Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Magnetic Resonance Imaging
2.3. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koppen, I.J.N.; Vriesman, M.H.; Saps, M.; Rajindrajith, S.; Shi, X.X.; van Etten-Jamaludin, F.S.; Di Lorenzo, C.; Benninga, M.A.; Tabbers, M.M. Prevalence of functional defecation disorders in children: A systematic review and meta-analysis. J. Pediatr. 2018, 198, 121–130. [Google Scholar] [CrossRef]
- Athanasakos, E.P.; Kemal, K.I.; Malliwal, R.S.; Scott, S.M.; Williams, N.S.; Aziz, Q.; Ward, H.C.; Knowles, C.H. Clinical and psychosocial functioning in adolescents and young adults with anorectal malformations and chronic idiopathic constipation. Br. J. Surg. 2013, 100, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Catto-Smith, T.; Gibb, S.; Chase, J.; Shin, Y.M.; Stanton, M.; King, S.; Sutcliffe, J.; Ong, S.Y.; Djaja, S.; et al. Chronic constipation: No longer stuck! Characterization of colonic dysmotility as a new disorder in children. J. Pediatr. Surg. 2004, 39, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Chase, J.W.; Clarke, M.C.C.; King, S.K.; Sutcliffe, J.; Gibb, S.; Catto-Smith, A.G.; Robertson, V.J.; Southwell, B.R. Slow-transit constipation in children: Our experience. Pediatr. Surg. Int. 2009, 25, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and rome iv. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef]
- Mirjalili, S.A.; Tarr, G.; Stringer, M.D. The length of the large intestine in children determined by computed tomography scan. Clin. Anat. 2017, 30, 887–893. [Google Scholar] [CrossRef]
- Mahadevan, V. Anatomy of the rectum and anal canal. Surgery 2020, 38, 7–11. [Google Scholar] [CrossRef][Green Version]
