogeneity (I2 = 0%, p = 0.938), the fixed-effects model was performed. The combined HR for EFS with the primary tumor SUVmean was 1.182 (95% CI = 0.899–1.544, p = 0.230) (Figure S2A). The Begg’s funnel plot and Egger’s test indicated no publication bias (p = 0.345), and the sensitivity analysis showed that the analysis was relatively stable (Figure S2B,C).
Three articles on MTV (measuring tumor tissue) were included in the study [12,16,18]. With no significant heterogeneity (I2 = 20.9%, p = 0.282), the combined HR for EFS with the highest MTV was 2.06 (95% CI = 1.21–3.51, p = 0.007) (Figure 2A). This indicated that patients with a high MTV had a higher risk of recurrence or progression than those with a low MTV. Because the data were limited, the publication bias was not evaluated.
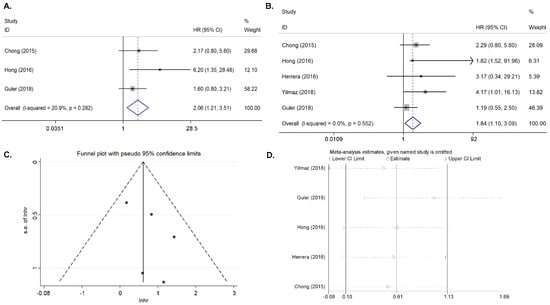
Figure 2.
Results for the primary MTV and TLG for DFS. (A) Forest plots of the HRs for DFS with MTV. (B) Forest plots of the HRs for DFS with TLG. (C) Begg’s funnel plot for TLG. (D) Sensitivity analysis of the influence of each individual study on the pooled HR for TLG.
Four articles on primary tumor TLG were included in the study [13,16,18,19]. In the absence of a significant heterogeneity (I2 = 0%, p = 0.552), the fixed-effects model was applied. The combined HR for EFS with the primary tumor TLG was 1.843 (95% CI = 1.100–3.086, p = 0.02). (Figure 2B). The Begg’s funnel plot and Egger’s test indicated no publication bias (p = 0.261), and the sensitivity analysis showed that the analysis was relatively stable (Figure 2C,D).
Five articles on nodal SUVmax were included in the study [13,14,20,22,23]. Where there was a significant heterogeneity (I2 = 69.4%, p = 0.011), the random-effects model was applied. The combined HR for EFS with the nodal SUVmax was 3.478(95% CI = 2.006–6.029, p < 0.001) (Figure S3A). The publication bias was significant based on the funnel graph (Figure S3B) and Egger’s test (p = 0.04). The sensitivity analysis proved that the results were relatively stable (Figure S3C).
3.4. Second Outcome: OS
Seven articles on SUVmax in tumor tissue were included in the study [11,14,15,21,23,24,25]. Where there was a moderate heterogeneity (I2 = 36.2%, p = 0.151), the fixed-effects model was applied when pooling the HR. The combined HR was 2.582 (95% CI = 1.936–3.443, p < 0.001), which meant that patients with a high SUVmax had a shorter survival time than those with a low SUVmax (Figure 3A). Begg’s funnel plot and Egger’s test indicated no publication bias (p = 0.113), and the sensitivity analysis showed that the analysis was relatively stable (Figure 3B,C).
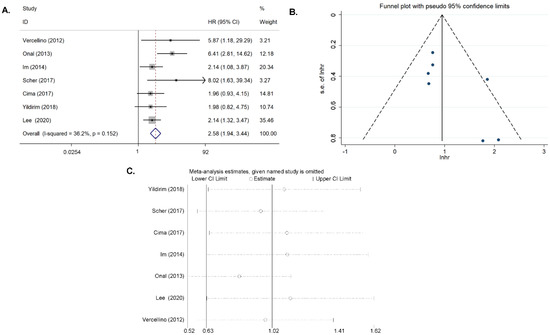
Figure 3.
Results for the primary SUVmax for OS. (A) Forest plots of the HRs. (B) Begg’s funnel plot. (C) Sensitivity analysis of the influence of each individual study on the pooled HR.
Two articles on primary tumor SUVmean were included in the study [15,19]. With no significant heterogeneity (I2 = 0.0%, p = 0.893), the combined HR for EFS with the highest MTV was 1.523 (95% CI = 0.764–3.038, p = 0.232) (Figure S4A). Because of the limited data, the publication bias and sensitivity analysis were not performed.
