Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient’s Clinical Variables and Transplant Outcome Complications
Abstract
1. Introduction
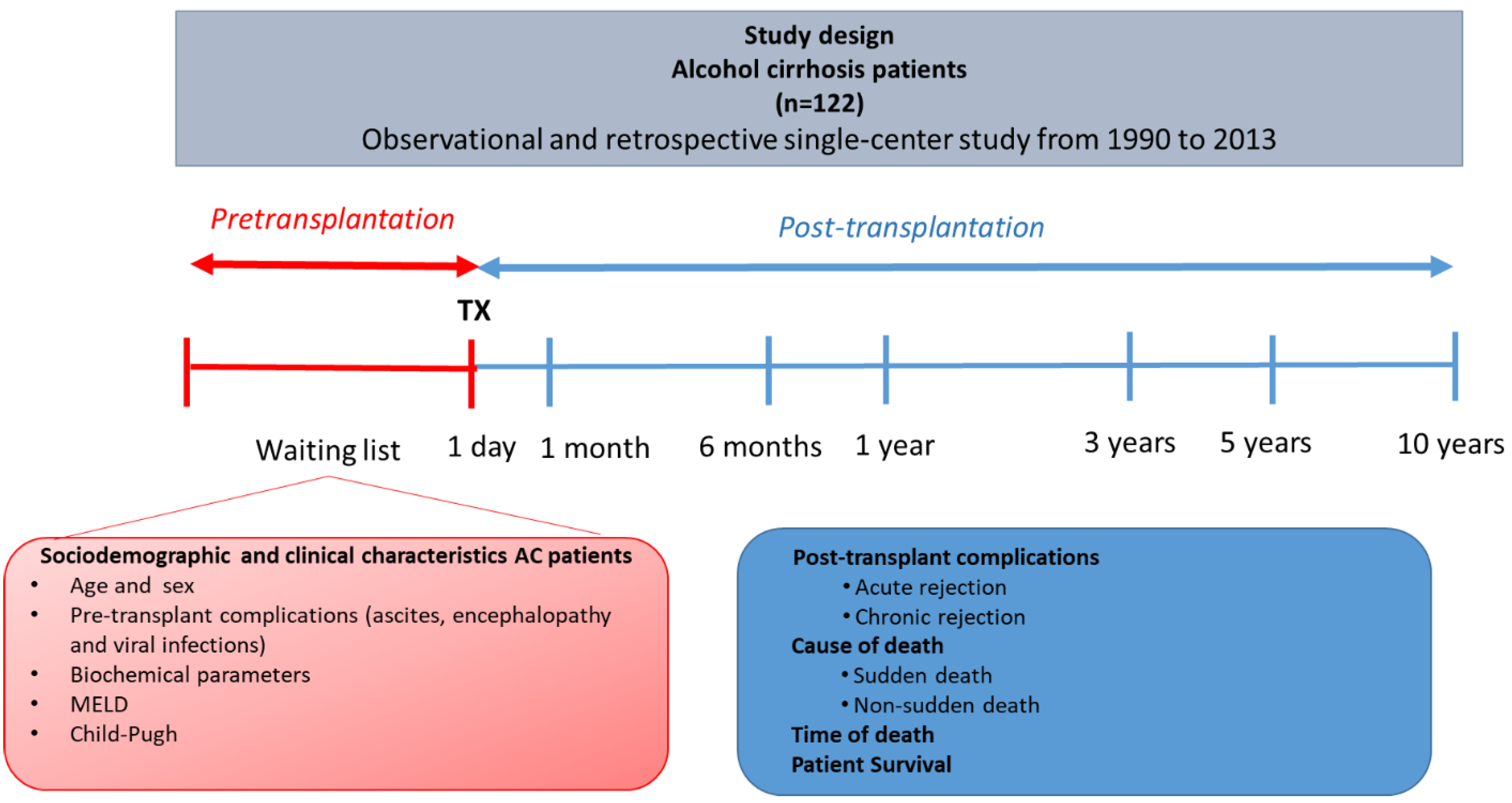
2. Material and Methods
2.1. Patient Enrollment
2.2. Diagnostic Criteria of Alcoholic Cirrhosis
2.3. Main Causes and Time of Death in Alcoholic Cirrhosis Patients
2.4. Viral Infection, Ascites, and Hepatic Encephalopathy Diagnosis
2.5. Ascites and Hepatic Encephalopathy Diagnosis
2.6. Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Characteristics
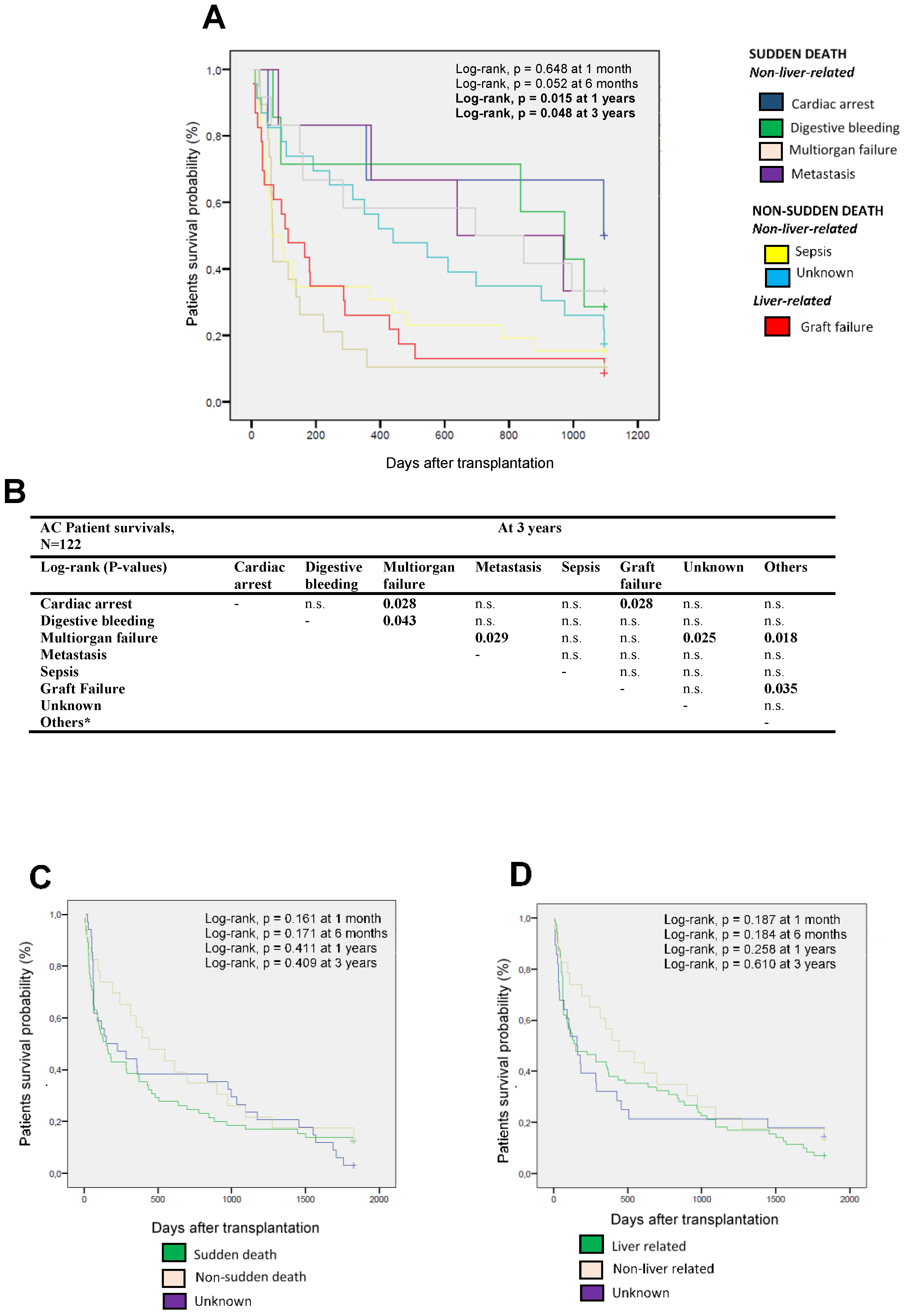
3.2. Different Causes of Death of AC Patients at the Short and Long Term
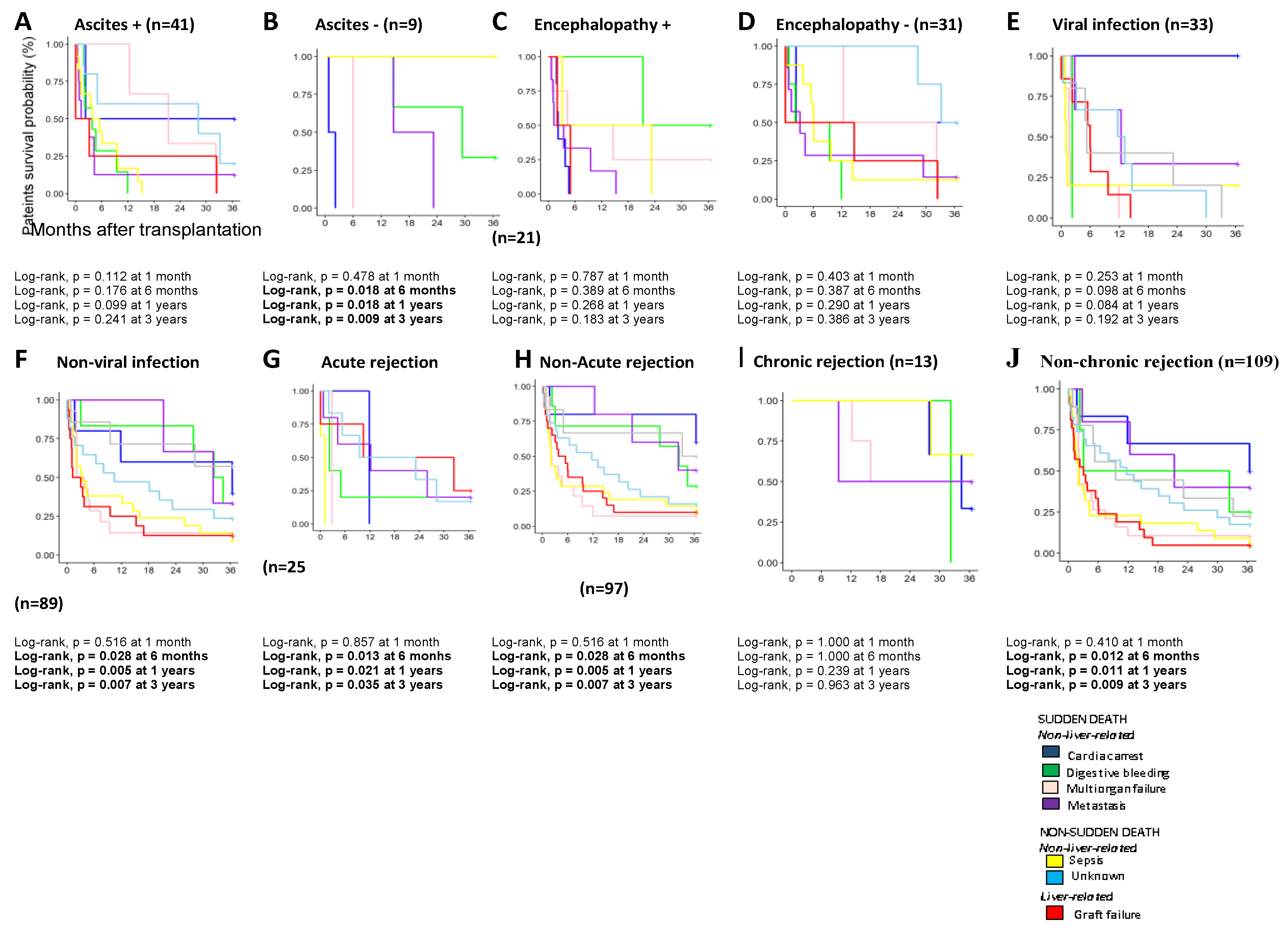
3.3. Analysis of the Causes of Death in AC Patients and the Pre- and Post-Transplant Complications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legaz, I.; Bolarin, J.M.; Campillo, J.A.; Moya, R.M.; Luna, A.; Osuna, E.; Minguela, A.; Sanchez-Bueno, F.; Alvarez, M.R.; Muro, M. Pretransplant ascites or