Influencing Factors on Postmortem Protein Degradation for PMI Estimation: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Data Source and Eligibility Criteria
2.2. Search Strategy
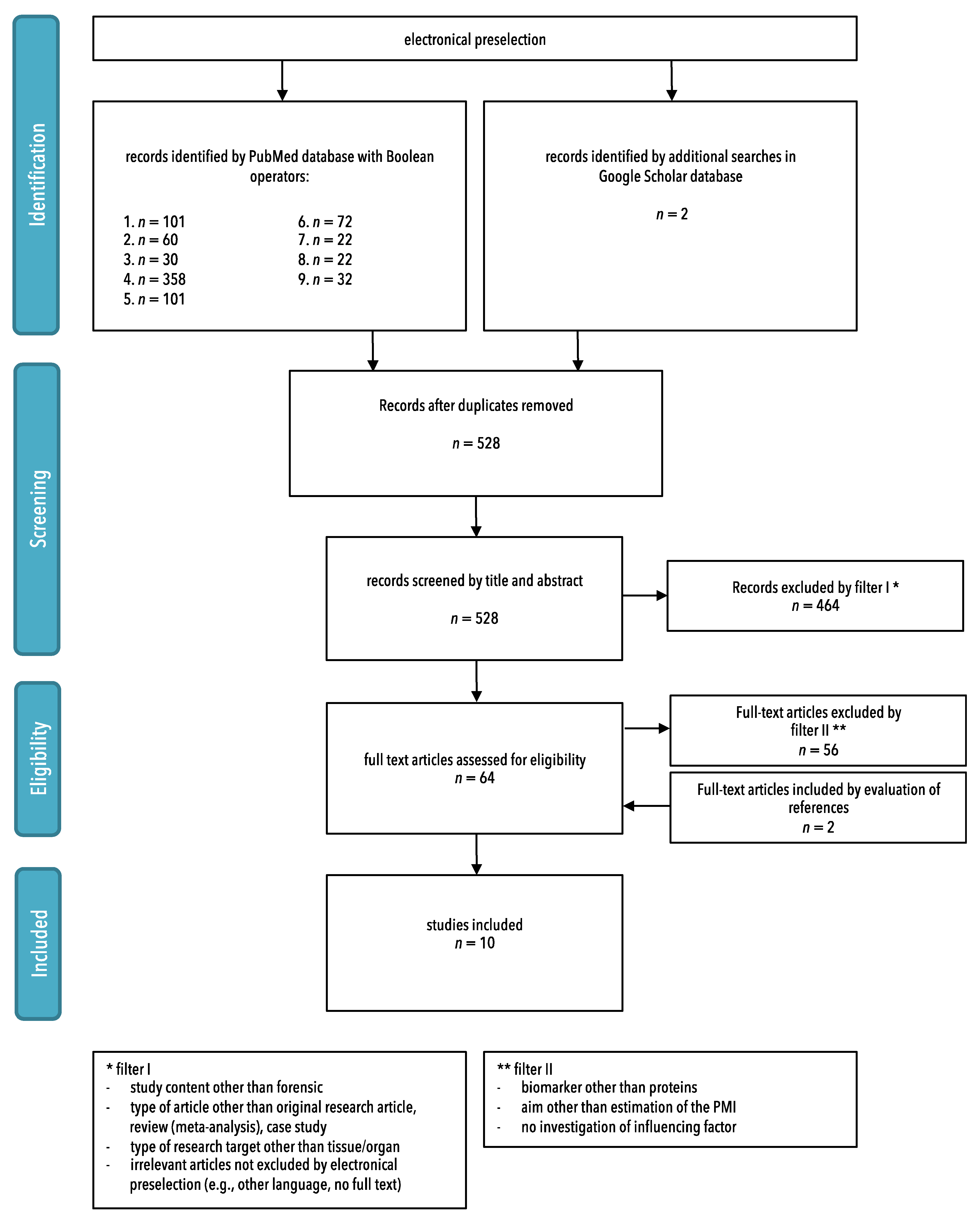
2.3. Study Selection
- -
- Study content other than forensic;
- -
- Type of article other than original research article, review (meta-analysis), or case study;
- -
- Type of research target other than tissue/organ;
- -
- Irrelevant articles not excluded by electronical preselection (e.g., other language, no full text).
- -
- Biomarker other than proteins;
- -
- Aim other than estimation of the PMI;
- -
- No investigation of influencing factors.
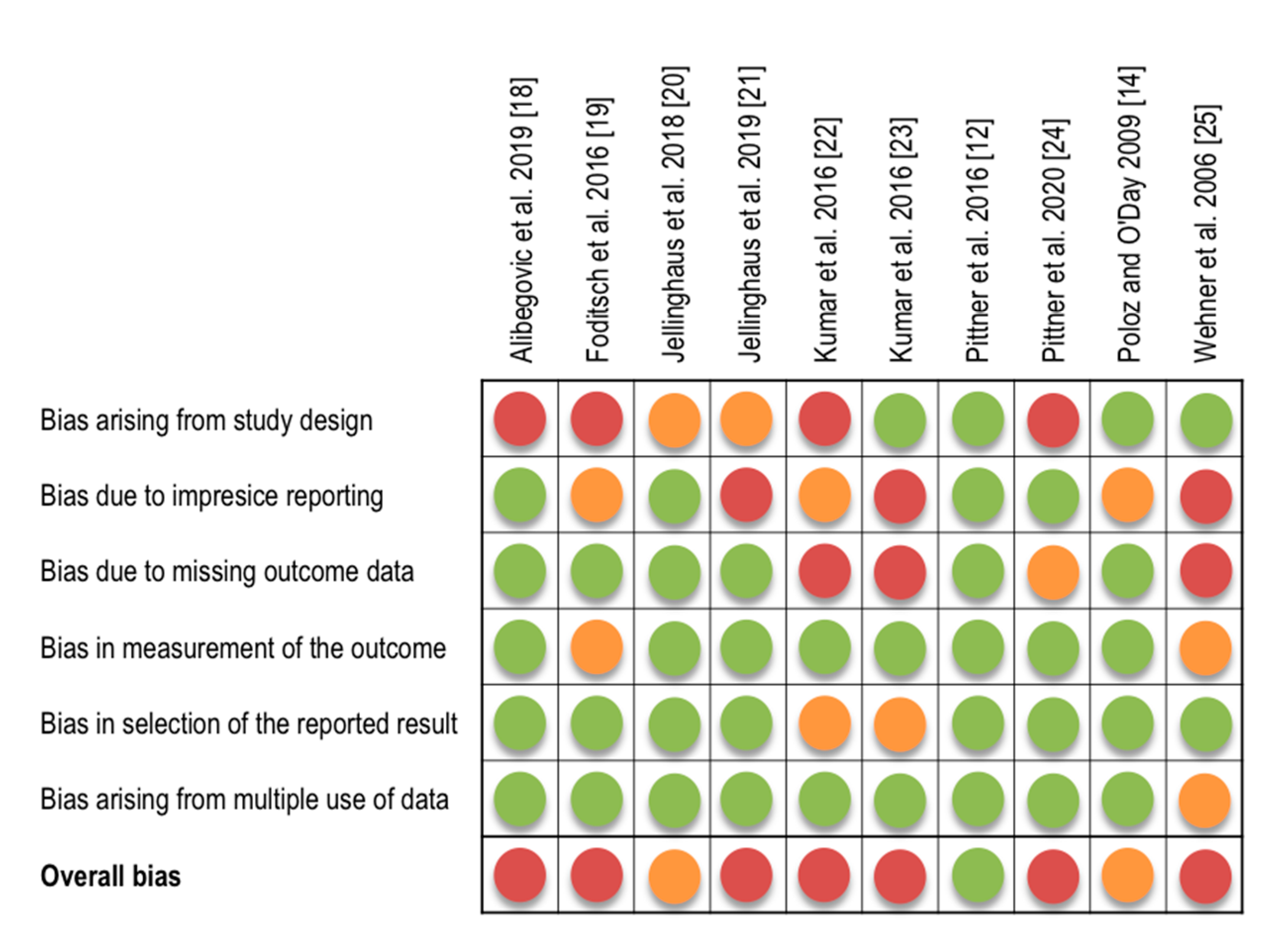
2.4. Risk of Bias Assessment
2.5. Data Extraction and Synthesis
3. Results
3.1. Study Selection
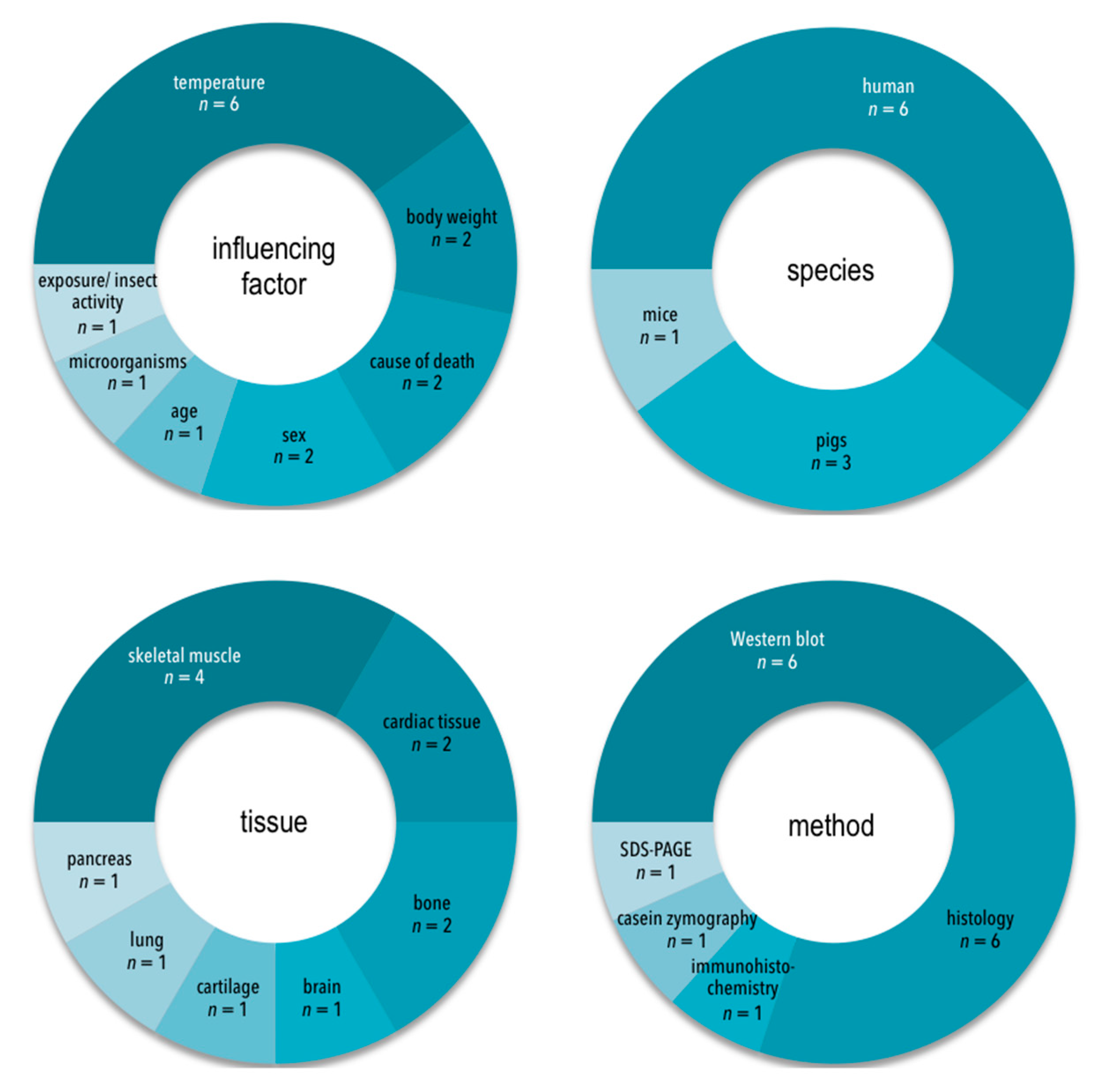
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment
3.4. Body of Evidence
3.4.1. Evidence Base
3.4.2. Consistency
3.4.3. Generalizability
4. Synthesis of Results
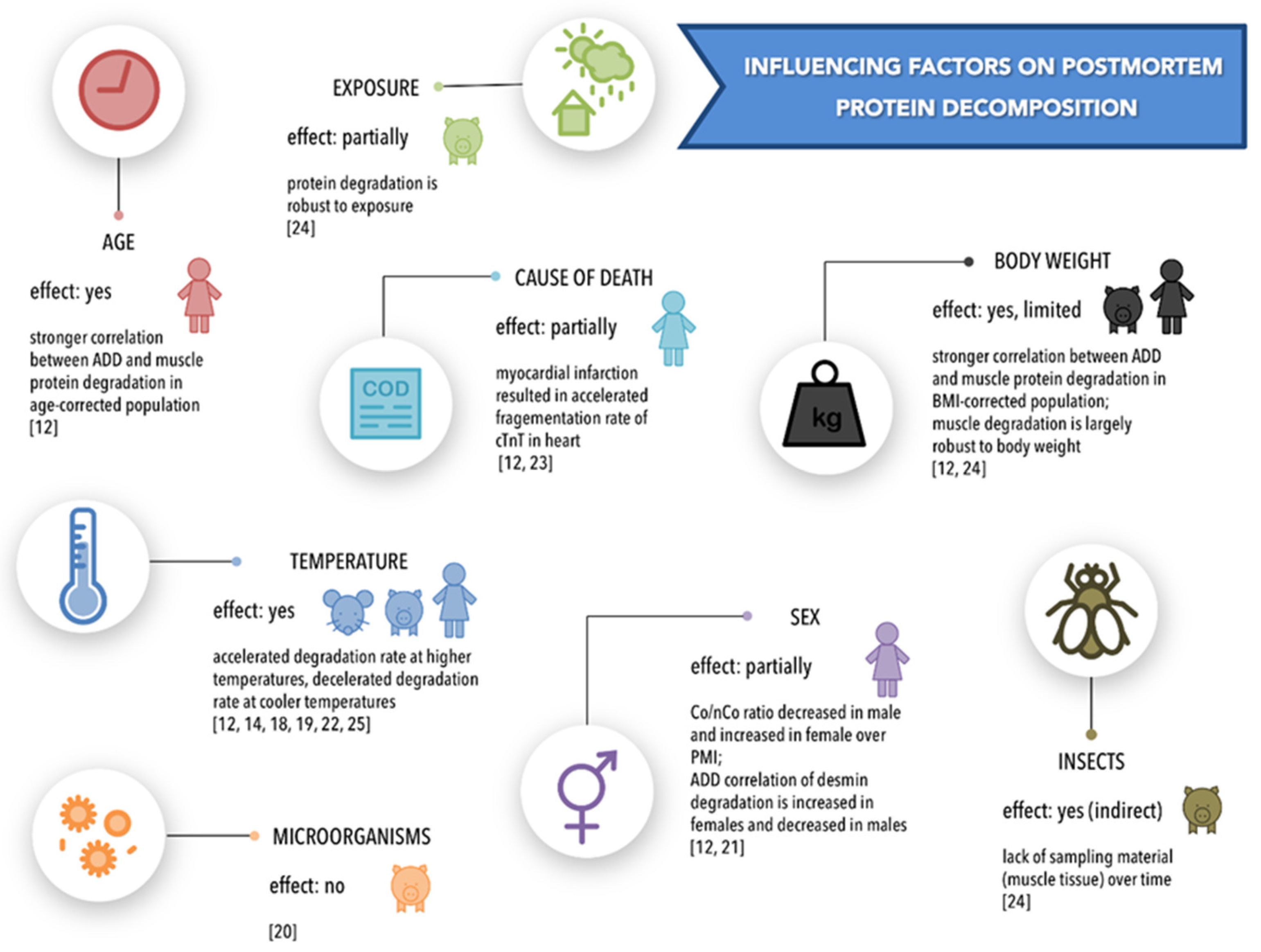
4.1. Temperature
4.2. Body Weight
4.3. Cause of Death
4.4. Sex
4.5. Age
4.6. Exposure/Environment and Insect Activity
4.7. Microorganisms
5. Discussion
5.1. The Effect of Temperature
5.2. The Effect of Body Mass
5.3. The Effect of Sex and Age
5.4. The Effect of Cause of Death
5.5. The Effects of Exposure, Insects and Microorganisms
6. Limitation
7. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donaldson, A.E.; Lamont, I.L. Biochemistry Changes That Occur after Death: Potential Markers for Determining Post-Mortem Interval. PLoS ONE 2013, 8, e82011. [Google Scholar] [CrossRef] [PubMed]
- Campobasso, C.P.; DI Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Almulhim, A.M.; Menezes, R.G. Evaluation of Postmortem Changes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Teo, C.H.; Hamzah, N.H.; Hing, H.L.; Hamzah, S.P.A.A. Decomposition Process and Post Mortem Changes: Review (Proses Pereputan Dan Perubahan Pasca Kematian: Ulasan). Sains Malays. 2014, 43, 1873–1882. [Google Scholar] [CrossRef]
- Zhou, C.; Byard, R.W. Factors and processes causing accelerated decomposition in human cadavers—An overview. J. Forensic Leg. Med. 2011, 18, 6–9. [Google Scholar] [CrossRef]
- Henssge, C. Death time estimation in case work. I. The rectal temperature time of death nomogram. Forensic Sci. Int. 1988, 38, 209–236. [Google Scholar] [CrossRef]
- Shedge, R.; Krishan, K.; Warrier, V.; Kanchan, T. Postmortem Changes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Madea, B. Methods for determining time of death. Forensic Sci. Med. Pathol. 2016, 12, 451–485. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using Accumulated Degree-Days to Estimate the Postmortem Interval from Decomposed Human Remains. J. Forensic Sci. 2005, 50, 1–9. [Google Scholar] [CrossRef]
- Henssge, C.; Knight, B.; Krompecher, T.H.; Madea, B.; Nokes, L. The Estimation of the Time Since Death in the Early Pos—Mortem Period; Eward Arnold: London, UK, 1995. [Google Scholar]