Two articles on TLG in tumor tissue were included in the study [15,19]. In the absence of heterogeneity (I2 = 0.0%, p = 0.672), the fixed-effects model was applied when pooling the HR. The combined HR for EFS with the nodal SUVmax was 1.371 (95% CI = 0.681–2.759, p = 0.377) (Figure S4B). Because of the limited data, the publication bias and sensitivity analysis were not performed.
Two articles on nodal SUVmax were included in the study [20,23]. Where there was no heterogeneity (I2 = 54.1%, p = 0.140), the fixed-effects model was applied when pooling the HR. The combined HR for EFS with the nodal SUVmax was 2.095 (95% CI = 2.027–2.166, p < 0.001) (Figure S4C). Because of the limited data, the publication bias and sensitivity analysis were not performed.
4. Discussion
In this system review and meta-analysis, we review the prognostic value of primary tumor SUVmax, SUVmean, MTV, TLG, and nodal SUVmax in patients treated with CCRT for LACC. Besides, the results of meta-analysis show that patients with a high primary SUVmax have a shorter OS and a higher risk of recurrence or progression than those with a low SUVmax. The synthesized results showed that the highest TLG and MTV were correlated with EFS, and the highest Nodal SUVmax was associated with OS in patients treated with CCRT for LACC. Due to the limited data, a publication bias, and heterogeneity, to explore whether MTV, TLG, and nodal SUVmax are significant markers and can act as prognostic indicators in clinical practice, further large-scale prospective studies are required.
This meta-analysis focused on the FDG PET/CT parameters as a categorical variable, but the results of the articles concerning FDG PET/CT parameters as a continuous variable exhibited an extreme discrepancy among different studies. For example, Carpenter et al. reported that primary tumor MTV and primary tumor TLG were significantly associated with OS in univariate analyses, whereas only primary tumor TLG were significantly correlated with OS in multivariate analyses [26]. Calles-Sastre et al. found that primary-tumor MTV and primary-tumor TLG showed a significant association with OS and with RFS in univariate analyses [27]. Scher et al. found that primary-tumor MTV and primary-tumor TLG were correlated with OS and DFS in univariate analyses, whereas primary-tumor TLG was the only FDG PET parameter significantly correlated with OS and DFS in multivariate analyses [25]. In addition, Liu et al. confirmed that primary-tumor MTV was a significant predictor of OS in univariate analyses [28]. Chong et al. reported that primary-tumor SUVmax, primary tumor MTV, and primary tumor TLG were significant prognostic factors for DFS in univariate analyses, but none of them was significant in multivariate analyses (Table S2) [22].
Apart from pretreatment FDG PET/CT parameters, some studies focused on the prognostic value of during-treatment FDG PET/CT parameters. Carpenter et al. reported that primary-tumor MTV and primary-tumor TLG during treatment were significantly associated with OS and DFS [26]. Liu et al. found that primary-tumor MTV during treatment was significantly associated with OS [28]. Krhili et al. confirmed that not only primary tumor MTV and primary-tumor TLG, but also SUVmax during treatment were significant prognostic factors for OS and DFS (Table S3) [29]. Besides, changes between pre- and intra-treatment FDG-PET/CT parameters were also confirmed to be prognostic factors in some studies. Park et al. confirmed that the percentage changes of SUVmax have a prognostic value for predicting DFS [30]. Oh et al. showed that a decrease of SUVmax was a statistically significant predictor of PFS [31]. However, Carpenter et al. reported no correlation among the changes in primary-tumor SUVmax, SUVmean, MTV, TLG, and survival [26].
Except for the pretreatment FDG PET/CT parameter, during-treatment FDG PET/CT parameter, and changes between pre- and intra-treatment FDG-PET/CT parameters, the one-two punch, using various types of prognostic factors to create a prognosis-predicting model, was a new orientation. For example, Hong et al. suggested a simple prognosis prediction model, using pretreatment FDG PET/CT and human papillomavirus (HPV) genotyping in patients with LACC treated with CCRT [32]. Lee et al. constructed a nomogram based on these six (including age, nodal SUVmax, primary-tumor SUVmax, size, stage, and SCC) and seven (including age, nodal SUVmax, primary-tumor SUVmax, size, stage, SCC, and high-risk HPV status) independent risk factors for two-year DFS and five-year OS, respectively [23]. Besides, radiomics is a new frontier for predicting the prognosis of patients with LACC. For example, Lucia et al. found that in LACC treated with CCRT, radiomics features, such as Grey Level Non-uniformity GLRLM from PET, are independent predictors of recurrence and loco-regional control with a significantly higher prognostic power than usual clinical parameters [17]. The predictive tool-enrolled FDG PET/CT parameter still needs more study.
