Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review
Abstract
1. Introduction
2. Aetiology and Risk Factors
3. Clinical Presentation and Classification
4. Investigations
4.1. Laboratory
4.2. Imaging
4.2.1. X-ray
4.2.2. Ultrasound
4.2.3. CT
Imaging Techniques
Imaging Findings
- the etiology (arterial ischemic, non-occlusive, reperfusive, venous);
- the location and extension of the intestinal damage;
- the phase of the damage (acute, subacute or chronic).
- 1.
- Etiology
- 2.
- Location and extension of the damage
- 3.
- Phase of the damage (acute, subacute or chronic)
4.2.4. MRI
Imaging Technique
Imaging Findings
4.2.5. Mesenteric Angiography
5. Differential Diagnosis
6. Colonoscopy
7. Treatment Possibilities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boley, S.J.; Schwartz, S.; Lash, J.; Sternhill, V. Reversible vascular occlusion of the colon. Surg. Gynecol. Obstet. 1963, 116, 53–60. [Google Scholar]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Genovese, E.A.; Mazzei, F.G.; Volterrani, L. Diagnosis of acute mesenteric ischemia/infarction in the era of multislice CT. Recenti Prog. Med. 2012, 103, 435–437. [Google Scholar] [CrossRef]
- Paterno, F.; Longo, W.E. The etiology and pathogenesis of vascular disorders of the intestine. Radiol. Clin. N. Am. 2008, 46, 877–885. [Google Scholar] [CrossRef]
- Taourel, P.; Aufort, S.; Merigeaud, S.; Doyon, F.C.; Hoquet, M.D.; Delabrousse, E. Imaging of ischemic colitis. Radiol. Clin. N. Am. 2008, 46, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Klempnauer, J.; Grothues, F.; Bektas, H.; Pichlmayr, R. Long-term results after surgery for acute mesenteric ischemia. Surgery 1997, 121, 239–243. [Google Scholar] [CrossRef]
- Longo, W.E.; Ward, D.; Vernava, A.M., III; Kaminski, D.L. Outcome of patients with total colonic ischemia. Dis. Colon Rectum 1997, 40, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Guttormson, N.L.; Bubrick, M.P. Mortality from ischemic colitis. Dis. Colon Rectum 1989, 32, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Matsushima, M.; Orihashi, Y.; Dekiden-Monma, M.; Mizukami, H.; Nakahara, F.; Nakamura, J.; Fujisawa, M.; Koike, J.; Suzuki, T.; et al. A Case-control Study on the Risk Factors for Ischemic Colitis. Tokai J. Exp. Clin. Med. 2018, 20, 111–116. [Google Scholar]
- Yadav, S.; Dave, M.; Edakkanambeth Varayil, J.; Harmsen, W.S.; Tremaine, W.J.; Zinsmeister, A.R.; Sweetser, S.R.; Melton, L.J., III; Sandborn, W.J.; Loftus, E.V., Jr. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 731–738. [Google Scholar] [CrossRef]
- Montoro, M.A.; Brandt, L.J.; Santolaria, S.; Gomollon, F.; Sánchez Puértolas, B.; Vera, J.; Bujanda, L.; Cosme, A.; Cabriada, J.L.; Durán, M.; et al. Workgroup for the Study of Ischaemic Colitis of the Spanish Gastroenterological Association (GTECIE-AEG). Clinical patterns and outcomes of ischaemic colitis: Results of the Working Group for the Study of Ischaemic Colitis in Spain (CIE study). Scand. J. Gastroenterol. 2011, 46, 236–246. [Google Scholar] [CrossRef]
- Baixauli, J.; Kiran, R.P.; Delaney, C.P. Investigation and management of ischemic colitis. Cleve Clin. J. Med. 2003, 70, 925–926. [Google Scholar] [CrossRef]
- Chung, J.W.; Cheon, J.H.; Park, J.J.; Jung, E.S.; Choi, E.H.; Kim, H. Development and validation of a novel prognostic scoring model for ischemic colitis. Dis. Colon Rectum 2010, 53, 1287–1294. [Google Scholar] [CrossRef]
- Terlouw, L.G.; Moelker, A.; Abrahamsen, J.; Acosta, S.; Bakker, O.J.; Baumgartner, I.; Boyer, L.; Corcos, O.; van Dijk, L.J.; Duran, M.; et al. European guidelines on chronic mesenteric ischaemia-joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United Eur. Gastroenterol. J. 2020, 8, 371–395. [Google Scholar] [CrossRef]