- Bauer, M.; Gramlich, I.; Polzin, S.; Patzelt, D. Quantification of mRNA degradation as possible indicator of postmortem interval—A pilot study. Leg. Med. 2003, 5, 220–227. [Google Scholar] [CrossRef]
- Pittner, S.; Ehrenfellner, B.; Monticelli, F.C.; Zissler, A.; Sänger, A.M.; Stoiber, W.; Steinbacher, P. Postmortem muscle protein degradation in humans as a tool for PMI delimitation. Int. J. Leg. Med. 2016, 130, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Pittner, S.; Ehrenfellner, B.; Zissler, A.; Racher, V.; Trutschnig, W.; Bathke, A.C.; Sänger, A.M.; Stoiber, W.; Steinbacher, P.; Monticelli, F.C. First application of a protein-based approach for time since death estimation. Int. J. Leg. Med. 2016, 131, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Poloz, Y.O.; O’Day, D.H. Determining time of death: Temperature-dependent postmortem changes in calcineurin A, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int. J. Leg. Med. 2009, 123, 305–314. [Google Scholar] [CrossRef]
- Zissler, A.; Stoiber, W.; Steinbacher, P.; Geissenberger, J.; Monticelli, F.C.; Pittner, S. Postmortem Protein Degradation as a Tool to Estimate the PMI: A Systematic Review. Diagnostics 2020, 10, 1014. [Google Scholar] [CrossRef]
- Pittner, S.; Monticelli, F.C.; Pfisterer, A.; Zissler, A.; Sänger, A.M.; Stoiber, W.; Steinbacher, P. Postmortem degradation of skeletal muscle proteins: A novel approach to determine the time since death. Int. J. Leg. Med. 2015, 130, 421–431. [Google Scholar] [CrossRef]
- Higgins, J.; Sterne, J.; Savović, J.; Page, M.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A Revised Tool for Assessing Risk of Bias in Randomized Trials. Cochrane Methods. Cochrane Database Syst. Rev. 2016, 10 (Suppl. 1). [Google Scholar] [CrossRef]
- Alibegović, A.; Blagus, R.; Martinez, I.Z. Safranin O without fast green is the best staining method for testing the degradation of macromolecules in a cartilage extracellular matrix for the determination of the postmortem interval. Forensic Sci. Med. Pathol. 2019, 16, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, E.E.; Saenger, A.M.; Monticelli, F.C. Skeletal muscle proteins: A new approach to delimitate the time since death. Int. J. Leg. Med. 2015, 130, 433–440. [Google Scholar] [CrossRef]