Our study is the first system review and meta-analysis to evaluate the prognostic value of FDG PET/CT metabolic parameters (including primary-tumor SUVmax, SUVmean, MTV, TLG, and nodal SUVmax) in patients with LACC treated with CCRT. We performed an extensive search addressing all the available databases, and our meta-analysis confirmed the significant association with the primary-tumor SUVmax and OS of patients with LACC treated with CCRT. Future studies could focus on the prognosis-predicting model based on the primary-tumor SUVmax using various types of prognostic factors. However, there were still some noteworthy limitations of this meta-analysis. First, the small article scale concerning TLG, MTV, and SUVmax did not support a high-quality and valuable conclusion, and large-scale prospective studies are urgently need to explore the clinical value of our findings. Besides, a significant heterogeneity existed in the results of this meta-analysis, and no subgroup analysis was performed for the limited studies. The potential source of the heterogeneity was probably four-fold: (1) The patients in each study had different FIGO stages, different histologic subtypes, different treatment strategies, and different follow-up endpoints (Table 1, Table 2 and Table 3); (2) different PET/CT techniques and image analyses existed in each study, which might have caused the heterogeneity in this analysis (Table S4); (3) the cut-off values for FDG PET/CT metabolic parameters were different in each study; and (4) the HR used in this meta-analysis was directly extracted from a Cox proportional hazards model (multivariate analysis and univariate analysis) or indirectly estimated from Kaplan-Meier curves.
5. Conclusions
A high primary SUVmax has a significant association with a shorter OS and EFS in patients treated with CCRT for LACC, which might serve as a prognostic predictor. Due to the limited data, a publication bias, and heterogeneity, to explore the correlation between survival and TLG, MTV, and nodal SUVmax, further large-scale studies are needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics11071258/s1, Table S1: Searching strategy. Table S2: Summary of prognostic results about pretreatment FDG PET parameters as a continuous variable. Table S3: Summary of prognostic results about FDG PET parameters during treatment. Table S4: Information about the PET/CT technique and image analysis. Figure S1: Results for the primary SUVmax for DFS. (A) Forest plots of the HRs for SUVmax. (B) Begg’s funnel plot for SUVmax. (C) Sensitivity analysis of the influence of each individual study on the pooled HR for SUVmax. Figure S2: Results for the nodal SUVmax for DFS. (A) Forest plots of the HRs. (B) Begg’s funnel plot. (C) Sensitivity analysis of the influence of each individual study on the pooled HR. Figure S3: Results for the primary SUVmax for OS. (A) Forest plots of the HRs. (B) Begg’s funnel plot. (C) Sensitive analysis of the influence of each individual study on the pooled HR. Figure S4: Results for the primary SUVmean, TLG, and nodal SUVmax, as well as for OS. (A) Forest plots of the HRs with primary SUVmean. (B) Forest plots of the HRs with primary TLG. (C) Forest plots of the HRs with nodal SUVmax.