- Brandt, L.J.; Feuerstadt, P.; Longstreth, G.F.; Boley, S.J. American College of Gastroenterology. ACG clinical guideline: Epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am. J. Gastroenterol. 2015, 110, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, G.; Nassar, A.; Subramonia, S. The Pathophysiology, Presentation and Management of Ischaemic Colitis: A Systematic Review. World J. Surg. 2020, 44, 927–938. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chung, W.C.; Lee, K.M.; Kim, H.J.; Paik, C.N.; Yang, J.M. Ischemic colitis due to obstruction of mesenteric and splenic veins: A case report. World J. Gastroenterol. 2008, 14, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, D.A.; Brandt, L.J. Colonic ischemia. J. Clin. Gastroenterol. 1998, 27, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Tendler, D.A. Ischemic colitis: A clinical review. South. Med. J. 2005, 98, 217–222. [Google Scholar] [CrossRef]
- Guerrini, S.; Bucalossi, A.; Cioffi Squitieri, N.; Mazzei, F.G.; Volterrani, L.; Mazzei, M.A. Ischemic colitis diagnosed by magnetic resonance imaging during lenalidomide treatment in a patient with relapsed multiple myeloma. Tumori 2016, 102. [Google Scholar] [CrossRef]
- Champagne, B.J.; Lee, E.C.; Valerian, B.; Mulhotra, N.; Mehta, M. Incidence of colonic ischemia after repair of ruptured abdominal aortic aneurysm with endograft. J. Am. Coll. Surg. 2007, 204, 597–602. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Imbriaco, G.; Mazzei, F.G.; Volterrani, L. Non-obstructive mesenteric ischemia after cardiovascular surgery: Not so uncommon. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 253–255. [Google Scholar] [CrossRef][Green Version]
- Mazzei, M.A.; Guerrini, S.; Gentili, F.; Monteleone, I.; Lucii, G.; Mazzei, F.G.; Volterrani, L. Non-occlusive Mesenteric Ischemia as a Fatal Complication in Acute Pancreatitis: A Brief Radiological Comment. Dig. Dis. Sci. 2020, 65, 1553–1555. [Google Scholar] [CrossRef]
- Elder, K.; Lashner, B.A.; Al, S.F. Clinical approach to colonic ischemia. Cleve Clin. J. Med. 2009, 76, 401–409. [Google Scholar] [CrossRef]
- Gore, R.M.; Yaghmai, V.; Thakrar, K.H.; Berlin, J.W.; Mehta, U.K.; Newmark, G.M.; Miller, F.H. Imaging in intestinal ischemic disorders. Radiol. Clin. N. Am. 2008, 46, 845–875. [Google Scholar] [CrossRef]
- Lange, J.F.; Komen, N.; Akkerman, G.; Nout, E.; Horstmanshoff, H.; Schlesinger, F.; Bonjer, J.; Kleinrensink, G.J. Riolan’s arch: Confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am. J. Surg. 2007, 193, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.; Wehrle, C.J.; van Fossen, K. Anatomy, Abdomen and Pelvis, Inferior Mesenteric Artery; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Saba, L.; Berritto, D.; Iacobellis, F.; Scaglione, M.; Castaldo, S.; Cozzolino, S.; Mazzei, M.A.; Di Mizio, V.; Grassi, R. Acute arterial mesenteric ischemia and reperfusion: Macroscopic and MRI findings, preliminary report. World J. Gastroenterol. 2013, 28, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Berritto, D.; Iacobellis, F.; Mazzei, M.A.; Volterrani, L.; Guglielmi, G.; Brunese, L.; Grassi, R. MDCT in ischaemic colitis: How to define the aetiology and acute, subacute and chronic phase of damage in the emergency setting. Br. J. Radiol. 2016, 89, 20150821. [Google Scholar] [CrossRef]
- Iacobellis, F.; Berritto, D.; Fleischmann, D.; Gagliardi, G.; Brillantino, A.; Mazzei, M.A.; Grassi, R. CT findings in acute, subacute, and chronic ischemic colitis: Suggestions for diagnosis. Biomed. Res. Int. 2014, 2014, 895248. [Google Scholar] [CrossRef] [PubMed]
- Brillantino, A.; Iacobellis, F.; Renzi, A.; Nasti, R.; Saldamarco, L.; Grillo, M.; Romano, L.; Castriconi, M.; Cittadini, A.; de Palma, M.; et al. Diagnostic value of arterial blood gas lactate concentration in the different forms of mesenteric ischemia. Eur J. Trauma Emerg. Surg. 2018, 44, 265–272. [Google Scholar] [CrossRef]
- Theodoropoulou, A.; Koutroubakis, I.E. Ischemic colitis: Clinical practice in diagnosis and treatment. World J. Gastroenterol. 2008, 14, 7302–7308. [Google Scholar] [CrossRef]
- Zou, X.; Cao, J.; Yao, Y.; Liu, W.; Chen, L. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: A report of 85 cases. Dig. Dis Sci. 2009, 54, 2009–2015. [Google Scholar] [CrossRef]
- Brandt, L.J.; Boley, S.J. Colonic ischemia. Surg. Clin. N. Am. 1992, 72, 203–229. [Google Scholar] [CrossRef]