- Jellinghaus, K.; Hachmann, C.; Hoeland, K.; Bohnert, M.; Wittwer-Backofen, U.; Höland, K. Collagen degradation as a possibility to determine the post-mortem interval (PMI) of animal bones: A validation study referring to an original study of Boaks et al. (2014). Int. J. Leg. Med. 2017, 132, 753–763. [Google Scholar] [CrossRef]
- Jellinghaus, K.; Urban, P.K.; Hachmann, C.; Bohnert, M.; Hotz, G.; Rosendahl, W.; Wittwer-Backofen, U. Collagen degradation as a possibility to determine the post-mortem interval (PMI) of human bones in a forensic context—A survey. Leg. Med. 2019, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ali, W.; Singh, U.S.; Kumar, A.; Bhattacharya, S.; Verma, A.K.; Rupani, R. Temperature-Dependent Postmortem Changes in Human Cardiac Troponin-T (cTnT): An Approach in Estimation of Time Since Death. J. Forensic Sci. 2015, 61, S241–S245. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, W.; Bhattacharya, S.; Verma, A.K. The effect of elapsed time on cardiac troponin-T (cTnT) degradation and its dependency on the cause of death. J. Forensic Leg. Med. 2016, 40, 16–21. [Google Scholar] [CrossRef]
- Pittner, S.; Bugelli, V.; Weitgasser, K.; Zissler, A.; Sanit, S.; Lutz, L.; Monticelli, F.; Campobasso, C.P.; Steinbacher, P.; Amendt, J. A field study to evaluate PMI estimation methods for advanced decomposition stages. Int. J. Leg. Med. 2020, 134, 1361–1373. [Google Scholar] [CrossRef]
- Wehner, F.; Steinriede, A.; Martin, D.; Wehner, H.-D. Two-Tailed Delimitation of the Time of Death by Immunohistochemical Detection of Somatostatin and GFAP. Forensic Sci. Med. Pathol. 2006, 2, 241–248. [Google Scholar] [CrossRef]
- Boaks, A.; Siwek, D.; Mortazavi, F. The temporal degradation of bone collagen: A histochemical approach. Forensic Sci. Int. 2014, 240, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.; Dutson, T.; Caldwell, J.; Carpenter, Z. Effect of temperature and pH on the post-mortem degradation of myofibrillar proteins. Meat Sci. 1983, 9, 157–179. [Google Scholar] [CrossRef]
- Bechtel, P.J.; Parrish, F.C. Effects of Postmortem Storage and Temperature on Muscle Protein Degradation: Analysis by SDS Gel Electrophoresis. J. Food Sci. 1983, 48, 294–295. [Google Scholar] [CrossRef]
- Pomponio, L.; Ertbjerg, P. The effect of temperature on the activity of μ- and m-calpain and calpastatin during post-mortem storage of porcine longissimus muscle. Meat Sci. 2012, 91, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tavichakorntrakool, R.; Prasongwattana, V.; Sriboonlue, P.; Puapairoj, A.; Pongskul, J.; Khuntikeo, N.; Hanpanich, W.; Yenchitsomanus, P.-T.; Wongkham, C.; Thongboonkerd, V. Serial analyses of postmortem changes in human skeletal muscle: A case study of alterations in proteome profile, histology, electrolyte contents, water composition, and enzyme activity. Proteom. Clin. Appl. 2008, 2, 1255–1264. [Google Scholar] [CrossRef]
- Ferrer, I.; Santpere, G.; Arzberger, T.; Bell, J.; Blanco, R.; Boluda, S.; Budka, H.; Carmona, M.; Giaccone, G.; Krebs, B.; et al. Brain Protein Preservation Largely Depends on the Postmortem Storage Temperature. J. Neuropathol. Exp. Neurol. 2007, 66, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.L.; Kaliszan, M. The post mortem temperature plateau and its role in the estimation of time of death. A review. Leg. Med. 2012, 14, 55–62. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; Leblanc, H.N.; Hall, M.J.R. Best practice in forensic entomology—standards and guidelines. Int. J. Leg. Med. 2006, 121, 90–104. [Google Scholar] [CrossRef]
- Matuszewski, S.; Hall, M.J.R.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef]
- Diedrich, M.; Tadic, J.; Mao, L.; Wacker, M.; Nebrich, G.; Hetzer, R.; Regitz-Zagrosek, V.; Klose, J. Heart protein expression related to age and sex in mice and humans. Int. J. Mol. Med. 2007, 20, 865–874. [Google Scholar] [CrossRef]
- Amin, H.A.A.; El-Hennawy, A.M.Y.; Nakhla, G.A.A.; Tabak, S.A.-H.; Hassan, H.H. Immuno-histochemistry in the detection of early myocardial infarction (a post-mortem study). Egypt. J. Forensic Sci. 2011, 1, 5–12. [Google Scholar] [CrossRef][Green Version]
- Fishbein, M.C.; Wang, T.; Matijasevic, M.; Hong, L.; Apple, F.S. Myocardial tissue troponins T and I. Cardiovasc. Pathol. 2003, 12, 65–71. [Google Scholar] [CrossRef]
- Ricchiuti, V.; Zhang, J.; Apple, F.S. Cardiac troponin I and T alterations in hearts with severe left ventricular remodeling. Clin. Chem. 1997, 43, 990–995. [Google Scholar] [CrossRef]
- Majola, T.; Kelly, J.; Van Der Linde, T. A Preliminary Study on the Influence of Direct Sunlight and Shade on Carcasses’ Decomposition and Arthropod Succession. Can. Soc. Forensic Sci. J. 2013, 46, 93–102. [Google Scholar] [CrossRef]
- Teo, C.H.; Pawita, A.H.; Khairul, O.; Ayunni, A.G.A.; Hazfalinda, H.N. Post mortem changes in relation to different types of clothing. Malays. J. Pathol. 2013, 35, 77–85. [Google Scholar] [PubMed]
