VEGF-Targeted Multispectral Optoacoustic Tomography and Fluorescence Molecular Imaging in Human Carotid Atherosclerotic Plaques
Abstract
:1. Introduction
2. Materials and Methods
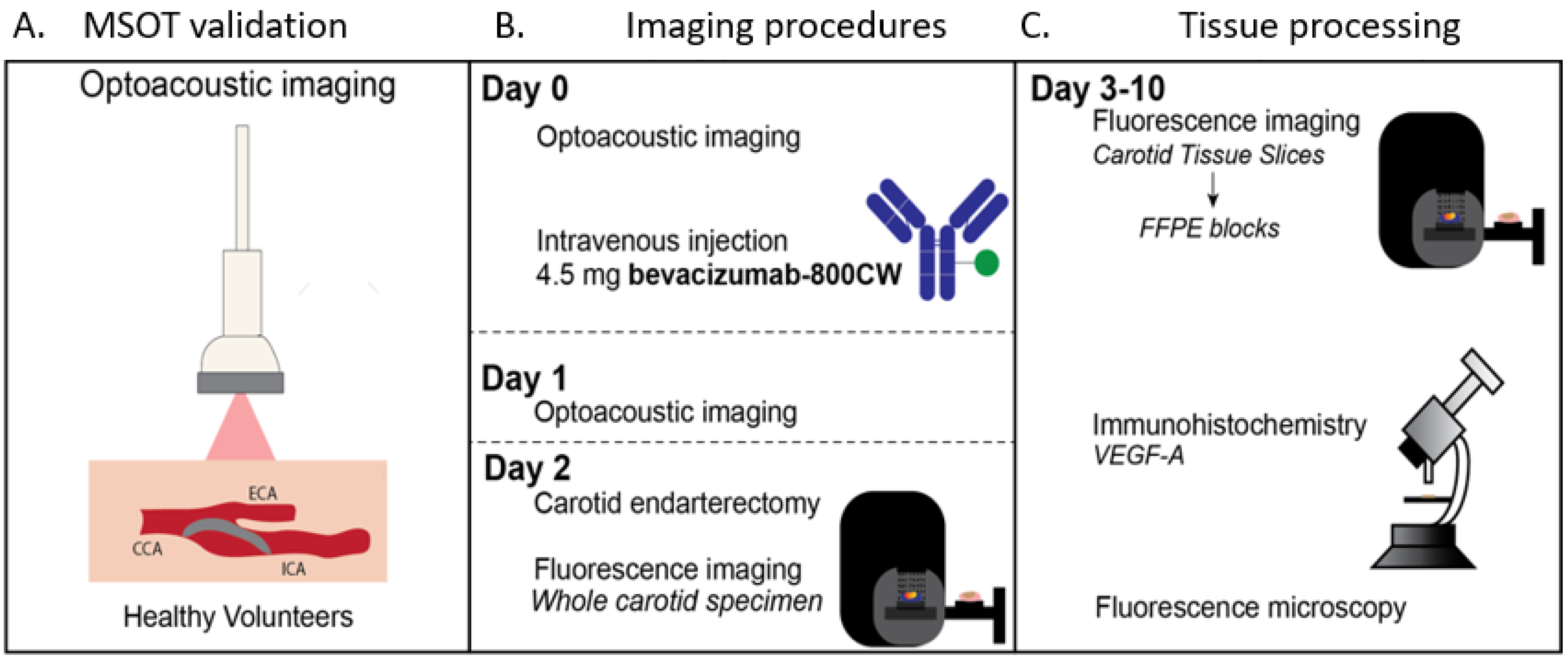
2.1. Clinical Trial Design
2.2. Safety Data
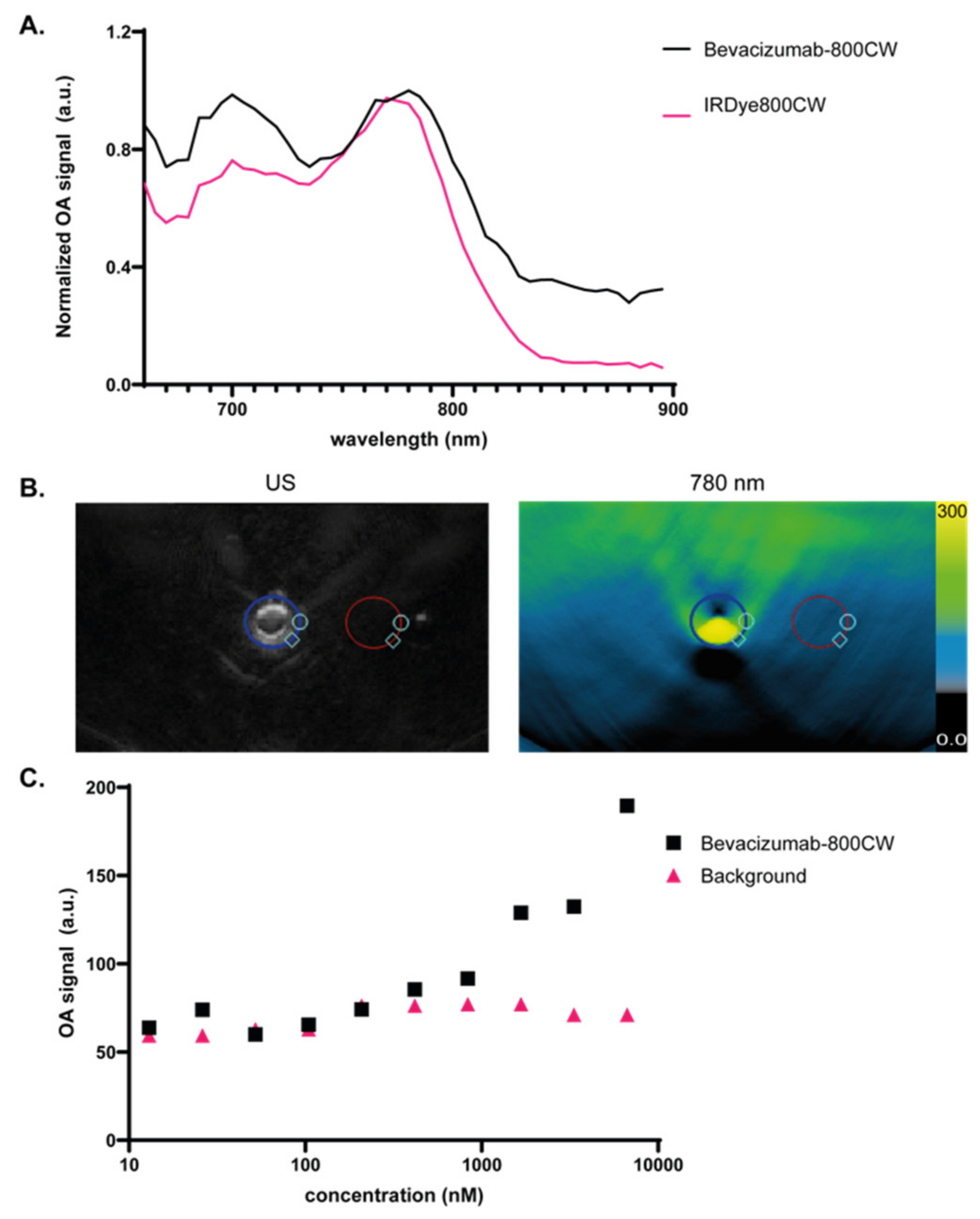
2.3. Optical Characterization of Bevacizumab-800CW
2.4. Optoacoustic Imaging
2.5. Ex Vivo Analyses of Surgical Specimen
2.6. Statistics
3. Results
3.1. Validation of Bevacizumab-800CW Detection Using a Clinical MSOT System
3.2. Subject Characteristics and Safety Data
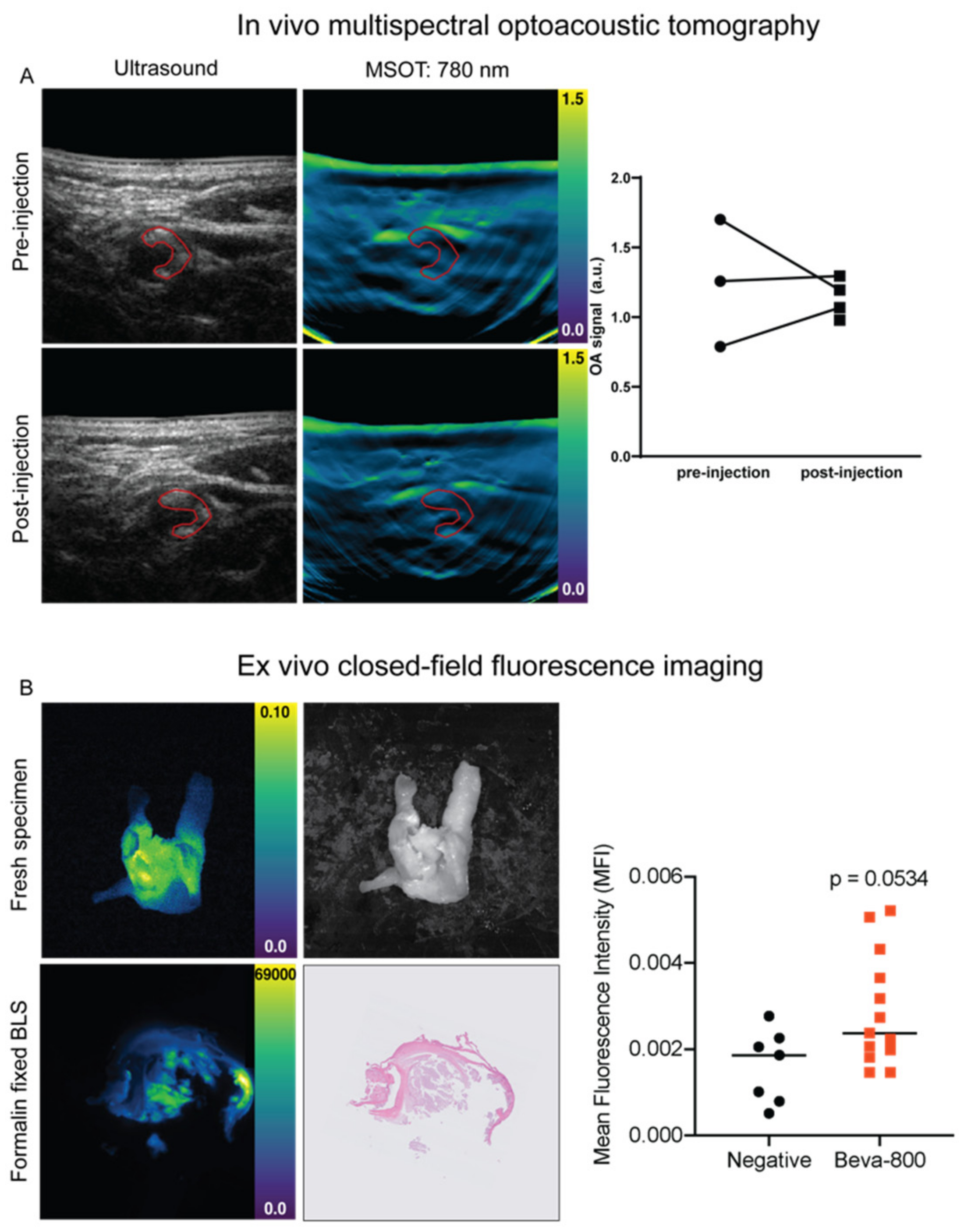
3.3. In Vivo MSOT
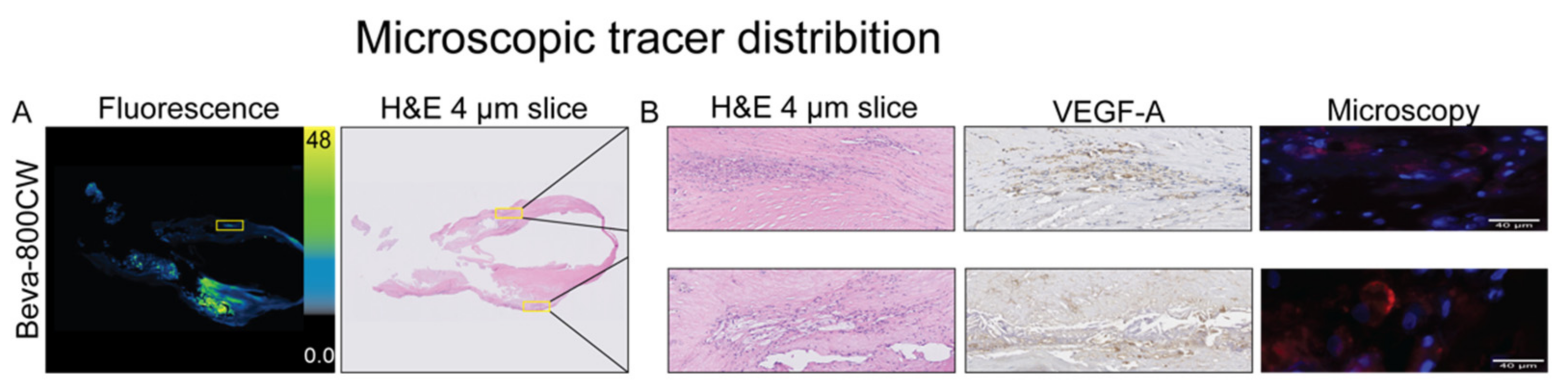
3.4. Ex Vivo Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ooi, Y.C.; Gonzalez, N.R. Management of Extracranial Carotid Artery Disease. Cardiol. Clin. 2015, 33, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, P.M.; Eliasziw, M.; Gutnikov, S.A.; Fox, A.J.; Taylor, D.W.; Mayberg, M.R.; Warlow, C.P.; Barnett, H.J.M. Analysis of Pooled Data from the Randomised Controlled Trials of Endarterectomy for Symptomatic Carotid Stenosis. Lancet 2003, 361, 107–116. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the Atherosclerotic Plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.W.; Slart, R.H.J.A.; Zeebregts, C.J.; Hillebrands, J.L.; Tio, R.A. Atherosclerotic Plaque Development and Instability: A Dual Role for VEGF. Ann. Med. 2009, 41, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Zeebregts, C.J.; Terwisscha Van Scheltinga, A.G.T.; Lub-de Hooge, M.N.; van Dam, G.M.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Tio, R.A.; Suurmeijer, A.J.H.; Boersma, H.H.; et al. Feasibility of Vascular Endothelial Growth Factor Imaging in Human Atherosclerotic Plaque Using 89Zr-Bevacizumab Positron Emission Tomography. Mol. Imaging 2013, 12. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic, I.; Merčep, E.; Schmedt, C.G.; Deán-Ben, X.L.; Razansky, D. Real-Time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regensburger, A.P.; Fonteyne, L.M.; Jungert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of Collagens by Multispectral Optoacoustic Tomography as an Imaging Biomarker for Duchenne Muscular Dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Timmermans, A.C.; Wouters, P.C.; Kacprowicz, M.; van Dam, G.M.; Ntziachristos, V. Optoacoustic Imaging of Human Vasculature: Feasibility by Using a Handheld Probe. Radiology 2016, 281, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Kruizinga, P.; van der Steen, A.F.W.; de Jong, N.; Springeling, G.; Robertus, J.L.; van der Lugt, A.; van Soest, G. Photoacoustic Imaging of Carotid Artery Atherosclerosis. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; van der Steen, A.F.W.; Regar, E.; van Soest, G. Emerging Technology Update: Intravascular Photoacoustic Imaging of Vulnerable Atherosclerotic Plaque. Interv. Cardiol. Rev. 2016, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Jüstel, D.; Prakash, J.; Karlas, A.; Helfen, A.; Masthoff, M.; Wildgruber, M.; Ntziachristos, V. Soft Ultrasound Priors in Optoacoustic Reconstruction: Improving Clinical Vascular Imaging. Photoacoustics 2020, 19, 100172. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; van Scheltinga, A.G.T.T.; Jansen, L.; de Vries, J.; Lub-De Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Qiu, S.Q.; Linssen, M.D.; Jansen, L.; Kelder, W.; de Vries, J.; Kruithof, I.; Zhang, G.J.; Robinson, D.J.; Nagengast, W.B.; et al. Implementation and Benchmarking of a Novel Analytical Framework to Clinically Evaluate Tumor-Specific Fluorescent Tracers. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration, HHS. International Conference on Harmonisation; Guidance on M3 (R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; Availability Notice. Fed. Regist. 2010, 75, 3471–3472. [Google Scholar]
- Ter Weele, E.J.; van Scheltinga, A.G.; Linssen, M.D.; Nagengast, W.B.; Lindner, I.; Jorritsma-Smit, A.; de Vries, E.G.; Kosterink, J.G.; Hooge, M.N.L. Development, Preclinical Safety, Formulation, and Stability of Clinical Grade Bevacizumab-800CW, a New near Infrared Fluorescent Imaging Agent for First in Human Use. Eur. J. Pharm. Biopharm. 2016, 104, 226–234. [Google Scholar] [CrossRef]
- National Institute of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 25 May 2021).