- Sun, D.; Wang, C.; Yang, L.; Liu, M.; Chen, F. The predictors of the severity of ischaemic colitis: A systematic review of 2823 patients from 22 studies. Colorectal Dis. 2016, 18, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Rania, H.; Mériam, S.; Rym, E.; Hyafa, R.; Amine, A.; Najet, B.H.; Lassad, G.; Mohamed, T.K. Ischemic colitis in five points: An update 2013. Tunis Med. 2014, 92, 299–303. [Google Scholar] [PubMed]
- Chiloiro, G.; Rodriguez-Carnero, P.; Lenkowicz, J.; Casà, C.; Masciocchi, C.; Boldrini, L.; Cusumano, D.; Dinapoli, N.; Meldolesi, E.; Carano, D.; et al. Delta Radiomics Can Predict Distant Metastasis in Locally Advanced Rectal Cancer: The Challenge to Personalize the Cure. Front. Oncol. 2020, 10, 595012. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer. Radiol. Med. 2021, 126, 421–429. [Google Scholar] [CrossRef]
- Faqar-Uz-Zaman, S.F.; Filmann, N.; Mahkovic, D.; von Wagner, M.; Detemble, C.; Kippke, U.; Marschall, U.; Anantharajah, L.; Baumartz, P.; Sobotta, P.; et al. Study protocol for a prospective, double-blinded, observational study investigating the diagnostic accuracy of an app-based diagnostic health care application in an emergency room setting: The eRadaR trial. BMJ Open 2021, 8, e041396. [Google Scholar] [CrossRef] [PubMed]
- Crimì, F.; Capelli, G.; Spolverato, G.; Bao, Q.R.; Florio, A.; Milite Rossi, S.; Cecchin, D.; Albertoni, L.; Campi, C.; Pucciarelli, S.; et al. MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol. Med. 2020, 125, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Gorincour, G.; Monneuse, O.; Ben Cheikh, A.; Avondo, J.; Chaillot, P.F.; Journe, C.; Youssof, E.; Lecomte, J.C.; Thomson, V. Management of abdominal emergencies in adults using telemedicine and artificial intelligence. J. Visc. Surg. 2021, S1878–S7886. [Google Scholar] [CrossRef]
- Boldrini, L.; Cusumano, D.; Chiloiro, G.; Casà, C.; Masciocchi, C.; Lenkowicz, J.; Cellini, F.; Dinapoli, N.; Azario, L.; Teodoli, S.; et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): A hypothesis-generating study for an innovative personalized medicine approach. Radiol. Med. 2019, 124, 145–153. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial intelligence: Radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol. Med. 2021, 126, 63–71. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Dai, Y.; Dong, J.; Wu, L.; Li, Y.; Cheng, Z.; Ding, Y.; Liu, Z. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: A pilot study. Chin. J. Cancer Res. 2018, 30, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Miele, V.; Giovagnoni, A. Artificial intelligence: A challenge for third millennium radiologist. Radiol. Med. 2019, 124, 241–242. [Google Scholar] [CrossRef]
- Shi, R.; Chen, W.; Yang, B.; Qu, J.; Cheng, Y.; Zhu, Z.; Gao, Y.; Wang, Q.; Liu, Y.; Li, Z.; et al. Prediction of KRAS, NRAS and BRAF status in colorectal cancer patients with liver metastasis using a deep artificial neural network based on radiomics and semantic features. Am. J. Cancer Res. 2020, 10, 4513–4526. [Google Scholar]
- Neri, E.; Coppola, F.; Miele, V.; Bibbolino, C.; Grassi, R. Artificial intelligence: Who is responsible for the diagnosis? Radiol. Med. 2020, 125, 517–521. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef]
- Song, K.D. Current status of deep learning applications in abdominal ultrasonography. Ultrasonography 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Q.; Xu, L.; Wang, Z.; Su, K.; Liu, R.; Yen, E.A.; Liu, S.; Qin, J.; Rong, Y.; et al. Integrating tumor and nodal radiomics to predict lymph node metastasis in gastric cancer. Radiother. Oncol. 2020, 150, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhu, X.; Yu, Z.; Wu, G.; Han, X.; Ren, J. Interventional radiology protocol for treatment of esophagogastric anastomotic leakage. Radiol. Med. 2019, 124, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Lombardo, P.; Cinque, T.; Tortora, G.; Romano, L. Acute colonic disease: How to image in emergency. Eur. J. Radiol. 2007, 61, 424–432. [Google Scholar] [CrossRef]
- Trotter, J.M.; Hunt, L.; Peter, M.B. Ischaemic colitis. BMJ 2016, 355, i6600. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Imbriaco, G.; Chieca, R.; Civitelli, S.; Savelli, V.; Mazzei, F.G.; Volterrani, L. Magnetic resonance imaging: Is there a role in clinical management for acute ischemic colitis? World J. Gastroenterol. 2013, 28, 1256–1263. [Google Scholar] [CrossRef]