- Brooks, J.W. Postmortem Changes in Animal Carcasses and Estimation of the Postmortem Interval. Vet. Pathol. 2016, 53, 929–940. [Google Scholar] [CrossRef]
| General Study Characteristics | Study Details and Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/ Year | Influencing Factor | Research Target (Species, Tissue) | Sample Size and Study Groups | Method | Storage Conditions | Investigated PMI | Sampling Site Details | Sample Number and Sampling Frequency | Investigated Proteins | Type of Measurement Procedure/ Data Processing | Type of Study Outcome | Main Study Outcome |
| Alibegovic et al., 2019 [18] | temperature | human, cartilage | 3 individuals | histology/ grading scale | varying before autopsy; laboratory-controlled after autopsy, storage of samples in tubes, 11 ± 2 °C, 35 ± 2 °C | estimated PMI (30-48 hpm) + 1–36 dpm | human trochlea, medial and lateral condyle | 3 samples per time point (3) and per temperature (2) | collagen, proteoglycan | qualitative assessment of histological staining intensity using Bern grading scale | significant decrease in staining intensity over PMI | no significant effect of temperature |
| Foditsch et al., 2016 [19] | temperature | pig, skeletal muscle | 2 individuals (1 per group) | SDS PAGE, Western blot | 4 ± 1 °C, 22 ± 2 °C | 4 °C: 0–21 dpm, 22 °C: 0–5 dpm | M. biceps femoris | 1 sample per temperature and per time point (time points not specified) | α-actinin, calsequestrin 1, desmin, nebulin, titin, SERCA-1, SERCA-2, tropomyosin, cardiac troponin T (cTNT), laminin, µ-calpain | qualitative assessment of band presence/ absence over PMI | effect of temperature to degradation events (decrease in/loss of protein, degradation products) over time | increased protein degradation at higher temperatures |
| Jellinghaus et al., 2018 [20] | micro- organism | pig, bone | individuals not known, 16 bones (8 per group) | histology/ digital imaging, histology/ photometry | buried in boxes; 13–34 °C (monitored); 2 groups with different water infusion | 0–3 months pm | right and left O. femoris | 8 samples per time point (4) | collagen | quantitative assessment (software) of histological staining | effect of micro- organismic presence to collagenous to non-collagenous protein (Co/NCo) ratio | no significant effect of micro- organism presence |