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic Status of Sentinel Lymph Nodes in Melanoma Determined Noninvasively with Multispectral Optoacoustic Imaging. Sci. Trans. Med. 2015, 7, 317. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, 37. [Google Scholar] [CrossRef]
- Fuenzalida Werner, J.P.; Huang, Y.; Mishra, K.; Janowski, R.; Vetschera, P.; Heichler, C.; Chmyrov, A.; Neufert, C.; Niessing, D.; Ntziachristos, V.; et al. Challenging a Preconception: Optoacoustic Spectrum Differs from the Optical Absorption Spectrum of Proteins and Dyes for Molecular Imaging. Anal. Chem. 2020, 92, 10717–10724. [Google Scholar] [CrossRef]
- Huisman, L.A.; Steinkamp, P.J.; Hillebrands, J.L.; Zeebregts, C.J.; Linssen, M.D.; Jorritsma-Smit, A.; Slart, R.H.J.A.; van Dam, G.M.; Boersma, H.H. Feasibility of Ex Vivo Fluorescence Imaging of Angiogenesis in (Non-) Culprit Human Carotid Atherosclerotic Plaques Using Bevacizumab-800CW. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Steinkamp, P.J.; Pranger, B.K.; Li, M.; Linssen, M.D.; Voskuil, F.J.; Been, L.B.; van Leeuwen, B.L.; Suurmeijer, A.J.H.; Nagengast, W.B.; Kruijff, S.K.; et al. Fluorescence-Guided Visualization of Soft Tissue Sarcomas by Targeting Vascular Endothelial Growth Factor-A: A Phase 1 Single-Center Clinical Trial. J. Nucl. Med. 2020, 62, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast Agents for Molecular Photoacoustic Imaging. Nat. Meth. 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mat. 2019, 31, 1805875. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | 5 volunteers |
|---|---|
| Age (years), mean (range) | 28 (23–38) |
| Sex: males, n (%) | 5 (100) |
| Weight (kg), mean (range) | 82 (74–86) |
| Height (cm), mean (range) | 186 (172–189) |
| Imaging time (min) mean (range) | 11 (5–12) |
| MSOT related adverse events | 0 |
| Characteristics | 4 patients |
| Age (years), mean (range) | 76 (58–82) |
| Sex: males, n (%) | 4 (100) |
| Weight (kg), mean (range) | 71.5 (65–80) |
| Height (cm), mean (range) | 172.5 (170–178) |
| Total infusion time (min) | 1 |
| Imaging time (min), mean (range) | 9 (5–12) |
| Adverse events (grade 1–4) | 0 |
| MSOT related adverse events | 0 |
| Total tissue slices analyzed | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinkamp, P.J.; Vonk, J.; Huisman, L.A.; Meersma, G.-J.; Diercks, G.F.H.; Hillebrands, J.-L.; Nagengast, W.B.; Zeebregts, C.J.; Slart, R.H.J.A.; Boersma, H.H.; et al. VEGF-Targeted Multispectral Optoacoustic Tomography and Fluorescence Molecular Imaging in Human Carotid Atherosclerotic Plaques. Diagnostics 2021, 11, 1227. https://doi.org/10.3390/diagnostics11071227
Steinkamp PJ, Vonk J, Huisman LA, Meersma G-J, Diercks GFH, Hillebrands J-L, Nagengast WB, Zeebregts CJ, Slart RHJA, Boersma HH, et al. VEGF-Targeted Multispectral Optoacoustic Tomography and Fluorescence Molecular Imaging in Human Carotid Atherosclerotic Plaques. Diagnostics. 2021; 11(7):1227. https://doi.org/10.3390/diagnostics11071227
Chicago/Turabian StyleSteinkamp, Pieter J., Jasper Vonk, Lydian A. Huisman, Gert-Jan Meersma, Gilles F. H. Diercks, Jan-Luuk Hillebrands, Wouter B. Nagengast, Clark J. Zeebregts, Riemer H. J. A. Slart, Hendrikus H. Boersma, and et al. 2021. "VEGF-Targeted Multispectral Optoacoustic Tomography and Fluorescence Molecular Imaging in Human Carotid Atherosclerotic Plaques" Diagnostics 11, no. 7: 1227. https://doi.org/10.3390/diagnostics11071227
APA StyleSteinkamp, P. J., Vonk, J., Huisman, L. A., Meersma, G.-J., Diercks, G. F. H., Hillebrands, J.-L., Nagengast, W. B., Zeebregts, C. J., Slart, R. H. J. A., Boersma, H. H., & van Dam, G. M. (2021). VEGF-Targeted Multispectral Optoacoustic Tomography and Fluorescence Molecular Imaging in Human Carotid Atherosclerotic Plaques. Diagnostics, 11(7), 1227. https://doi.org/10.3390/diagnostics11071227







