Evaluation of VIDAS® Diagnostic Assay Prototypes Detecting Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM and IgG Antibodies
Abstract
1. Introduction
2. Materials and Methods
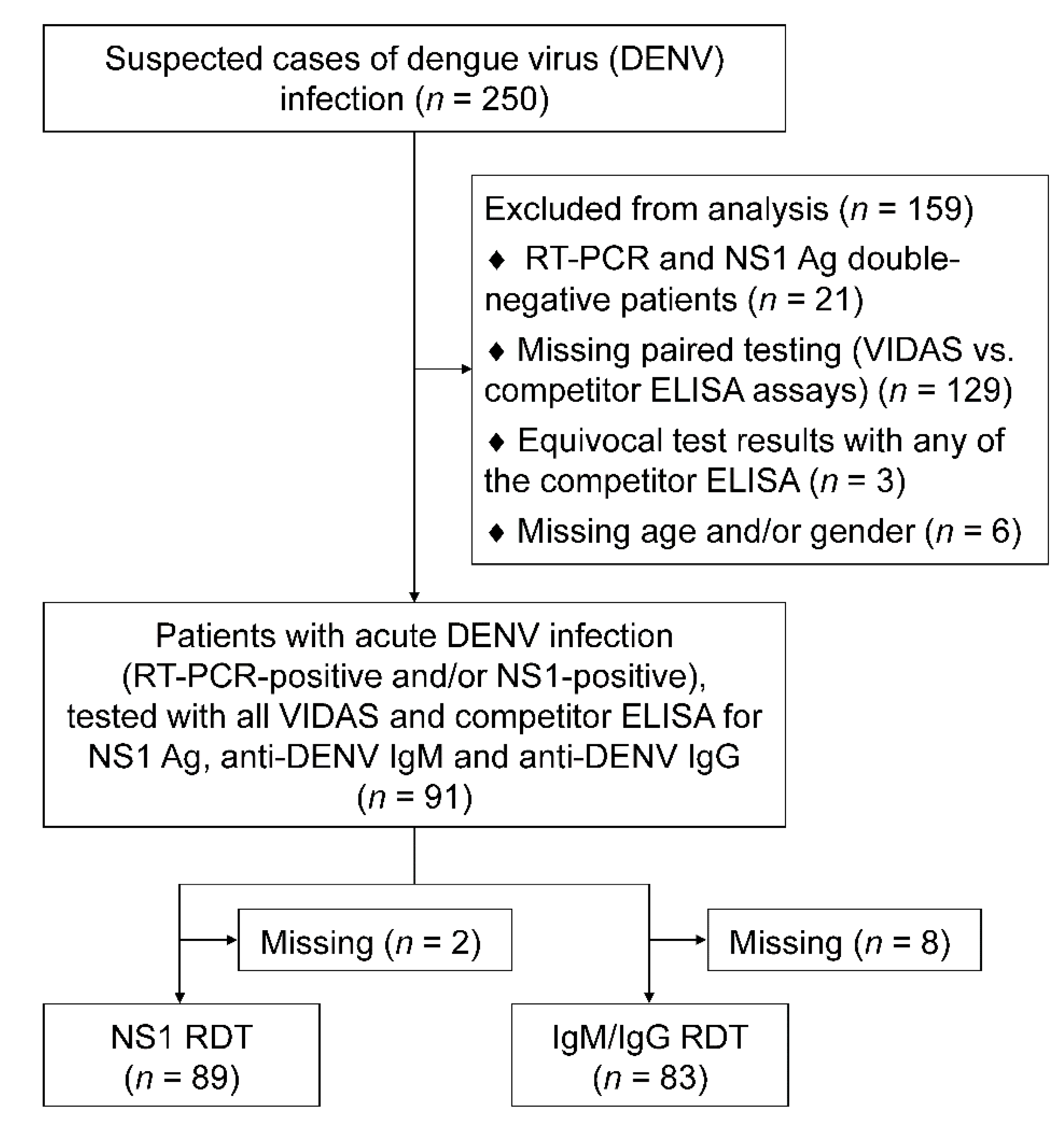
2.1. Patients and Samples
2.2. Study Design and Definition
2.3. VIDAS® Assays
2.4. Competitor Assays
2.5. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
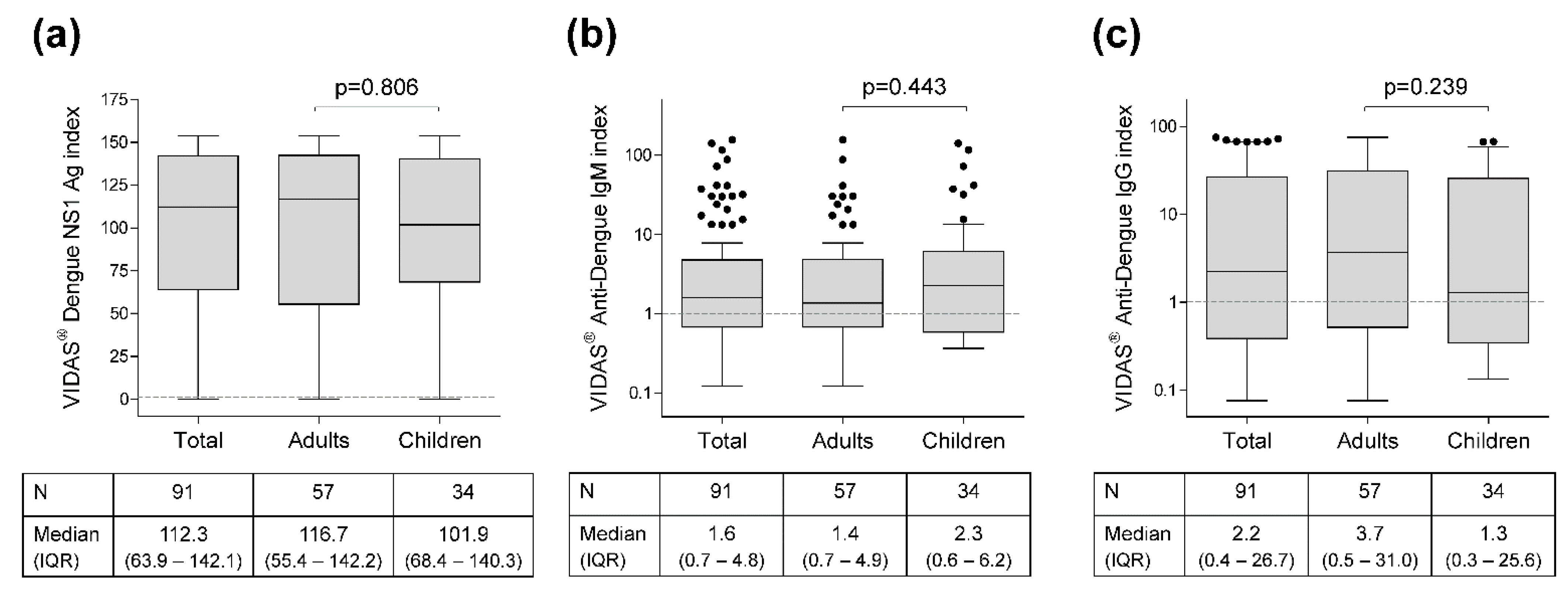
3.2. VIDAS® Index Distribution
3.3. Positive Agreement of Dengue NS1 Antigen Assays with RT-PCR as Gold Standard
3.4. Comparison of VIDAS® DENGUE NS1 Ag Assay with Competitor Assays
3.5. Comparison of VIDAS® Anti-DENGUE IgM Assay with Competitor Assays
3.6. Comparison of VIDAS® Anti-DENGUE IgG Assay with Competitor Assays
3.7. Negative Agreement of the VIDAS® NS1 Antigen and Anti-DENV IgM and IgG Assays with Competitor Assays Conducted on Healthy Donor Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control; World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR): Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- Ahmed, A.M.; Mohammed, A.T.; Vu, T.T.; Khattab, M.; Doheim, M.F.; Ashraf-Mohamed, A.; Abdelhamed, M.M.; Shamandy, B.E.; Dawod, M.T.; Alesaei, W.A.; et al. Prevalence and Burden of Dengue Infection in Europe: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2020, 30, e2093. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. Dengue Position Paper. Available online: http://www.who.int/immunization/policy/position_papers/dengue/en/ (accessed on 13 January 2021).
- Center for Disease Control and Prevention Testing for Dengue Virus. Dengue. CDC. Available online: https://www.cdc.gov/dengue/healthcare-providers/testing/index.html (accessed on 15 January 2021).