Author Contributions
Conceptualization, Q.L. and L.Z.; methodology, L.H.; software, Q.W.; investigation, L.H.; data curation, L.H. and Q.W.; writing—original draft preparation, L.H. and L.Z.; writing—review and editing, X.F., Y.Z. and Y.W.; funding acquisition, Q.L. and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Basic Research Program of Shaanxi (2020JQ-952, 2017ZDJC-11, 2018JM7073). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used to support the findings of this study are included in the article.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001, 358, 781–786. [Google Scholar] [CrossRef]
- Endo, D.; Todo, Y.; Okamoto, K.; Minobe, S.; Kato, H.; Nishiyama, N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: A retrospective analysis in a Japanese cohort. J. Gynecol. Oncol. 2015, 26, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, L.C.; Zhang, Y.; Zhao, L.N.; Li, W.W.; Ping, L.J.; Dang, Y.Z.; Hu, J.; Shi, M. The Prognosis and Risk Stratification Based on Pelvic Lymph Node Characteristics in Patients with Locally Advanced Cervical Squamous Cell Carcinoma Treated with Concurrent Chemoradiotherapy. Int. J. Gynecol. Cancer 2016, 26, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wang, L.; Lin, J.C.; Jan, J.S. The prognostic factors for locally advanced cervical cancer patients treated by intensity-modulated radiation therapy with concurrent chemotherapy. J. Formos. Med. Assoc. 2015, 114, 231–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teh, J.; Yap, S.P.; Tham, I.; Sethi, V.K.; Chua, E.J.; Yeo, R.; Ho, T.H.; Tay, E.H.; Chia, Y.N.; Soh, L.T.; et al. Concurrent chemoradiotherapy incorporating high-dose rate brachytherapy for locally advanced cervical carcinoma: Survival outcomes, patterns of failure, and prognostic factors. Int. J. Gynecol. Cancer 2010, 20, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Prior, J.O. The role of PET/CT in cervical cancer. Front. Oncol. 2013, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yang, R.; Tian, J.; Fan, L. The role of F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT) in the screening of cervical cancer: A literature review. Cell Biochem. Biophys. 2014, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Gandy, N.; Arshad, M.A.; Park, W.E.; Rockall, A.G.; Barwick, T.D. FDG-PET Imaging in Cervical Cancer. Semin. Nucl. Med. 2019, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Yoon, H.J.; Lee, E.S.; Kim, T.S.; Kim, J.Y.; Chung, J.K.; Kim, S.K.; Park, S.Y. Prognostic implication of retrocrural lymph node involvement revealed by (18)F-FDG PET/CT in patients with uterine cervical cancer. Nucl. Med. Commun. 2014, 35, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Jeong, S.Y.; Park, S.H.; Lee, Y.H.; Lee, S.W.; Hong, D.G.; Kim, J.C.; Lee, Y.S.; Cho, Y.L. Comparison of the Prognostic Value of F-18 Pet Metabolic Parameters of Primary Tumors and Regional Lymph Nodes in Patients with Locally Advanced Cervical Cancer Who Are Treated with Concurrent Chemoradiotherapy. PLoS ONE 2015, 10, e0137743. [Google Scholar] [CrossRef]
- Yilmaz, B.; Dag, S.; Ergul, N.; Cermik, T.F. The efficacy of pretreatment and after treatment 18F-FDG PET/CT metabolic parameters in patients with locally advanced squamous cell cervical cancer. Nucl. Med. Commun. 2019, 40, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Akkus Yildirim, B.; Onal, C.; Erbay, G.; Cem Guler, O.; Karadeli, E.; Reyhan, M.; Koc, Z. Prognostic values of ADCmean and SUVmax of the primary tumour in cervical cancer patients treated with definitive chemoradiotherapy. J. Obstet. Gynaecol. 2019, 39, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Cima, S.; Perrone, A.M.; Castellucci, P.; Macchia, G.; Buwenge, M.; Cammelli, S.; Cilla, S.; Ferioli, M.; Ferrandina, G.; Galuppi, A.; et al. Prognostic Impact of Pretreatment Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography SUVmax in Patients with Locally Advanced Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Guler, O.C.; Torun, N.; Yildirim, B.A.; Onal, C. Pretreatment metabolic tumour volume and total lesion glycolysis are not independent prognosticators for locally advanced cervical cancer patients treated with chemoradiotherapy. Br. J. Radiol. 2018, 91, 20170552. [Google Scholar] [CrossRef] [PubMed]