- Schindera, S.T.; Triller, J.; Vock, P.; Hoppe, H. Detection of hepatic portal venous gas: Its clinical impact and outcome. Emerg. Radiol. 2006, 12, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.L.; Sprayregen, S.; Bakal, C.W. Radiology in intestinal ischemia. Plain film, contrast, and other imaging studies. Surg. Clin. N. Am. 1992, 72, 107–124. [Google Scholar] [CrossRef]
- Reginelli, A.; Genovese, E.; Cappabianca, S.; Iacobellis, F.; Berritto, D.; Fonio, P.; Coppolino, F.; Grassi, R. Intestinal Ischemia: US-CT findings correlations. Crit. Ultrasound J. 2013, S7. [Google Scholar] [CrossRef] [PubMed]
- Di Serafino, M.; Vallone, G. The role of point of care ultrasound in radiology department: Update and prospective. A statement of Italian college ultrasound. Radiol. Med. 2020. [Google Scholar] [CrossRef]
- Baud, C.; Saguintaah, M.; Veyrac, C.; Couture, A.; Ferran, J.L.; Barnéon, G.; Veyrac, M. Sonographic diagnosis of colitis in children. Eur. Radiol. 2004, 14, 2105–2119. [Google Scholar] [CrossRef]
- Coppolino, F.; Gatta, G.; Di Grezia, G.; Reginelli, A.; Iacobellis, F.; Vallone, G.; Giganti, M.; Genovese, E. Gastrointestinal perforation: Ultrasonographic diagnosis. Crit. Ultrasound J. 2013, 5, S4. [Google Scholar] [CrossRef]
- Grassi, R.; Pinto, A.; Rossi, G.; Rotondo, A. Conventional plain-film radiology, ultrasonography and CT in jejuno-ileal perforation. Acta Radiol. 1998, 39, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Brillantino, A.; Iacobellis, F.; Reginelli, A.; Monaco, L.; Sodano, B.; Tufano, G.; Tufano, A.; Maglio, M.; de Palma, M.; Di Martino, N.; et al. Preoperative assessment of simple and complex anorectal fistulas: Tridimensional endoanal ultrasound? Magnetic resonance? Both? Radiol. Med. 2019, 124, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Stabile Ianora, A.A.; Rubini, G.; Losco, M.; Niccoli Asabella, A.; Fonio, P.; Moschetta, M. Aspetto atipico della pneumatosi intestinale in TC multidetettore [Atypical appearance of pneumatosis intestinalis at multidetector CT]. Recenti Prog. Med. 2012, 103, 542–545. [Google Scholar] [CrossRef]
- Acampora, C.; Di Serafino, M.; Iacobellis, F.; Trovato, P.; Barbuto, L.; Sangiuliano, N.; Costigliola, L.; Romano, L. Insight into Dunbar syndrome: Color-Doppler ultrasound findings and literature review. J. Ultrasound 2020. [Google Scholar] [CrossRef]
- Grazzini, G.; Danti, G.; Cozzi, D.; Lanzetta, M.M.; Addeo, G.; Falchini, M.; Masserelli, A.; Pradella, S.; Miele, V. Diagnostic imaging of gastrointestinal neuroendocrine tumours (GI-NETs): Relationship between MDCT features and 2010 WHO classification. Radiol. Med. 2019, 124, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, C.; Argalia, G.; Polonara, G.; Giovagnoni, A.; Giuseppetti, G.M. Contrast-enhanced ultrasound in delayed splenic vascular injury and active extravasation diagnosis. Radiol. Med. 2019, 124, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, C.; Argalia, G.; Graziani, B.; Salmistraro, D.; Giuseppett, G.M.; Giovagnoni, A. Contrast-enhanced ultrasound in the evaluation of splenic injury healing time and grade. Radiol. Med. 2019, 124, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Guerri, S.; Danti, G.; Frezzetti, G.; Lucarelli, E.; Pradella, S.; Miele, V. Clostridium difficile colitis: CT findings and differential diagnosis. Radiol. Med. 2019, 124, 1185–1198. [Google Scholar] [CrossRef]
- Salerno, S.; Lo Re, G.; Bellini, D.; Rengo, M.; Marrale, M.; Terranova, M.C.; Scopelliti, L.; Laghi, A. Patient centring and scan length: How inaccurate practice impacts on radiation dose in CT colonography (CTC). Radiol. Med. 2019, 124, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Trinci, M.; Cirimele, V.; Cozzi, D.; Galluzzo, M.; Miele, V. Diagnostic accuracy of pneumo-CT-cystography in the detection of bladder rupture in patients with blunt pelvic trauma. Radiol. Med. 2020, 125, 907–917. [Google Scholar] [CrossRef]