- Pan American Health Organization; World Health Organization. Dengue: Guidelines for Patient Care in the Region of the Americas; World Health Organization (WHO): Geneva, Switzerland, 2016; ISBN 978-92-75-31890-4. [Google Scholar]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of Diagnostic Tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef]
- Blacksell, S.D. Commercial Dengue Rapid Diagnostic Tests for Point-of-Care Application: Recent Evaluations and Future Needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Depelsenaire, A.C.I.; Young, P.R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J. Infect. Dis. 2017, 215, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Chanama, S.; Anantapreecha, S.; A-nuegoonpipat, A.; Sa-gnasang, A.; Kurane, I.; Sawanpanyalert, P. Analysis of Specific IgM Responses in Secondary Dengue Virus Infections: Levels and Positive Rates in Comparison with Primary Infections. J. Clin. Virol. 2004, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hunsperger, E.A.; Yoksan, S.; Buchy, P.; Nguyen, V.C.; Sekaran, S.D.; Enria, D.A.; Pelegrino, J.L.; Vázquez, S.; Artsob, H.; Drebot, M.; et al. Evaluation of Commercially Available Anti-Dengue Virus Immunoglobulin M Tests. Emerg. Infect. Dis. 2009, 15, 436–440. [Google Scholar] [CrossRef]
- Chien, Y.-W.; Liu, Z.-H.; Tseng, F.-C.; Ho, T.-C.; Guo, H.-R.; Ko, N.-Y.; Ko, W.-C.; Perng, G.C. Prolonged Persistence of IgM against Dengue Virus Detected by Commonly Used Commercial Assays. BMC Infect. Dis. 2018, 18, 156. [Google Scholar] [CrossRef]
- Kikuti, M.; Cruz, J.S.; Rodrigues, M.S.; Tavares, A.S.; Paploski, I.A.D.; Silva, M.M.O.; Santana, P.M.; Tauro, L.B.; Silva, G.A.O.F.; Campos, G.S.; et al. Accuracy of the SD BIOLINE Dengue Duo for Rapid Point-of-Care Diagnosis of Dengue. PLoS ONE 2019, 14, e0213301. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Jarman, R.G.; Bailey, M.S.; Tanganuchitcharnchai, A.; Jenjaroen, K.; Gibbons, R.V.; Paris, D.H.; Premaratna, R.; de Silva, H.J.; Lalloo, D.G.; et al. Evaluation of Six Commercial Point-of-Care Tests for Diagnosis of Acute Dengue Infections: The Need for Combining NS1 Antigen and IgM/IgG Antibody Detection to Achieve Acceptable Levels of Accuracy. Clin. Vaccine Immunol. 2011, 18, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.-Y.; Valks, A.; Young, P.R.; et al. The Diagnostic Sensitivity of Dengue Rapid Test Assays Is Significantly Enhanced by Using a Combined Antigen and Antibody Testing Approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Jarman, R.G.; Gibbons, R.V.; Tanganuchitcharnchai, A.; Mammen, M.P.; Nisalak, A.; Kalayanarooj, S.; Bailey, M.S.; Premaratna, R.; de Silva, H.J.; et al. Comparison of Seven Commercial Antigen and Antibody Enzyme-Linked Immunosorbent Assays for Detection of Acute Dengue Infection. Clin. Vaccine Immunol. 2012, 19, 804–810. [Google Scholar] [CrossRef]
- Pal, S.; Dauner, A.L.; Valks, A.; Forshey, B.M.; Long, K.C.; Thaisomboonsuk, B.; Sierra, G.; Picos, V.; Talmage, S.; Morrison, A.C.; et al. Multicountry Prospective Clinical Evaluation of Two Enzyme-Linked Immunosorbent Assays and Two Rapid Diagnostic Tests for Diagnosing Dengue Fever. J. Clin. Microbiol. 2015, 53, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Blessmann, J.; Winkelmann, Y.; Keoviengkhone, L.; Sopraseuth, V.; Kann, S.; Hansen, J.; El Halas, H.; Emmerich, P.; Schmidt-Chanasit, J.; Schmitz, H.; et al. Assessment of Diagnostic and Analytic Performance of the SD Bioline Dengue Duo Test for Dengue Virus (DENV) Infections in an Endemic Area (Savannakhet Province, Lao People’s Democratic Republic). PLoS ONE 2020, 15, e0230337. [Google Scholar] [CrossRef]