| Jellinghaus et al., 2019 [21] | sex | human, bone | 48 individuals | histology/digital imaging, histology/photometry | outdoor; cemetery and archeological samples (museum) | up to 171 years pm | O. femoris | 48 samples at different time points | collagen | quantitative assessment (software) of histological staining | effect of gender to collagenous to non-collagenous protein (Co/Nco) ratio | decrease in ratio of Co/NCo concentration in males, increase in females |
| Kumar et al., 2016 [22] | temperature | human, heart | 6 individuals | Western blot | 12 °C, 20 ± 2 °C, 25 °C, 37 °C | unclear: probably up to 189 hpm | n.a. | not specified; several samples at several time points and temperatures | cTnT | not defined, probably percentage of intact protein | effect of temperature to degradation events (decrease in/loss of protein, degradation products) over time | increased protein degradation at higher temperatures |
| Kumar et al., 2016 [23] | cause of death | human, heart | 50 individuals (10 per group) | Western blot | varying | unclear | n.a. | not specified; per group apparently different number of samples and different time points | cTnT | not defined, probably percentage of intact protein | effect of cause of death to degradation events (decrease in/loss of protein, degradation products) over time | dependence of protein degradation upon cause of death; |
| Pittner et al., 2016 [12] | age, body mass index, cause of death, sex, temperature (ADD) | human, skeletal muscle | 40 individuals | casein zymography, Western blot | varying, accumulated degree days calculated | 4–93 hpm | M. vastus lateralis | 40 samples at different time points | desmin, calpain-1, calpain-2, cTnT, tropomyosin | presence and absence probability of bands at different accumulated degree days; correlation of band presence and absence with ADD | effect of age, BMI, sex and cause of death (COD) on timing and confidence intervals of degradation events | stronger correlation of degradation events with ADD in age and BMI corrected groups, no (major) effects by sex and COD |
| Pittner et al., 2020 [24] | body weight, exposure/environment, insect activity | pig, skeletal muscle | 8 individuals (4 per group) | Western blot | outdoor; rectal and ambient temperature recorded | 1–16 dpm | M. quadriceps femoris | 8 samples (1 per animal/ 2 per group) per time point (10) | tropomyosin, desmin, vinculin, cTnT | quantitative (threshold) assessment of band presence/absence over PMI; temporal dependence of degradation events (changing probability of band presence over time) | effect of insect activity, body weight and exposure to protein degradation | loss of tissue hinders protein degradation analysis; robustness to body weight and exposure |