- Avesani, G.; Arshad, M.; Lu, H.; Fotopoulou, C.; Cannone, F.; Melotti, R.; Aboagye, E.; Rockall, A. Radiological assessment of Peritoneal Cancer Index on preoperative CT in ovarian cancer is related to surgical outcome and survival. Radiol. Med. 2020, 125, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.; Bronico, I.; Maestroni, U.; Ziglioli, F.; Silini, E.M.; Buti, S.; de Filippo, M. Small renal masses (≤ 4 cm): Differentiation of oncocytoma from renal clear cell carcinoma using ratio of lesion to cortex attenuation and aorta-lesion attenuation difference (ALAD) on contrast-enhanced CT. Radiol. Med. 2020, 125, 1280–1287. [Google Scholar] [CrossRef]
- Hu, J.; Liu, W.; Xie, S.; Li, M.; Wang, K.; Li, W. Abdominal perivascular epithelioid cell tumor (PEComa) without visible fat: A clinicopathologic and radiological analysis of 16 cases. Radiol. Med. 2021, 126, 189–199. [Google Scholar] [CrossRef]
- Millor, M.; Bartolomé, P.; Pons, M.J.; Bastarrika, G.; Beloqui, Ó.; Cano, D.; González, I.; Vivas, I. Whole-body computed tomography: A new point of view in a hospital check-up unit? Our experience in 6516 patients. Radiol. Med. 2019, 124, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Romano, L.; Grassi, R. Multidetector row computed tomography findings from ischemia to infarction of the large bowel. Eur. J. Radiol. 2007, 61, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Schueller, G.; Scaglione, M.; Linsenmaier, U.; Schueller-Weidekamm, C.; Andreoli, C.; de Vargas Macciucca, M.; Gualdi, G. The key role of the radiologist in the management of polytrauma patients: Indications for MDCT imaging in emergency radiology. Radiol. Med. 2015, 120, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, F.; Perillo, A.; Iadevito, I.; Tanga, M.; Romano, L.; Grassi, R.; Nicola, R.; Scaglione, M. Imaging of Oncologic Emergencies. Semin. Ultrasound CT MR 2018, 39, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Miele, V.; Andreoli, C.; Grassi, R. The management of emergency radiology: Key facts. Eur. J. Radiol. 2006, 59, 311–314. [Google Scholar] [CrossRef]
- Sheafor, D.H.; Kovacs, M.D.; Burchett, P.; Picard, M.M.; Davis, B.; Hardie, A.D. Impact of low-kVp scan technique on oral contrast density at abdominopelvic CT. Radiol. Med. 2018, 123, 918–925. [Google Scholar] [CrossRef]
- Schieda, N.; Fasih, N.; Shabana, W. Triphasic CT in the diagnosis of acute mesenteric ischaemia. Eur. Radiol. 2013, 23, 1891–1900. [Google Scholar] [CrossRef]
- Ofer, A.; Abadi, S.; Nitecki, S.; Karram, T.; Kogan, I.; Leiderman, M.; Shmulevsky, P.; Israelit, S.; Engel, A. Multidetector CT angiography in the evaluation of acute mesenteric ischemia. Eur. Radiol. 2009, 19, 24–30. [Google Scholar] [CrossRef]
- Lassandro, F.; Mangoni de Santo Stefano, M.L.; Porto, A.M.; Grassi, R.; Scaglione, M.; Rotondo, A. Intestinal pneumatosis in adults: Diagnostic and prognostic value. Emerg. Radiol. 2010, 17, 361–365. [Google Scholar] [CrossRef]
- Moschetta, M.; Stabile Ianora, A.A.; Pedote, P.; Scardapane, A.; Angelelli, G. Prognostic value of multidetector computed tomography in bowel infarction. Radiol. Med. 2009, 114, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Mandato, Y.; Solazzo, A.; Berritto, D.; Iacobellis, F.; Grassi, R. Errors in the radiological evaluation of the alimentary tract: Part II. Semin. Ultrasound CT MR 2012, 33, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Mandato, Y.; Reginelli, A.; Galasso, R.; Iacobellis, F.; Berritto, D.; Cappabianca, S. Errors in the radiological evaluation of the alimentary tract: Part I. Semin. Ultrasound CT MR 2012, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oliva, I.B.; Davarpanah, A.H.; Rybicki, F.J.; Desjardins, B.; Flamm, S.D.; Francois, C.J.; Gerhard-Herman, M.D.; Kalva, S.P.; Ashraf Mansour, M.; Mohler, E.R., III; et al. ACR Appropriateness Criteria® imaging of mesenteric ischemia. Abdom. Imaging 2013, 38, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Abujudeh, H.H.; Nazarian, R.M.; Thrall, J.H. Ischemic colitis: Spectrum of CT findings, sites of involvement and severity. Emerg. Radiol. 2015, 22, 357–365. [Google Scholar] [CrossRef]
- Di Grezia, G.; Gatta, G.; Rella, R.; Iacobellis, F.; Berritto, D.; Musto, L.A.; Grassi, R. MDCT in acute ischaemic left colitis: A pictorial essay. Radiol. Med. 2019, 124, 103–108. [Google Scholar] [CrossRef]