- Pal, S.; Dauner, A.L.; Mitra, I.; Forshey, B.M.; Garcia, P.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; Wu, S.-J.L. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE 2014, 9, e113411. [Google Scholar] [CrossRef]
- Gaikwad, S.; Sawant, S.S.; Shastri, J.S. Comparison of Nonstructural Protein-1 Antigen Detection by Rapid and Enzyme-Linked Immunosorbent Assay Test and Its Correlation with Polymerase Chain Reaction for Early Diagnosis of Dengue. J. Lab. Physicians 2017, 9, 177–181. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, J.H.; Park, H.S.; Park, K.H.; Bae, H.; Yun, S.; Choi, A.R.; Cho, S.Y.; Park, C.; Lee, D.G.; et al. Comparison of Six Commercial Diagnostic Tests for the Detection of Dengue Virus Non-Structural-1 Antigen and IgM/IgG Antibodies. Ann. Lab. Med. 2019, 39, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Hunsperger, E.A.; Yoksan, S.; Buchy, P.; Nguyen, V.C.; Sekaran, S.D.; Enria, D.A.; Vazquez, S.; Cartozian, E.; Pelegrino, J.L.; Artsob, H.; et al. Evaluation of Commercially Available Diagnostic Tests for the Detection of Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM Antibody. PLoS Negl. Trop. Dis. 2014, 8, e3171. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, C.; Okandze, A.; Matheus, S.; Djossou, F.; Nacher, M.; Mahamat, A. Prospective Evaluation of the SD BIOLINE Dengue Duo Rapid Test during a Dengue Virus Epidemic. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2441–2447. [Google Scholar] [CrossRef]
- Lao, M.; Caro, V.; Thiberge, J.-M.; Bounmany, P.; Vongpayloth, K.; Buchy, P.; Duong, V.; Vanhlasy, C.; Hospied, J.-M.; Thongsna, M.; et al. Co-Circulation of Dengue Virus Type 3 Genotypes in Vientiane Capital, Lao PDR. PLoS ONE 2014, 9, e115569. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, D.; Northill, J.A.; Pyke, A.; Smith, G.A. Single Rapid TaqMan Fluorogenic Probe Based PCR Assay That Detects All Four Dengue Serotypes. J. Med. Virol. 2002, 66, 524–528. [Google Scholar] [CrossRef]
- Ito, M.; Takasaki, T.; Yamada, K.-I.; Nerome, R.; Tajima, S.; Kurane, I. Development and Evaluation of Fluorogenic TaqMan Reverse Transcriptase PCR Assays for Detection of Dengue Virus Types 1. J. Clin. Microbiol. 2004, 42, 5935–5937. [Google Scholar] [CrossRef]
- Combe, M.; Lacoux, X.; Martinez, J.; Méjan, O.; Luciani, F.; Daniel, S. Expression, Refolding and Bio-Structural Analysis of a Tetravalent Recombinant Dengue Envelope Domain III Protein for Serological Diagnosis. Protein. Expr. Purif. 2017, 133, 57–65. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Lee, S.J.; Chanthongthip, A.; Taojaikong, T.; Thongpaseuth, S.; Hübscher, T.; Newton, P.N. Comparison of Performance of Serum and Plasma in Panbio Dengue and Japanese Encephalitis Virus Enzyme-Linked Immunosorbent Assays. Am. J. Trop. Med. Hyg. 2012, 87, 573–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parkash, O.; Shueb, R.H. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses 2015, 7, 877. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.H.; Sekaran, S.D.; Azizan, A. Dengue Virus NS1 Protein as a Diagnostic Marker: Commercially Available ELISA and Comparison to QRT-PCR and Serological Diagnostic Assays Currently Used by the State of Florida. J. Trop. Med. 2017, 2017, 8072491. [Google Scholar] [CrossRef]