encephalopathy and their influence on survival and liver graft rejection in alcoholic cirrhosis disease. Arch. Med. Sci. 2021, 17, 682–693. [Google Scholar] [CrossRef]
- Legaz, I.; Navarro-Noguera, E.; Bolarín, J.M.; García-Alonso, A.M.; Maldonado, A.L.; Mrowiec, A.; Campillo, J.A.; Gimeno, L.; Moya-Quiles, R.; Álvarez-López, M.D.R.; et al. Epidemiology, Evolution, and Long-Term Survival of Alcoholic Cirrhosis Patients Submitted to Liver Transplantation in Southeastern Spain. Alcohol. Clin. Exp. Res. 2016, 40, 794–805. [Google Scholar] [CrossRef]
- Legaz, I.; López-Álvarez, M.R.; Campillo, J.A.; Moya-Quiles, M.R.; Bolarín, J.M.; de la Peña, J.; Salgado, G.; Gimeno, L.; García-Alonso, A.M.; Muro, M.; et al. KIR Gene Mismatching and KIR/C Ligands in Liver Transplantation: Consequences for Short-Term Liver Allograft Injury. Transplantation 2013, 95, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Vikhert, A.M.; Tsiplenkova, V.G.; Cherpachenko, N.M. Alcoholic cardiomyopathy and sudden cardiac death. J. Am. Coll. Cardiol. 1986, 8, 3A–11A. [Google Scholar] [CrossRef]
- George, A.; Figueredo, V.M. Alcohol and arrhythmias: A comprehensive review. J. Cardiovasc. Med. 2010, 11, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Lundberg, W.B. Ventricular arrhythmias associated with the ingestion of alcohol. Ann. Intern. Med. 1972, 77, 247–248. [Google Scholar] [CrossRef]
- Ettinger, P.O.; Wu, C.F.; Cruz, C.D.L.; Weisse, A.B.; Ahmed, S.S.; Regan, T.J. Arrhythmias and the “Holiday Heart”: Alcoholassociated cardiac rhythm disorders. Am. Heart J. 1978, 95, 555–562. [Google Scholar] [CrossRef]
- Sano, F.; Ohira, T.; Kitamura, A.; Imano, H.; Cui, R.; Kiyama, M.; Okada, T.; Yamagishi, K.; Sankai, T.; Tanigawa, T.; et al. Heavy alcohol consumption and risk of atrial fibrillation: The circulatory risk in communities study (circs). Circ. J. 2014, 78, 955–961. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Jurisch, D.; Neef, M.; Hagendorff, A. Alkohol und Rhythmusstörungen. