Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function
Abstract
:1. Background
- (1)
- (2)
- (3)
- (4)
- (5)
- Questionnaires, interviews, or a combination of both [18].
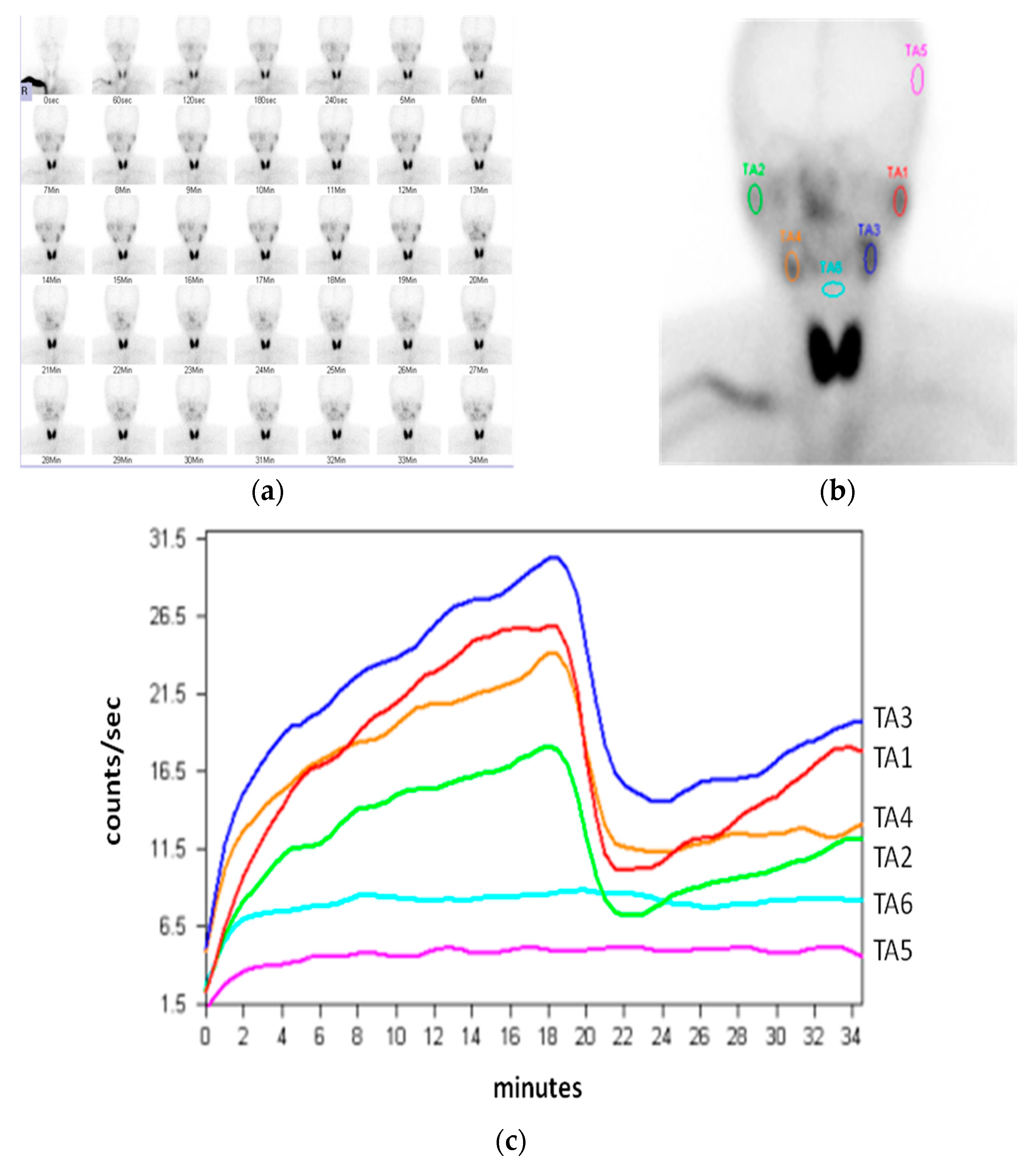
2. Mechanism and Procedure of Sialoscintigraphy
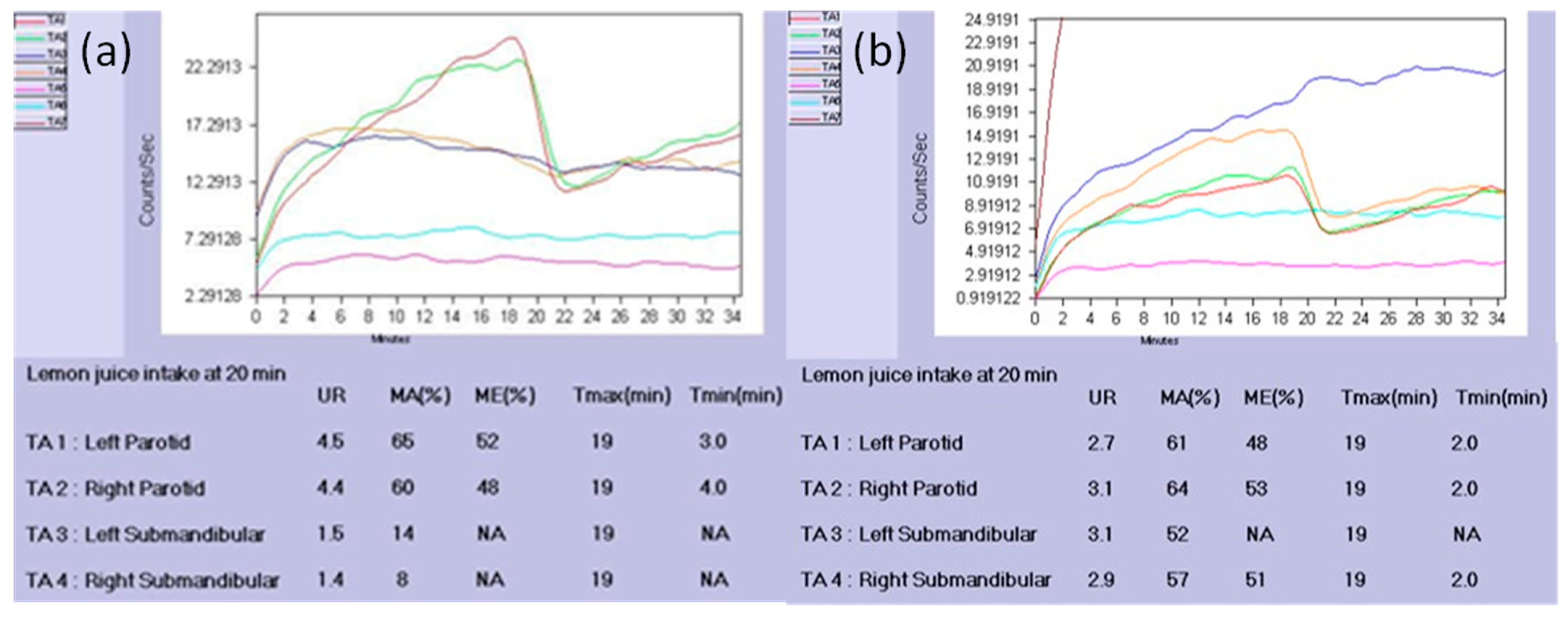
3. Comparing Quantitative with Visual Evaluations
4. Applications of Sialoscintigraphy to Different Salivary Functional Disorders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locker, D. Xerostomia in older adults: A longitudinal study. Gerodontology 1995, 12, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Slade, G.D.; Carter, K. A longitudinal study of medication exposure and xerostomia among older people. Gerodontology 2006, 23, 205–213. [Google Scholar] [CrossRef]
- Gibson, B.; Periyakaruppiah, K.; Thornhill, M.H.; Baker, S.R.; Robinson, P.G. Measuring the symptomatic, physical, emotional and social impacts of dry mouth: A qualitative study. Gerodontology 2020, 37, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Löfgren, C.D.; Isberg, P.-E.; Christersson, C. Screening for oral dryness in relation to salivary flow rate addresses the need for functional tests of saliva. Oral Health Prev. Dent. 2010, 8, 243–252. [Google Scholar]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Jager, D.H.J.; Bots, C.P.; Forouzanfar, T.; Brand, H.S. Clinical oral dryness score: Evaluation of a new screening method for oral dryness. Odontology 2018, 106, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löfgren, C.D.; Wickström, C.; Sonesson, M.; Lagunas, P.T.; Christersson, C. A systematic review of methods to diagnose oral dryness and salivary gland function. BMC Oral Health 2012, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Bermejo-Fenoll, A. A simple test for salivary gland hypofunction using Oral Schirmer’s test. J. Oral Pathol. Med. 2006, 35, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Minagi, H.O.; Yamanaka, Y.; Sakai, T. Evaluation of the Saxon test for patients with hyposalivation without Sjögren’s syndrome. J. Oral Rehabil. 2020, 47, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Almståhl, A.; Wikström, M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch. Oral Biol. 2003, 48, 337–344. [Google Scholar] [CrossRef]
- Osailan, S.; Pramanik, R.; Shirodaria, S.; Challacombe, S.; Proctor, G.; Challacombe, S.; Osailan, S.; Pramanik, R.; Shirodaria, S.; Challacombe, S.; et al. Investigating the relationship between hyposalivation and mucosal wetness. Oral Dis. 2011, 17, 109–114. [Google Scholar] [CrossRef]
- El-Miedany, Y.M.; El-Hady, S.M.; El-Baddin, M.A. Validity of the saliva ferning test for the diagnosis of dry mouth in Sjogren’s syndrome. Rev. Rhum. Engl. Ed. 1999, 66, 73–78. [Google Scholar]
- Fukushima, Y.; Sano, Y.; Isozaki, Y.; Endo, M.; Tomoda, T.; Kitamura, T.; Sato, T.; Kamijo, Y.; Haga, Y.; Yoda, T. A pilot clinical evaluation of oral mucosal dryness in dehydrated patients using a moisture-checking device. Clin. Exp. Dent. Res. 2019, 5, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Herscovici, D.; Rosenberg, M. A simple technique for the determination of salivary gland hypofunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 175–178. [Google Scholar] [CrossRef]
- Sanchez-Guerrero, J.; Aguirre-García, E.; Pérez-Dosal, M.R.; Kraus, A.; Cardiel, M.H.; Soto-Rojas, A.E. The wafer test: A semi-quantitative test to screen for xerostomia. Rheumatology 2002, 41, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Jousse-Joulin, S.; d’Agostino, M.A.; Nicolas, C.; Naredo, E.; Ohrndorf, S.; Backhaus, M.; Tamborrini, G.; Chary-Valckenaere, I.; Terslev, L.; Iagnocco, A.; et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: An OMERACT ultrasound working group reliability exercise. Ann. Rheum. Dis. 2019, 78, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, M.S.; Glaudemans, A.W.; van der Vegt, B.; Mossel, E.; Kroese, F.G.; Bootsma, H.; Vissink, A. Imaging in Primary Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; van der Putten, G.J.; de Baat, C.; Ikebe, K.; Matsuda, K.I.; Enoki, K.; Hopcraft, M.S.; Ling, G.Y. Shortening the xerostomia inventory. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Kishimoto, T.; Iwawaki, Y.; Fujimoto, K.; Ishida, Y.; Watanabe, M.; Nagao, K.; Ichikawa, T. Reliability of Screening Methods to Diagnose Oral Dryness and Evaluate Saliva Secretion. Dent. J. 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Baba, S.; Isoda, T.; Kitamura, Y.; Abe, K.; Sasaki, M.; Honda, H. A Functional Scoring System Based on Salivary Gland Scintigraphy for Evaluating Salivary Gland Dysfunction Secondary to 131I therapy in Patients with Differentiated Thyroid Carcinoma. J. Clin. Diagn. Res. 2017, 11, TC23–TC28. [Google Scholar] [CrossRef]
- Nakayama, M.O.; Nakajima, K.; Takahashi, K. Approach to Diagnosis of Salivary Gland Disease from Nuclear Medicine Images, Salivary Glands—New Approaches in Diagnostics and Treatment, Işıl Adadan Güvenç, IntechOpen. 2017. Available online: https://www.intechopen.com/books/salivary-glands-new-approaches-in-diagnostics-and-treatment/approach-to-diagnosis-of-salivary-gland-disease-from-nuclear-medicine-images (accessed on 23 April 2021). [CrossRef] [Green Version]
- Chen, Y.-C.; Han, D.-Y.; Chang, C.-C.; Su, C.-H.; Hung, S.-H.; Hsu, C.-H. The establishment and application of sialoscintigraphic reference values from patients with obstructive sialadenitis. Nucl. Med. Commun. 2020, 41, 308–313. [Google Scholar] [CrossRef]
- Akker, H.P.V.D. Aspects of Salivary Gland Scintigraphy with 99mtc-Pertechnetate; Amsterdam University: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Aung, W.; Murata, Y.; Ishida, R.; Takahashi, Y.; Okada, N.; Shibuya, H. Study of quantitative oral radioactivity in salivary gland scintigraphy and determination of the clinical stage of Sjogren’s syndrome. J. Nucl. Med. 2001, 42, 38–43. [Google Scholar] [PubMed]
- Schall, G.L.; Anderson, L.G.; Wolf, R.O.; Herdt, J.R.; Tarpley, T.M.; Cummings, N.A.; Zeiger, L.S.; Talal, N. Xerostomia in Sjögren’s syndrome. Evaluation by sequential salivary scintigraphy. JAMA 1971, 216, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; García, L.; Rayo, J.I.; Serrano, J.; Domínguez, M.L.; Moreno, M. Diagnostic contribution of quantitative analysis of salivary scintigraphy in patients with suspected Sjogren’s syndrome. Rev. Esp. Med. Nucl. Imagen. Mol. 2016, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Blanco, A.; Martínez-Lázaro, R. Reproducibility of the qualitative interpretation of dynamic salivary radionuclide scans with excretory stimulation. Acta Otorrinolaringol. Española 2000, 51, 143–148. [Google Scholar]
- Vinagre, F.; Santos, M.J.; Prata, A.; da Silva, J.C.; Santos, A.I. Assessment of salivary gland function in Sjogren’s syndrome: The role of salivary gland scintigraphy. Autoimmun. Rev. 2009, 8, 672–676. [Google Scholar] [CrossRef]
- Zhu, G.W.; Gao, Z.; Feng, H.B.; Qiu, J.J. Quantitative analysis for modified Schall’s classification by stimulation test with dynamic scintigraphy in Sjogren’s syndrome. Int. J. Rheum. Dis. 2020, 23, 381–391. [Google Scholar] [CrossRef]
- Daniels, T.E.; Powell, M.R.; Sylvester, R.A.; Talal, N. An evaluation of salivary scintigraphy in Sjögren’s syndrome. Arthritis Rheum. 1979, 22, 809–814. [Google Scholar] [CrossRef]
- Mishkin, F.S. Radionuclide salivary gland imaging. Semin. Nucl. Med. 1981, 11, 258–265. [Google Scholar] [CrossRef]
- Kohn, W.G.; Ship, J.A.; Atkinson, J.C.; Patton, L.L.; Fox, P.C. Salivary gland 99mTc-scintigraphy: A grading scale and correlation with major salivary gland flow rates. J. Oral Pathol. Med. 1992, 21, 70–74. [Google Scholar] [CrossRef]
- D’Arbonneau, F.; Ansart, S.; Berre, R.L.; Dueymes, M.; Youinou, P.; Pennec, Y.L. Thyroid dysfunction in primary Sjogren’s syndrome: A long-term followup study. Arthritis Rheum. 2003, 49, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Umehara, I.; Yamada, I.; Murata, Y.; Takahashi, Y.; Okada, N.; Shibuya, H. Quantitative evaluation of salivary gland scintigraphy in Sjorgen’s syndrome. J. Nucl. Med. 1999, 40, 64–69. [Google Scholar] [PubMed]
- Adams, B.K.; Al Attia, H.M.; Parkar, S. Salivary gland scintigraphy in Sjogren’s syndrome: Are quantitative indices the answer? Nucl. Med. Commun. 2003, 24, 1011–1016. [Google Scholar] [CrossRef]
- Nishiyama, S.; Miyawaki, S.; Yoshinaga, Y. A study to standardize quantitative evaluation of parotid gland scintigraphy in patients with Sjogren’s syndrome. J. Rheumatol. 2006, 33, 2470–2474. [Google Scholar] [PubMed]
- Kaldeway, H.P.; ter Borg, E.-J.; van de Garde, E.M.; Habraken, J.B.; van Buul, M.M. Validation of quantitative salivary gland scintigraphy in relation to the American–European concensus criteria for Sjögren’s syndrome. Nucl. Med. Commun. 2019, 40, 343–348. [Google Scholar] [CrossRef]
- Kim, H.A.; Yoon, S.H.; Yoon, J.K.; Lee, S.J.; Jo, K.S.; Lee, D.H.; Suh, C.H.; An, Y.S. Salivary gland scintigraphy in Sjogren’s syndrome. Comparison of the diagnostic performance of visual and semiquantitative analysis. Nuklearmedizin 2014, 53, 139–145. [Google Scholar] [CrossRef]
- García-González, M.; González-Soto, M.J.; Rodríguez-Bethencourt, M.; Ángeles, G.; Ferraz-Amaro, I. The validity of salivary gland scintigraphy in Sjögren’s syndrome diagnosis: Comparison of visual and excretion fraction analyses. Clin. Rheumatol. 2020, 40, 1923–1931. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Perez-De-Lis, M.; Diaz-Lagares, C.; Bove, A.; Soto, M.-J.; Jimenez, I.; Belenguer, R.; Siso, A.; Muxí, A.; et al. Clinical and Prognostic Significance of Parotid Scintigraphy in 405 Patients with Primary Sjögren’s Syndrome. J. Rheumatol. 2010, 37, 585–590. [Google Scholar] [CrossRef]
- Tzioufas, A.G. Salivary gland imaging techniques for the diagnosis of Sjögren’s syndrome. Int. J. Clin. Rheumatol. 2009, 4, 321–327. [Google Scholar] [CrossRef]
- Booker, J.; Howarth, D.; Taylor, L.; Voutnis, D.; Sutherland, D. Appropriate utilization of semi-quantitative analysis in salivary scintigraphy. Nucl. Med. Commun. 2004, 25, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, Y.; Hardwick, L. Individual Diagnosis, Prognosis and Counselling for Caries Prevention. Carries Res. 1978, 12 (Suppl. 1), 94–102. [Google Scholar] [CrossRef]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef]