- Shih, H.-I.; Hsu, H.-C.; Wu, C.-J.; Lin, C.-H.; Chang, C.-M.; Tu, Y.-F.; Hsieh, C.-C.; Chi, C.-H.; Sung, T.-C. Applications of a Rapid and Sensitive Dengue DUO Rapid Immunochromatographic Test Kit as a Diagnostic Strategy during a Dengue Type 2 Epidemic in an Urban City. PLoS ONE 2016, 11, e0158437. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Wang, J.; Peng, H.; Che, X.; Chen, X.; Zhou, Y. NS1-Based Tests with Diagnostic Utility for Confirming Dengue Infection: A Meta-Analysis. Int. J. Infect. Dis. 2014, 26, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.V.; Harris, E. Recent Advances in Understanding the Adaptive Immune Response to Zika Virus and the Effect of Previous Flavivirus Exposure. Virus Res. 2018, 254, 27–33. [Google Scholar] [CrossRef]
- Lustig, Y.; Keler, S.; Kolodny, R.; Ben-Tal, N.; Atias-Varon, D.; Shlush, E.; Gerlic, M.; Munitz, A.; Doolman, R.; Asraf, K.; et al. Potential Antigenic Cross-Reactivity between SARS-CoV-2 and Dengue Viruses. Clin. Infect. Dis. 2020, ciaa1207. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, P.; Murawski, C.; Ehmen, C.; von Possel, R.; Pekarek, N.; Oestereich, L.; Duraffour, S.; Pahlmann, M.; Struck, N.; Eibach, D.; et al. Limited Specificity of Commercially Available SARS-CoV-2 IgG ELISAs in Serum Samples of African Origin. Trop. Med. Int. Health 2021, 26, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.S.E.; Félix, A.C.; de Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of Serological Cross-Reactivity between Yellow Fever and Other Flaviviruses. Int. J. Infect Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef]
| Study population, N (%) | 91 (100.0%) |
| Adults | 57 (62.6%) |
| Children | 34 (37.4%) |
| Age in years, median (range) | |
| Total | 21.0 (5–63) |
| Adults | 27.0 (19–63) |
| Children | 11.5 (5–18) |
| Gender, N (%) | |
| Female | 51 (56.0%) |
| Male | 40 (44.0%) |
| Serotype, N (%) | |
| DENV-1 | 3 (3.3%) |
| DENV-2 | 4 (4.4%) |
| DENV-3 | 10 (11.0%) |
| DENV-4 | 3 (3.3%) |
| Unknown | 71 (78.0%) |
| Time to symptom onset in days, median (range) | |
| Total (N = 87) | 4.0 (0.0–12.0) |
| Adults (N = 54) | 4.5 (0.0–12.0) |
| Children (N = 33) | 3.0 (0.0–8.0) |
| Unknown (N = 4) | N/A |
| Acute infection status, N (%) | |
| PCR+/NS1+ (FOCUS) | 47 (51.6%) |
| PCR−/NS1+ (FOCUS) | 35 (38.5%) |
| PCR+/NS1− (FOCUS) | 9 (9.9%) |
| Reference Test | Population | VIDAS | FOCUS | RDT 1 | |||
|---|---|---|---|---|---|---|---|
| n/N | % [95% CI] | n/N | % [95% CI] | n/N | % [95% CI] | ||
| RT-PCR | Total | 48/56 | 85.7% [74.3–92.6] | 47/56 | 83.9% [72.2–91.3] | 40/55 | 72.7% [59.8–82.7] |
| Adults | 31/39 | 79.5% [64.5–89.2] | 31/39 | 79.5% [64.5–89.2] | 24/38 | 63.2% [47.3–76.6] | |
| Children | 17/17 | 100.0% [80.5–100.0] | 16/17 | 94.1% [73.0–99.0] | 16/17 | 94.1% [73.0–99.0] | |
| Reference Test | Population | Positive Agreement | Negative Agreement | Overall Agreement | |||
|---|---|---|---|---|---|---|---|
| n/N | % [95% CI] | n/N | % [95% CI] | n/N | % [95% CI] | ||
| Dengue NS1 Antigen DxSelect™ (Focus) | Total | 80/82 | 97.6% [91.5–99.7] | 7/9 | 77.8% [45.3–93.7] | 87/91 | 95.6% [89.1–98.8] |
| Adults | 48/49 | 98.0% [89.1–99.9] | 7/8 | 87.5% [52.9–97.8] | 55/57 | 96.5% [87.9–99.6] | |
| Children | 32/33 | 97.0% [84.2–99.9] | 0/1 | 0.0% [0.0–97.5] | 32/34 | 94.1% [80.9–98.4] | |