- Underhill, B.M.L. Intestinal length in man. Br. Med. J. 1955, 2, 1243–1246. [Google Scholar] [CrossRef]
- Hounnou, G.; Destrieux, C.; Desme, J.; Bertrand, P.; Velut, S. Anatomical study of the length of the human intestine. Surg. Radiol. Anat. 2002, 24, 290–294. [Google Scholar] [CrossRef]
- Saunders, B.P.; Masaki, T.; Sawada, T.; Halligan, S.; Phillips, R.K.S.; Muto, T. A peroperative comparison of western and oriental colonic anatomy and mesenteric attachments. Int. J. Colorectal Dis. 1995, 10, 216–221. [Google Scholar] [CrossRef]
- Sadahiro, S.; Ohmura, T.; Yamada, Y.; Saito, T.; Taki, Y. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg. Radiol. Anat. 1992, 14, 251–257. [Google Scholar] [CrossRef]
- Sharif, H.; Devadason, D.; Abrehart, N.; Stevenson, R.; Marciani, L. Imaging measurement of whole gut transit time in paediatric and adult functional gastrointestinal disorders: A systematic review and narrative synthesis. Diagnostics 2019, 9, 221. [Google Scholar] [CrossRef]
- Orellana, B.; Monclus, E.; Brunet, P.; Navazo, I.; Bendezu, A.; Azpiroz, F. A scalable approach to T2-MRI colon segmentation. Med. Image Anal. 2020, 63. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Sandberg, T.H.; Poulsen, J.L.; Gram, M.; Frokjaer, J.B.; Ostergaard, L.R.; Krogh, K.; Brock, C.; Drewes, A.M. Quantification and variability in colonic volume with anovel magnetic resonance imaging method. Neurogastroenterol. Motil. 2015, 27, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.E.; Marciani, L.; Garsed, K.C.; Hoad, C.L.; Thongborisute, W.; Roberts, E.; Gowland, P.A.; Spiller, R.C. Fasting and postprandial volumes of the undisturbed colon: Normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil. 2014, 26, 124–130. [Google Scholar] [CrossRef]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef]