- Lucia, F.; Visvikis, D.; Desseroit, M.C.; Miranda, O.; Malhaire, J.P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of outcome using pretreatment (18)F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Jung, U.S.; Min, K.J.; Lee, J.K.; Kim, S.; Eo, J.S. Prognostic value of total lesion glycolysis measured by 18F-FDG PET/CT in patients with locally advanced cervical cancer. Nucl. Med. Commun. 2016, 37, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Breuneval, T.; Prior, J.O.; Bourhis, J.; Ozsahin, M. [(18)F]FDG-PET/CT metabolic parameters as useful prognostic factors in cervical cancer patients treated with chemo-radiotherapy. Radiat. Oncol. 2016, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Guler, O.C.; Reyhan, M.; Yapar, A.F. Prognostic value of 18F-fluorodeoxyglucose uptake in pelvic lymph nodes in patients with cervical cancer treated with definitive chemoradiotherapy. Gynecol. Oncol. 2015, 137, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Reyhan, M.; Parlak, C.; Guler, O.C.; Oymak, E. Prognostic value of pretreatment 18F-fluorodeoxyglucose uptake in patients with cervical cancer treated with definitive chemoradiotherapy. Int. J. Gynecol. Cancer 2013, 23, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Lee, W.K.; Jeong, S.Y.; Park, S.H.; Lee, Y.H.; Lee, S.W.; Hong, D.G.; Kim, J.C.; Lee, Y.S. Prognostic value of intratumoral metabolic heterogeneity on F-18 fluorodeoxyglucose positron emission tomography/computed tomography in locally advanced cervical cancer patients treated with concurrent chemoradiotherapy. Oncotarget 2017, 8, 90402–90412. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Chong, G.O.; Jeong, S.Y.; Lee, H.J.; Park, S.H.; Ryu, J.M.; Choi, Y.S.; Kang, S.; Koo, Y.J.; Lee, D.H.; et al. Prognosis-Predicting Model Based on [(18)F]fluorodeoxyglucose PET Metabolic Parameters in Locally Advanced Cervical Cancer Patients Treated with Concurrent Chemoradiotherapy: Multi-Center Retrospective Study. J. Clin. Med. 2020, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; Groheux, D.; Thoury, A.; Delord, M.; Schlageter, M.H.; Delpech, Y.; Barre, E.; Baruch-Hennequin, V.; Tylski, P.; Homyrda, L.; et al. Hypoxia imaging of uterine cervix carcinoma with (18)F-FETNIM PET/CT. Clin. Nucl. Med. 2012, 37, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Scher, N.; Castelli, J.; Depeursinge, A.; Bourhis, J.; Prior, J.O.; Herrera, F.G.; Ozsahin, M. ((18)F)-FDG PET/CT parameters to predict survival and recurrence in patients with locally advanced cervical cancer treated with chemoradiotherapy. Cancer Radiother. 2018, 22, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.J.; Jacobs, C.D.; Wong, T.Z.; Craciunescu, O.; Chino, J.P. Changes on Midchemoradiation Therapy Fluorodeoxyglucose Positron Emission Tomography for Cervical Cancer Are Associated with Prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Calles-Sastre, L.; Mucientes-Rasilla, J.; San-Frutos Llorente, L.M.; Royuela, A.; Garcia-Espantaleon Navas, M.; Herrero Gamiz, S.; Perez-Medina, T. Prognostic significance of metabolic tumor volume and total lesion glycolysis in patients with advanced cervical carcinoma. Rev. Esp. Med. Nucl. Imagen Mol. 2019, 38, 17–21. [Google Scholar] [PubMed]
- Liu, F.Y.; Lai, C.H.; Yang, L.Y.; Wang, C.C.; Lin, G.; Chang, C.J.; Chang, W.Y.; Huang, S.H.; Huang, Y.E.; Peng, N.J.; et al. Utility of (18)F-FDG PET/CT in patients with advanced squamous cell carcinoma of the uterine cervix receiving concurrent chemoradiotherapy: A parallel study of a prospective randomized trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Krhili, S.; Muratet, J.P.; Roche, S.; Pointreau, Y.; Yossi, S.; Septans, A.L.; Denis, F. Use of Metabolic Parameters as Prognostic Factors During Concomitant Chemoradiotherapy for Locally Advanced Cervical Cancer. Am. J. Clin. Oncol. 2017, 40, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kim, C.K.; Park, B.K. Prognostic value of diffusion-weighted magnetic resonance imaging and (18)F-fluorodeoxyglucose-positron emission tomography/computed tomography after concurrent chemoradiotherapy in uterine cervical cancer. Radiother. Oncol. 2016, 120, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Lee, J.E.; Huh, S.J.; Park, W.; Nam, H.; Choi, J.Y.; Kim, B.T. Prognostic significance of tumor response as assessed by sequential 18F-fluorodeoxyglucose-positron emission tomography/computed tomography during concurrent chemoradiation therapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.M.; Park, S.H.; Chong, G.O.; Lee, Y.H.; Jeong, J.H.; Lee, S.W.; Lee, J.; Ahn, B.C.; Jeong, S.Y. Enhancing prognosis prediction using pre-treatment nodal SUVmax and HPV status in cervical squamous cell carcinoma. Cancer Imaging 2019, 19, 43. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).