| NS1 RDT 1 | Total | 70/70 | 100.0% [94.9–100.0] | 9/19 | 47.4% [27.3–68.3] | 79/89 | 88.8% [80.5–93.8] |
| Adults | 41/41 | 100.0% [91.4–100.0] | 8/15 | 53.3% [30.1–75.2] | 49/56 | 87.5% [76.4–93.8] | |
| Children | 29/29 | 100.0% [88.1–100.0] | 1/4 | 25.0% [4.6–69.9] | 30/33 | 90.9% [76.4–96.9] | |
| Reference Test | Population | Positive Agreement | Negative Agreement | Overall Agreement | |||
|---|---|---|---|---|---|---|---|
| n/N | % [95% CI] | n/N | % [95% CI] | n/N | % [95% CI] | ||
| Panbio Dengue IgM Capture ELISA | Total | 40/49 | 81.6% [68.6–90.0] | 26/42 | 61.9% [46.8–75.0] | 66/91 | 72.5% [62.6–80.6] |
| Adults | 25/31 | 80.6% [63.7–90.8] | 16/26 | 61.5% [42.5–77.6] | 41/57 | 71.9% [59.2–81.9] | |
| Children | 15/18 | 83.3% [60.8–94.2] | 10/16 | 62.5% [38.6–81.5] | 25/34 | 73.5% [56.9–85.4] | |
| IgM RDT 1 | Total | 31/36 | 86.1% [71.3–93.9] | 26/47 | 55.3% [41.2–68.6] | 57/83 | 68.7% [58.1–77.6] |
| Adults | 21/25 | 84.0% [65.3–93.6] | 16/28 | 57.1% [39.1–73.5] | 37/53 | 69.8% [56.5–80.5] | |
| Children | 10/11 | 90.9% [62.3–98.4] | 10/19 | 52.6% [31.7–72.7] | 20/30 | 66.7% [48.8–80.8] | |
| Reference Test | Population | Positive Agreement | Negative Agreement | Overall Agreement | |||
|---|---|---|---|---|---|---|---|
| n/N | % [95% CI] | n/N | % [95% CI] | n/N | % [95% CI] | ||
| Panbio Dengue IgG Indirect ELISA | Total | 55/72 | 76.4% [65.4–84.7] | 15/19 | 78.9% [56.7–91.5] | 70/91 | 76.9% [67.3–84.4] |
| Adults | 37/46 | 80.4% [66.8–89.3] | 8/11 | 72.7% [43.4–90.3] | 45/57 | 78.9% [66.7–87.5] | |
| Children | 18/26 | 69.2% [50.0–83.5] | 7/8 | 87.5% [52.9–97.8] | 25/34 | 73.5% [56.9–85.4] | |
| IgG RDT 1 | Total | 33/37 | 89.2% [75.3–95.7] | 24/46 | 52.2% [38.1–65.9] | 57/83 | 68.7% [58.1–77.6] |
| Adults | 27/29 | 93.1% [78.0–98.1] | 14/24 | 58.3% [38.8–75.5] | 41/53 | 77.4% [64.5–86.5] | |
| Children | 6/8 | 75.0% [40.9-92.9] | 10/22 | 45.5% [26.9–65.3] | 16/30 | 53.3% [36.1–69.8] | |
| Age in Years, Median (Range) | 32.0 (18–69) |
|---|---|
| Gender, N (%) | |
| Female | 34 (66.7%) |
| Male | 17 (33.3%) |
| Source of samples, N (%) | |
| EFS, France | 51 (100.0%) |
| Population | VIDAS® NS1 | VIDAS® IgM | VIDAS® IgG | |||
|---|---|---|---|---|---|---|
| n/N | % [95% CI] | n/N | % [95% CI] | n/N | % [95% CI] | |
| Adult healthy donors 1 | 51/51 | 100.0% [93.0–100.0] | 51/51 | 100.0% [93.0–100.0] | 49/51 | 96.1% [86.5–99.5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somlor, S.; Brossault, L.; Grandadam, M. Evaluation of VIDAS® Diagnostic Assay Prototypes Detecting Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM and IgG Antibodies. Diagnostics 2021, 11, 1228. https://doi.org/10.3390/diagnostics11071228
Somlor S, Brossault L, Grandadam M. Evaluation of VIDAS® Diagnostic Assay Prototypes Detecting Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM and IgG Antibodies. Diagnostics. 2021; 11(7):1228. https://doi.org/10.3390/diagnostics11071228
Chicago/Turabian StyleSomlor, Somphavanh, Ludovic Brossault, and Marc Grandadam. 2021. "Evaluation of VIDAS® Diagnostic Assay Prototypes Detecting Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM and IgG Antibodies" Diagnostics 11, no. 7: 1228. https://doi.org/10.3390/diagnostics11071228
APA StyleSomlor, S., Brossault, L., & Grandadam, M. (2021). Evaluation of VIDAS® Diagnostic Assay Prototypes Detecting Dengue Virus NS1 Antigen and Anti-Dengue Virus IgM and IgG Antibodies. Diagnostics, 11(7), 1228. https://doi.org/10.3390/diagnostics11071228