- Aksoy, T.; Kiratli, P.O.; Erbas, B. Correlations between histopathologic and scintigraphic parameters of salivary glands in patients with Sjögren’s syndrome. Clin. Rheumatol. 2012, 31, 1365–1370. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ye, X.; Meng, Y.; Zhao, Y.-N.; Liu, D.-G.; Yu, G.-Y. Evaluation of Parotid Gland Function Before and After Endoscopy-Assisted Stone Removal. J. Oral Maxillofac. Surg. 2019, 77, 328.e1–328.e9. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.W.C.; Leung, K.Y. A Review on the Assessment of Radiation Induced Salivary Gland Damage After Radiotherapy. Front. Oncol. 2019, 9, 1090. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-Q.; Feng, H.-J.; Ouyang, W.; Sun, Y.-G.; Chen, P.; Wang, J.; Xian, J.-L.; Huang, L.-H. Systematic evaluation of salivary gland damage following I-131 therapy in differentiated thyroid cancer patients by quantitative scintigraphy and clinical follow-up. Nucl. Med. Commun. 2015, 36, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Badam, R.K. Assessment of Salivary Gland Function Using Salivary Scintigraphy in Pre and Post Radioactive Iodine Therapy in Diagnosed Thyroid Carcinoma Patients. J. Clin. Diagn. Res. 2016, 10, ZC60–ZC62. [Google Scholar] [CrossRef]
- Chung, M.K.; Kim, D.H.; Ahn, Y.C.; Choi, J.Y.; Kim, E.H.; Son, Y.-I. Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 2016, 155, 423–430. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.A.; Russo, N.; Griffith, S.; Braun, T.; Eisbruch, A.; d’Silva, N.J. Recovery of salivary epidermal growth factor in parotid saliva following parotid sparing radiation therapy: A proof-of-principle study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 64–70. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Choi, J.-S.; Bin Hong, S.; Hyun, I.Y.; Lim, J.-Y. Salivary gland function after sialendoscopy for treatment of chronic radioiodine-induced sialadenitis. Head Neck 2016, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Dang, L.H.; Chen, L.-C.; Chang, C.-C.; Han, D.-Y.; Hsu, C.-H.; Ding, Y.-F.; Su, C.-H.; Hung, S.-H. Office-based salivary gland ductal irrigation in patients with chronic sialoadenitis: A preliminary study. J. Formos. Med. Assoc. 2021, 120, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chen, H.-Y.; Hsu, C.-H. Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function. Diagnostics 2021, 11, 1173. https://doi.org/10.3390/diagnostics11071173
Chen Y-C, Chen H-Y, Hsu C-H. Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function. Diagnostics. 2021; 11(7):1173. https://doi.org/10.3390/diagnostics11071173
Chicago/Turabian StyleChen, Yen-Chun, Hsin-Yung Chen, and Chung-Huei Hsu. 2021. "Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function" Diagnostics 11, no. 7: 1173. https://doi.org/10.3390/diagnostics11071173
APA StyleChen, Y.-C., Chen, H.-Y., & Hsu, C.-H. (2021). Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function. Diagnostics, 11(7), 1173. https://doi.org/10.3390/diagnostics11071173