- Menke, J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: Systematic review and meta-analysis. Radiology 2010, 256, 93–101. [Google Scholar] [CrossRef]
- Cornacchia, S.; Errico, R.; Balzano, R.F.; Fusco, V.; Maldera, A.; Pierpaoli, E.; Ferrari, C.; Rubini, G.; Guglielmi, G. Medical radiological procedures: Which information would be chosen for the report? Radiol. Med. 2019, 124, 783–793. [Google Scholar] [CrossRef]
- Iacobellis, F.; Berritto, D.; Somma, F.; Cavaliere, C.; Corona, M.; Cozzolino, S.; Fulciniti, F.; Cappabianca, S.; Rotondo, A.; Grassi, R. Magnetic resonance imaging: A new tool for diagnosis of acute ischemic colitis? World J. Gastroenterol. 2012, 18, 1496–1501. [Google Scholar] [CrossRef]
- Faggian, A.; Berritto, D.; Iacobellis, F.; Reginellim, A.; Cappabianca, S.; Grassi, R. Imaging Patients with Alimentary Tract Perforation: Literature Review. Semin. Ultrasound CT MR 2016, 37, 66–69. [Google Scholar] [CrossRef]
- Reginelli, A.; Iacobellis, F.; Berritto, D.; Gagliardi, G.; Di Grezia, G.; Rossi, M.; Fonio, P.; Grassi, R. Mesenteric ischemia: The importance of differential diagnosis for the surgeon. BMC Surg. 2013, S51. [Google Scholar] [CrossRef] [PubMed]
- Berritto, D.; Iacobellis, F.; Somma, F.; Corona, M.; Faggian, A.; Iacomino, A.; Feragalli, B.; Saba, L.; La Porta, M.; Grassi, R. 7T mMR in the assessment of acute arterial mesenteric ischemia in a rat model. J. Biol. Regul. Homeost. Agents 2013, 27, 771–779. [Google Scholar]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Vindigni, C.; Imbriaco, G.; Gentili, F.; Berritto, D.; Mazzei, F.G.; Grassi, R.; Volterrani, L. Reperfusion in non-occlusive mesenteric ischaemia (NOMI): Effectiveness of CT in an emergency setting. Br. J. Radiol. 2016, 89, 20150956. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Gentili, F.; Mazzei, F.G.; Grassi, R.; Volterrani, L. Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery: Don’t forget the reperfusion process! Br. J. Radiol. 2019, 20180736. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Mazzei, F.G.; Marrelli, D.; Imbriaco, G.; Guerrini, S.; Vindigni, C.; Civitelli, S.; Roviello, F.; Grassi, R.; Volterrani, L. Computed tomographic evaluation of mesentery: Diagnostic value in acute mesenteric ischemia. J. Comput. Assist. Tomogr. 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Macari, M.; Balthazar, E.J. CT of bowel wall thickening: Significance and pitfalls of interpretation. AJR Am. J. Roentgenol. 2001, 176, 1105–1116. [Google Scholar] [CrossRef]
- Copin, P.; Zins, M.; Nuzzo, A.; Purcell, Y.; Beranger-Gibert, S.; Maggiori, L.; Corcos, O.; Vilgrain, V.; Ronot, M. Acute mesenteric ischemia: A critical role for the radiologist. Diagn. Interv. Imaging 2018, 99, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Somma, F.; Berritto, D.; Iacobellis, F.; Landi, N.; Cavaliere, C.; Corona, M.; Russo, S.; Di Mizio, R.; Rotondo, A.; Grassi, R. 7T μMRI of mesenteric venous ischemia in a rat model: Timing of the appearance of findings. Magn. Reson. Imaging 2013, 31, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Gentili, F.; Volterrani, L. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia: A Necessary Clarification. AJR Am. J. Roentgenol. 2019, W93–W94. [Google Scholar] [CrossRef]
- Lourenco, P.D.M.; Rawski, R.; Mohammed, M.F.; Khosa, F.; Nicolaou, S.; McLaughlin, P. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia. AJR Am. J. Roentgenol. 2018, 211, 564–570. [Google Scholar] [CrossRef]
- Potretzke, T.A.; Brace, C.L.; Lubner, M.G.; Sampson, L.A.; Willey, B.J.; Lee, F.T., Jr. Early small-bowel ischemia: Dual-energy CT improves conspicuity compared with conventional CT in a swine model. Radiology 2015, 275, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Buffa, V.; Solazzo, A.; D’Auria, V.; del Prete, A.; Vallone, A.; Luzietti, M.; Madau, M.; Grassi, R.; Miele, V. Dual-source dual-energy CT: Dose reduction after endovascular abdominal aortic aneurysm repair. Radiol. Med. 2014, 119, 934–941. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. Radiol. Med. 2019, 124, 1281–1295. [Google Scholar] [CrossRef]