- Wilkinson-Smith, V.; Dellschaft, N.; Ansell, J.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment. Pharmacol. Ther. 2019, 49, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Garsed, K.C.; Hoad, C.L.; Fields, A.; Fordham, I.; Pritchard, S.E.; Placidi, E.; Murray, K.; Chaddock, G.; Costigan, C.; et al. Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: Comparison of split vs single dose. Neurogastroenterol. Motil. 2014, 26, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Chaddock, G.; Marciani, L.; Costigan, C.; Paul, J.; Cox, E.; Hoad, C.; Menys, A.; Pritchard, S.; Garsed, K.; et al. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol. Motil. 2016, 28, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Chaddock, G.; Marciani, L.; Costigan, C.; Cox, E.; Hoad, C.; Pritchard, S.; Gowland, P.; Spiller, R. Distinct abnormalities of small bowel and regional colonic volumes in subtypes of irritable bowel syndrome revealed by MRI. Am. J. Gastroenterol. 2017, 112, 346–355. [Google Scholar] [CrossRef]
- Bendezu, R.A.; Mego, M.; Monclus, E.; Merino, X.; Accarino, A.; Malagelada, J.R.; Navazo, I.; Azpiroz, F. Colonic content: Effect of diet, meals, and defecation. Neurogastroenterol. Motil. 2017, 29, e12930. [Google Scholar] [CrossRef]