| Poloz and O’Day, 2009 [14] | temperature | mouse, lung and skeletal muscle | 40 individuals | Western blot | laboratory- controlled; 5 °C, 10 °C, 21 °C | 0–96 hpm | n.a. | 4 samples per time point (4) and per temperature (3) | Calcineurin A (CnA), Myristoylated alanine-rich C-kinase substrate (MARCKS), Calcium/ calmodulin- dependent protein kinase II (CaMKII), Protein phosphatase 2A (PP2A) | band intensity, % of intact protein | effect of temperature to degradation (decrease in band intensity, degradation products) over time | increased protein degradation at higher temperatures |
| Wehner et al., 2006 [25] | temperature | human, brain and pancreas | 500 individuals | immuno- histochemistry | varying | 1–23 ± 1 dpm | frontal cortex, n.a. | number of samples per time point is unknown; 1 sample per tissue per individual | glial fibrillary acidic protein (GFAP), somatostatin | qualitative assessment of positive and negative immunostaining | effect of temperature (summer vs. winter seasons) to degradation events (presence and absence of staining) | faster decomposition in warmer season of the year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zissler, A.; Stoiber, W.; Geissenberger, J.; Steinbacher, P.; Monticelli, F.C.; Pittner, S. Influencing Factors on Postmortem Protein Degradation for PMI Estimation: A Systematic Review. Diagnostics 2021, 11, 1146. https://doi.org/10.3390/diagnostics11071146
Zissler A, Stoiber W, Geissenberger J, Steinbacher P, Monticelli FC, Pittner S. Influencing Factors on Postmortem Protein Degradation for PMI Estimation: A Systematic Review. Diagnostics. 2021; 11(7):1146. https://doi.org/10.3390/diagnostics11071146
Chicago/Turabian StyleZissler, Angela, Walter Stoiber, Janine Geissenberger, Peter Steinbacher, Fabio C. Monticelli, and Stefan Pittner. 2021. "Influencing Factors on Postmortem Protein Degradation for PMI Estimation: A Systematic Review" Diagnostics 11, no. 7: 1146. https://doi.org/10.3390/diagnostics11071146
APA StyleZissler, A., Stoiber, W., Geissenberger, J., Steinbacher, P., Monticelli, F. C., & Pittner, S. (2021). Influencing Factors on Postmortem Protein Degradation for PMI Estimation: A Systematic Review. Diagnostics, 11(7), 1146. https://doi.org/10.3390/diagnostics11071146