- Brandt, L.J.; Feuerstadt, P.; Blaszka, M.C. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: A study of 313 cases supported by histology. Am. J. Gastroenterol. 2010, 105, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Ciolina, M.; Caruso, D.; de Santis, D.; Zerunian, M.; Rengo, M.; Alfieri, N.; Musio, D.; de Felice, F.; Ciardi, A.; Tombolini, V.; et al. Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: Role of perfusion parameters in the assessment of response to treatment. Radiol. Med. 2019, 124, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Masselli, G.; de Vincentiis, C.; Aloi, M.; Guida, M.; Cao, R.; Cartocci, G.; Miele, V.; Grassi, R. Detection of Crohn’s disease with diffusion images versus contrast-enhanced images in pediatric using MR enterography with histopathological correlation. Radiol. Med. 2019, 124, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic contrast-enhanced MRI in oncology: How we do it. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Principi, M.; Pedote, P.; Pignataro, P.; Francavilla, M.; Sardaro, A.; Scardapane, A. Prevalence and clinical significance of incidental extra-intestinal findings in MR enterography: Experience of a single University Centre. Radiol. Med. 2021, 126, 181–188. [Google Scholar] [CrossRef]
- Fornell-Perez, R.; Vivas-Escalona, V.; Aranda-Sanchez, J.; Gonzalez-Dominguez, M.C.; Rubio-Garcia, J.; Aleman-Flores, P.; Lozano-Rodriguez, A.; Porcel-de-Peralta, G.; Loro-Ferrer, J.F. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: The role of diffusion-weighted imaging. Radiol. Med. 2020, 125, 522–530. [Google Scholar] [CrossRef]
- Albano, D.; Stecco, A.; Micci, G.; Sconfienza, L.M.; Colagrande, S.; Reginelli, A.; Grassi, R.; Carriero, A.; Midiri, M.; Lagalla, R.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: An Italian survey. Radiol. Med. 2021, 126, 299–305. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef]
- Chung, J.J.; Semelka, R.C.; Martin, D.R.; Marcos, H.B. Colon diseases: MR evaluation using combined T2-weighted single-shot echo train spin-echo and gadolinium-enhanced spoiled gradient-echo sequences. J. Magn. Reson. Imaging 2000, 12, 297–305. [Google Scholar] [CrossRef]
- Grassi, R.; Cavaliere, C.; Cozzolino, S.; Mansi, L.; Cirillo, S.; Tedeschi, G.; Franchi, R.; Russo, P.; Cornacchia, S.; Rotondo, A. Small animal imaging facility: New perspectives for the radiologist. Radiol. Med. 2009, 114, 152–167. [Google Scholar] [CrossRef]
- Berritto, D.; Somma, F.; Landi, N.; Cavaliere, C.; Corona, M.; Russo, S.; Fulciniti, F.; Cappabianca, S.; Rotondo, A.; Grassi, R. Seven-Tesla micro-MRI in early detection of acute arterial ischaemia: Evolution of findings in an in vivo rat model. Radiol. Med. 2011, 116, 829–841. [Google Scholar] [CrossRef]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol. Med. 2020, 125, 356–364. [Google Scholar] [CrossRef]
- Iacobellis, F.; Iadevito, I.; Romano, F.; Altiero, M.; Bhattacharjee, B.; Scaglione, M. Perforated Appendicitis: Assessment with Multidetector Computed Tomography. Semin. Ultrasound CT MR 2016, 37, 31–36. [Google Scholar] [CrossRef]
- Plastaras, L.; Vuitton, L.; Badet, N.; Koch, S.; Di Martino, V.; Delabrousse, E. Acute colitis: Differential diagnosis using multidetector CT. Clin. Radiol. 2015, 70, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Berritto, D.; Crincoli, R.; Iacobellis, F.; Iasiello, F.; Pizza, N.L.; Lassandro, F.; Musto, L.; Grassi, R. Primary pneumatosis intestinalis of small bowel: A case of a rare disease. Case Rep. Surg. 2014, 2014, 350312. [Google Scholar] [CrossRef] [PubMed]
- Lassandro, F.; Valente, T.; Rea, G.; Lassandro, G.; Golia, E.; Brunese, L.; Laghi, A. Imaging assessment and clinical significance of pneumatosis in adult patients. Radiol. Med. 2015, 120, 96–104. [Google Scholar] [CrossRef]
- Lassandro, F.; Scaglione, M.; Rossi, G.; Grassi, R.; Romano, L. Portomesenteric vein gas: Diagnostic and prognostic value. Emerg. Radiol. 2002, 9, 96–99. [Google Scholar] [CrossRef]
- İnce, A.T.; Baysal, B.; Kayar, Y.; Arabacı, E.; Bilgin, M.; Hamdard, J.; Yay, A.; Şentürk, H. Comparison of tomographic and colonoscopic diagnoses in the presence of colonic wall thickening. Int. J. Clin. Exp. Med. 2014, 7, 4413–4419. [Google Scholar] [PubMed]