- KeuzenkampJansen, C.W.; Fijnvandraat, C.J.; Kneepkens, C.M.F.; Douwes, A.C. Diagnostic dilemmas and results of treatment for chronic constipation. Arch. Dis. Child. 1996, 75, 36–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharif, H.; Abrehart, N.; Hoad, C.L.; Murray, K.; Perkins, A.C.; Smith, M.; Gowland, P.A.; Spiller, R.C.; Harris, R.; Kirkham, S.; et al. Feasibility study of a new magnetic resonance imaging mini-capsule device to measure whole gut transit time in paediatric constipation. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Eggers, H.; Brendel, B.; Duijndam, A.; Herigault, G. Dual-echo Dixon imaging with flexible choice of echo times. Magn. Reson. Med. 2011, 65, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Dellschaft, N.S.; Hoad, C.L.; Marciani, L.; Ban, L.; Prayle, A.P.; Barr, H.L.; Jaudszus, A.; Mainz, J.G.; Spiller, R.C.; et al. Postprandial changes in gastrointestinal function and transit in cystic fibrosis assessed by Magnetic Resonance Imaging. J. Cyst. Fibros. 2021, in press. [Google Scholar]
- El Edelbi, R.; Lindemalm, S.; Eksborg, S. Estimation of body surface area in various childhood ages-validation of the Mosteller formula. Acta Paediatr. 2012, 101, 540–544. [Google Scholar] [CrossRef]
- Devroede, G.; Soffie, M. Colonic absorption in idiopathic constipation. Gastroenterology 1973, 64, 552–561. [Google Scholar] [CrossRef]
- Inoh, Y.; Kanoshima, K.; Ohkuma, K.; Fuyuki, A.; Uchiyama, S.; Ohkubo, H.; Higurashi, T.; Iida, H.; Nonaka, T.; Fujita, K.; et al. Assessment of colonic contents in patients with chronic constipation using MRI. J. Clin. Biochem. Nutr. 2018, 62, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Chen, J.; Chen, X.F.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016, 150, 367–379. [Google Scholar] [CrossRef]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
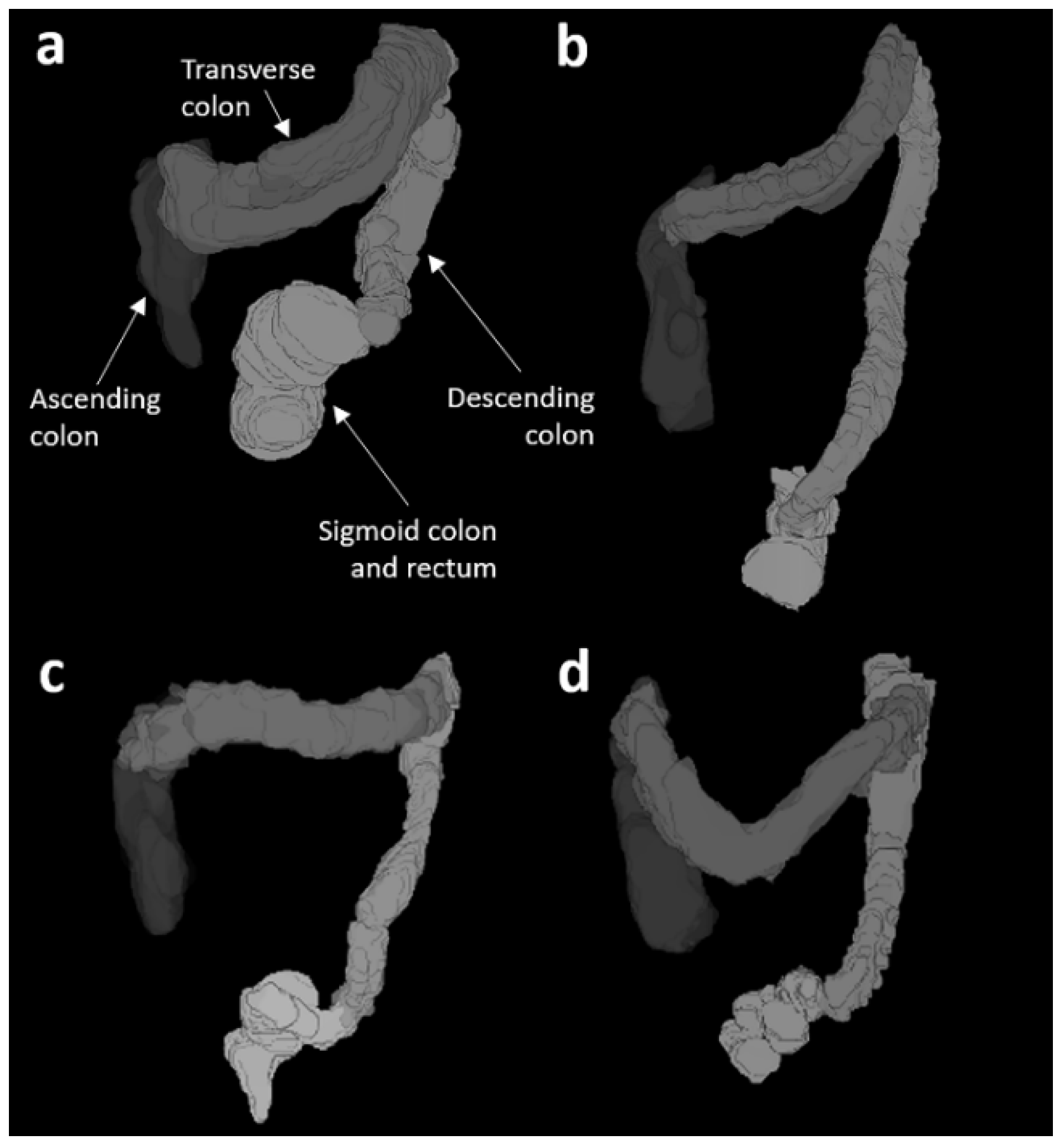