- Flynn, A.D.; Valentine, J.F. Update on the Diagnosis and Management of Colon Ischemia. Curr. Treat. Options Gastroenterol. 2016, 14, 128–139. [Google Scholar] [CrossRef]
- Choi, S.R.; Jee, S.R.; Song, G.A.; Park, S.J.; Lee, J.H.; Song, C.S.; Park, H.U. Predictive Factors for Severe Outcomes in Ischemic Colitis. Gut Liver 2015, 9, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Yang, S.S.; Jung, S.W.; Park, J.H.; Im, Y.C.; Kim, K.Y. Poor prognostic factors in patients who underwent surgery for acute non-occlusive ischemic colitis. World J. Emerg. Surg. 2015, 10, 12. [Google Scholar] [CrossRef] [PubMed]
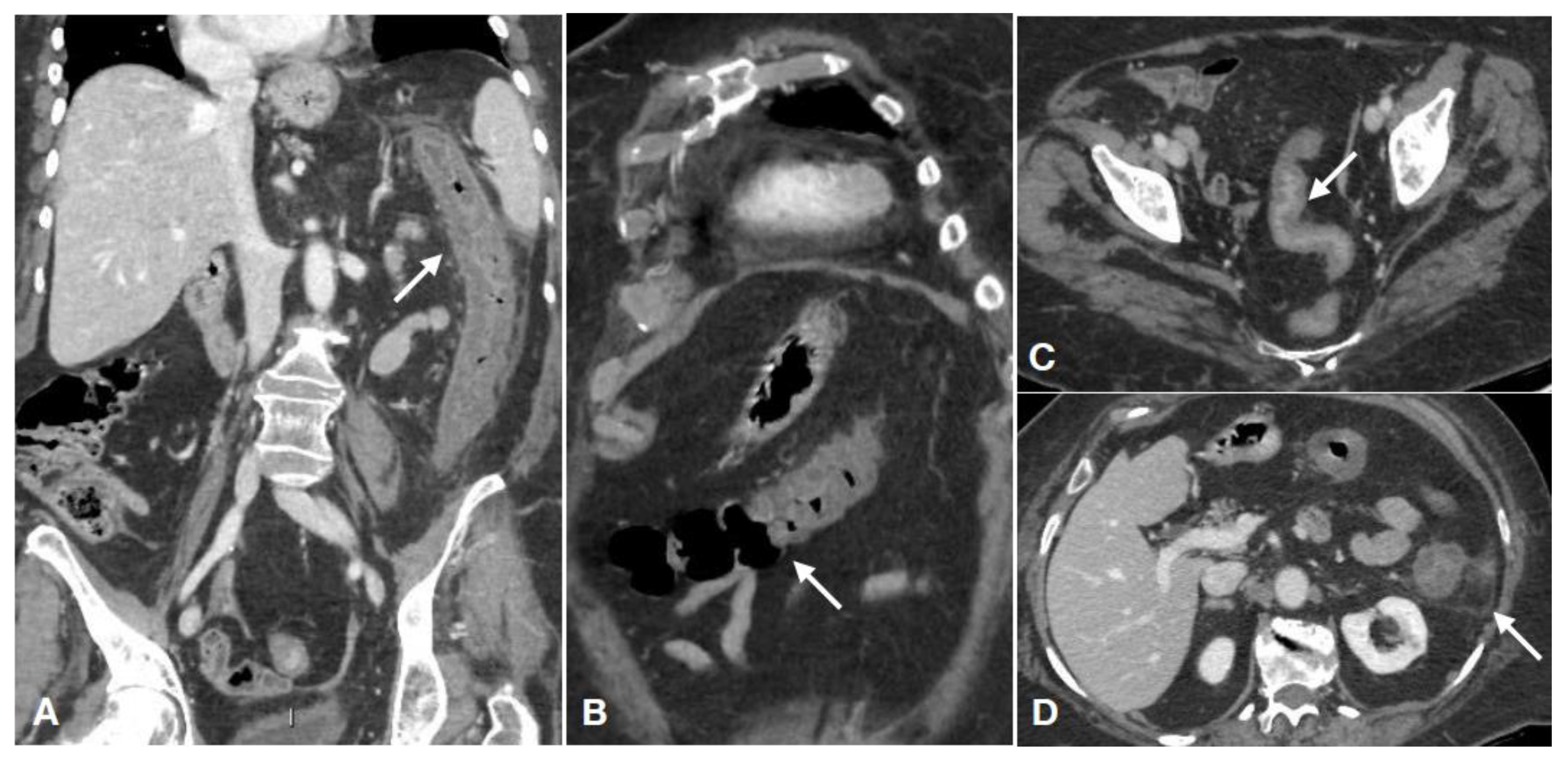
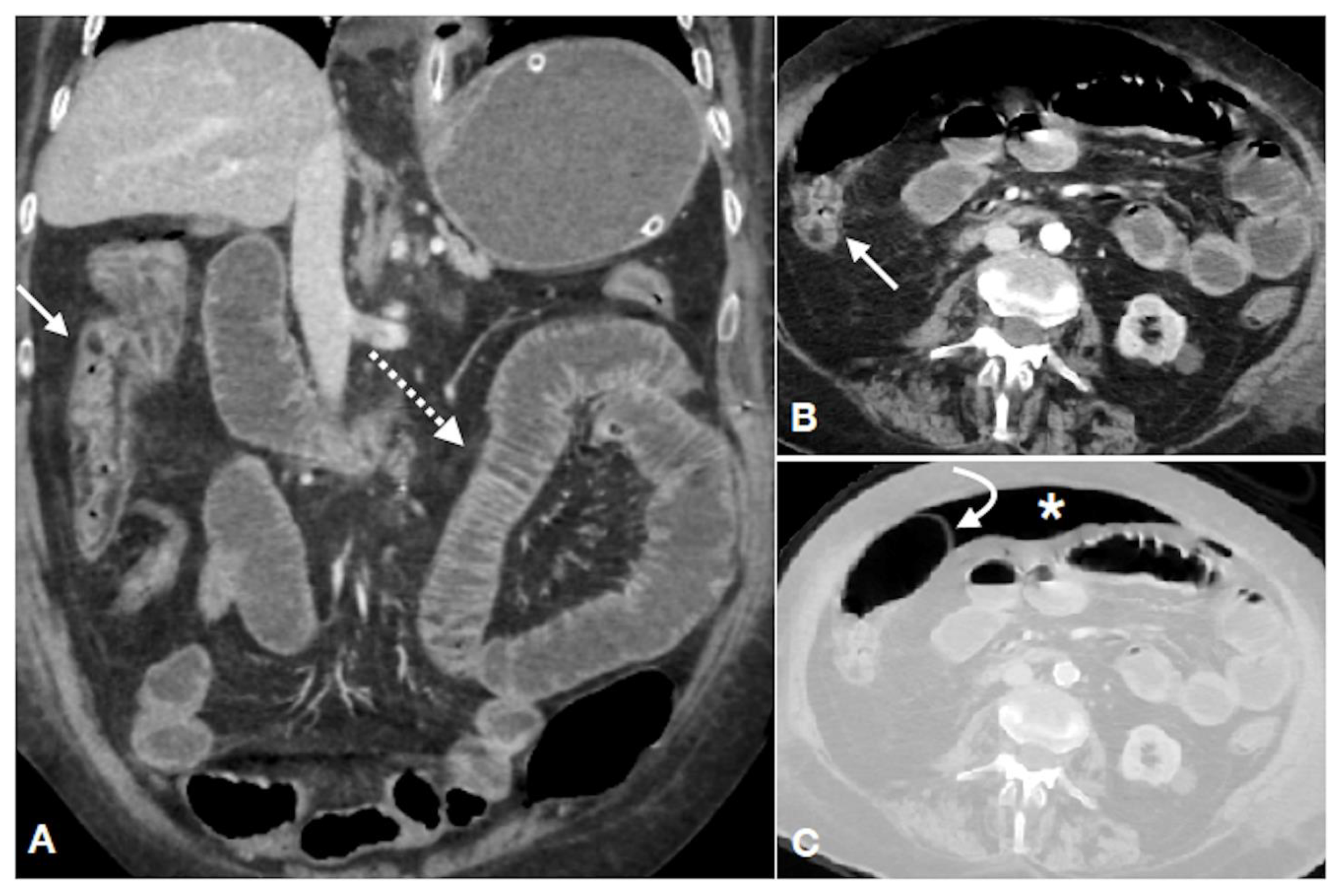
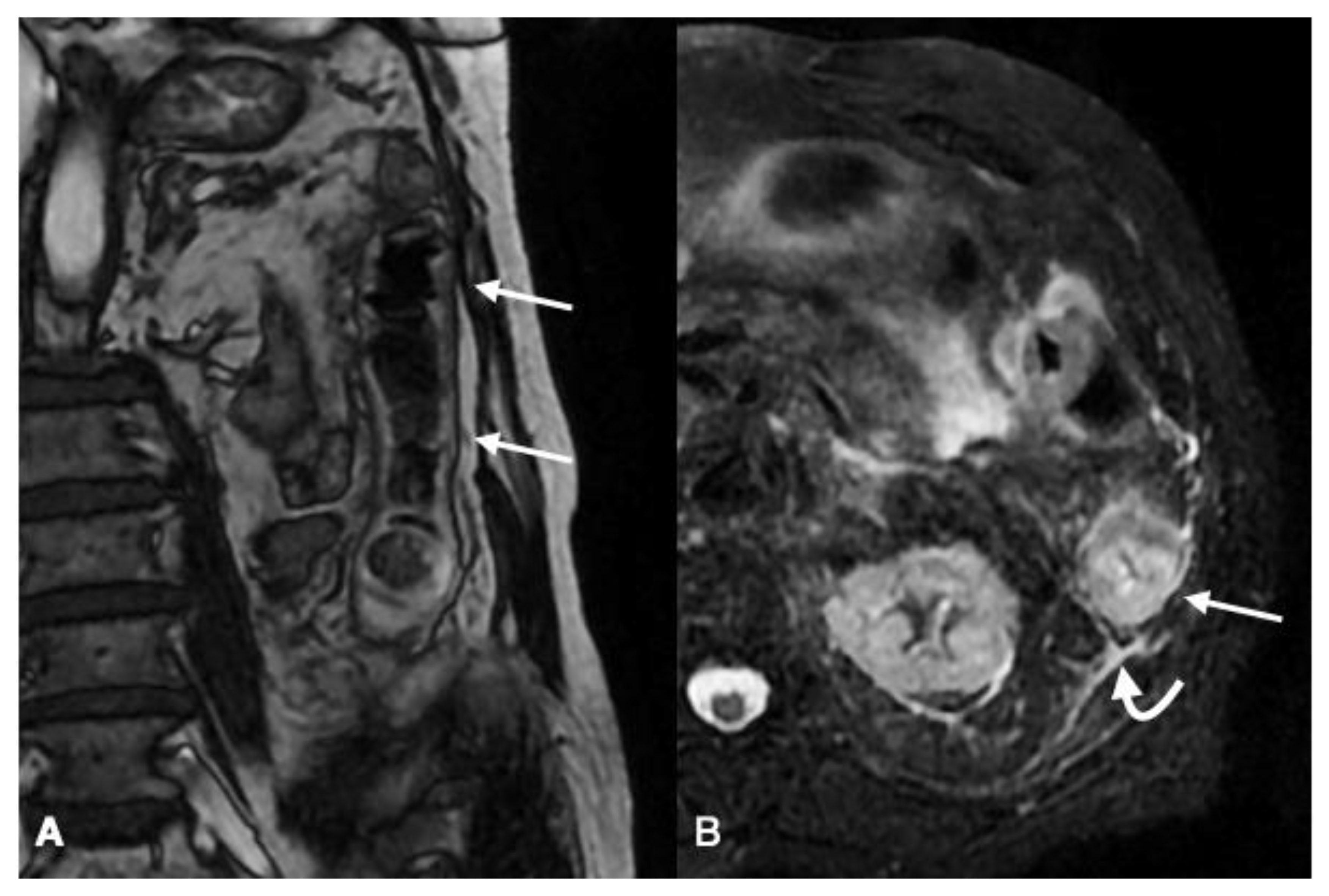
| Gasless Abdomen | Paucity of Intestinal Gas Due to Spastic Reflex Ileus |
|---|---|
| Large bowel dilation | Distention of the colonic lumen, similar to that seen with toxic megacolon inflammatory bowel disease may occur in fulminant ischemic colitis. |
| Bowel distension proximal to the involved colon | |
| “Colonic thumbprinting” | The most specific finding of colonic ischemia. It appears as loss of haustration and dilation of the colon with rounded densities along the colon wall. These multiple round, smooth soft-tissue densities projecting into the air-filled colonic lumen are caused by submucosal edema or hemorrhage. |
| Rigidity, narrowing and lack of haustrations and a tubular appearance of the bowel | May develop as the edema progresses. |
| Hepatic portal venous gas Intestinal pneumatosis Free air in the peritoneal cavity | Associated with bowel necrosis. These are signs of advanced ischemia that should be further investigated with CT examination |
| Damage | Radiological Findings and Characteristics |
|---|---|
| Type of damage Arterial ischemic type (less frequent) Reperfusive type (more frequent) Venous congestion (rare) | Thin or “paper thin” colonic wall; |
| Unenhanced colonic wall at enhanced CT; | |
| Dilation of the lumen, only gas-filled; | |
| Wall pneumatosis; | |
| Pneumoperitoneum; | |
| Parenchymal ischemia of liver/kidney/spleen; | |
| SMA/IMA or relative branches obstruction; | |
| Peritoneal/retroperitoneal free fluid (late finding); | |
| Thickened colonic wall; | |
| Mucosal hyperdensity (“little rose” sign); | |
| Lumen caliber reduction; | |
| Stratified enhanced wall (“target sign”); | |
| Fat stranding; | |
| SMA/IMA or relative branches obstruction; | |
| Pericolic fluid; | |
| SMV/IMV or relative branches obstruction; | |
| Bowel wall findings similar to the reperfusive type; | |
| Peritoneal/retroperitoneal free fluid | |
| Location of the damage | Descending colon; |
| Sigmoid colon; | |
| Right colon; | |
| Entire colon | |
| Extension of the damage | Single segment involvement; |
| Multisegmental pattern: | |
| Contiguous multisegmental involvement | |
| Skipped segments involvement | |
| Phases of the damage | Acute |
| Subacute | |
| Chronic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobellis, F.; Narese, D.; Berritto, D.; Brillantino, A.; Di Serafino, M.; Guerrini, S.; Grassi, R.; Scaglione, M.; Mazzei, M.A.; Romano, L. Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review. Diagnostics 2021, 11, 998. https://doi.org/10.3390/diagnostics11060998
Iacobellis F, Narese D, Berritto D, Brillantino A, Di Serafino M, Guerrini S, Grassi R, Scaglione M, Mazzei MA, Romano L. Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review. Diagnostics. 2021; 11(6):998. https://doi.org/10.3390/diagnostics11060998
Chicago/Turabian StyleIacobellis, Francesca, Donatella Narese, Daniela Berritto, Antonio Brillantino, Marco Di Serafino, Susanna Guerrini, Roberta Grassi, Mariano Scaglione, Maria Antonietta Mazzei, and Luigia Romano. 2021. "Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review" Diagnostics 11, no. 6: 998. https://doi.org/10.3390/diagnostics11060998
APA StyleIacobellis, F., Narese, D., Berritto, D., Brillantino, A., Di Serafino, M., Guerrini, S., Grassi, R., Scaglione, M., Mazzei, M. A., & Romano, L. (2021). Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review. Diagnostics, 11(6), 998. https://doi.org/10.3390/diagnostics11060998