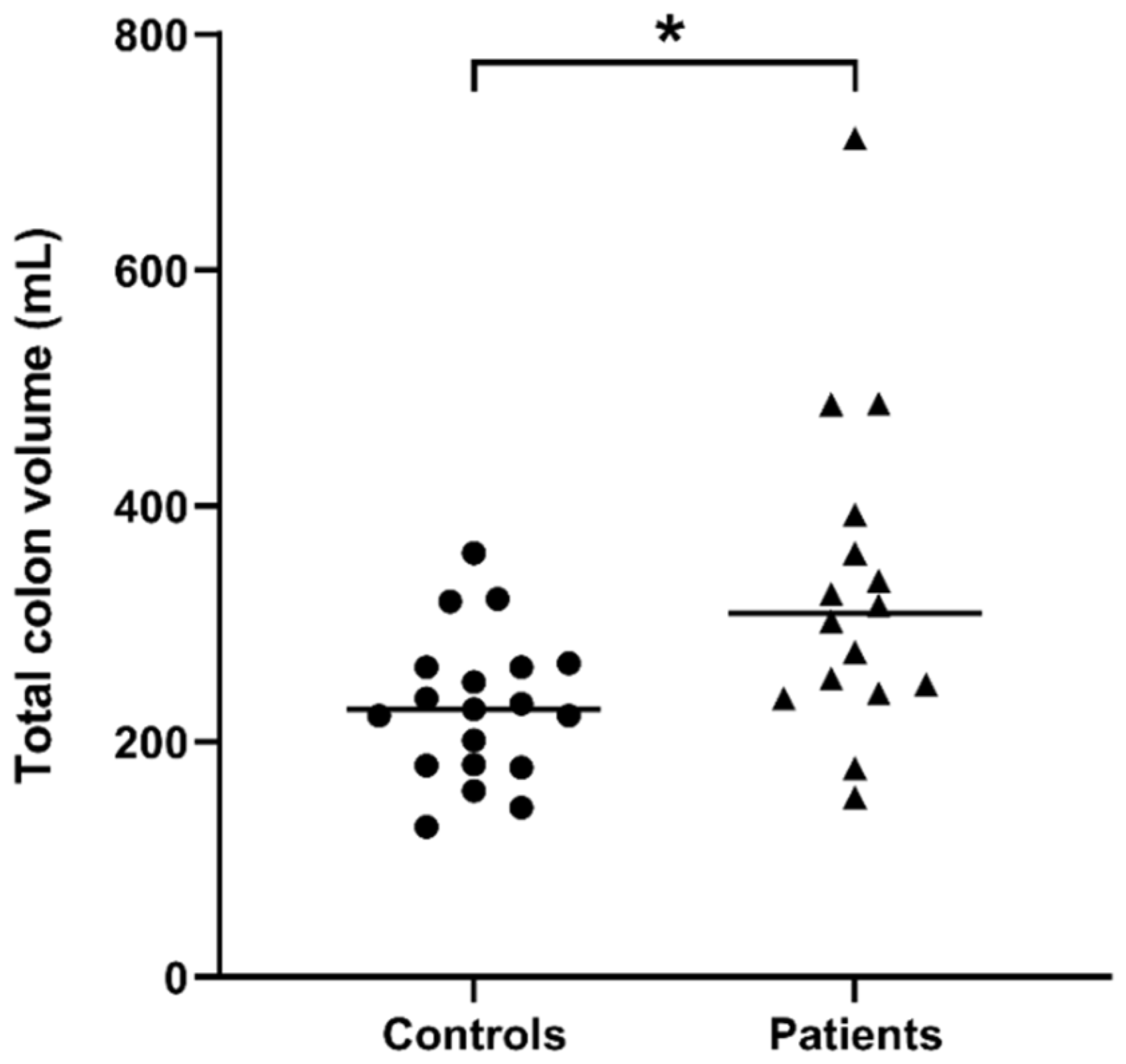
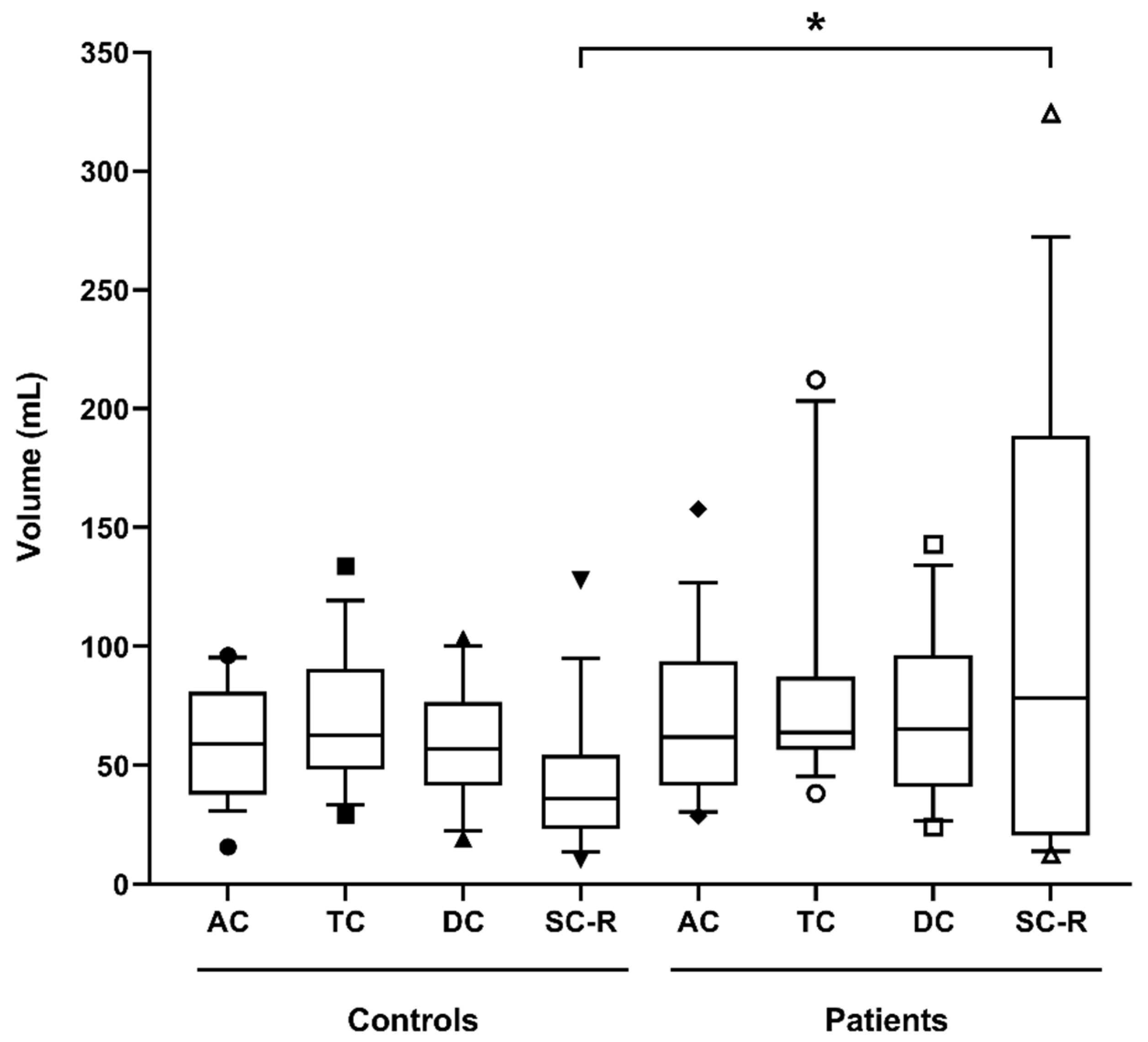

| Ascending Colon (mL) | Transverse Colon (mL) | Descending Colon (mL) | Sigmoid Colon and Rectum (mL) | Total Colon (mL) | |
|---|---|---|---|---|---|
| Controls | 59 (38–81) | 63 (48–91) | 57 (41–77) | 36 (23–54) | 227 (180–263) |
| Patients | 62 (41–94) | 64 (56–87) | 65 (41–96) | 78 (20–189) | 309 (243–384) |
| p-value | n/s | n/s | n/s | p < 0.05 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, H.; Hoad, C.L.; Abrehart, N.; Gowland, P.A.; Spiller, R.C.; Kirkham, S.; Loganathan, S.; Papadopoulos, M.; Benninga, M.A.; Devadason, D.; et al. Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics 2021, 11, 974. https://doi.org/10.3390/diagnostics11060974
Sharif H, Hoad CL, Abrehart N, Gowland PA, Spiller RC, Kirkham S, Loganathan S, Papadopoulos M, Benninga MA, Devadason D, et al. Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics. 2021; 11(6):974. https://doi.org/10.3390/diagnostics11060974
Chicago/Turabian StyleSharif, Hayfa, Caroline L. Hoad, Nichola Abrehart, Penny A. Gowland, Robin C. Spiller, Sian Kirkham, Sabarinathan Loganathan, Michalis Papadopoulos, Marc A. Benninga, David Devadason, and et al. 2021. "Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI" Diagnostics 11, no. 6: 974. https://doi.org/10.3390/diagnostics11060974
APA StyleSharif, H., Hoad, C. L., Abrehart, N., Gowland, P. A., Spiller, R. C., Kirkham, S., Loganathan, S., Papadopoulos, M., Benninga, M. A., Devadason, D., & Marciani, L. (2021). Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics, 11(6), 974. https://doi.org/10.3390/diagnostics11060974







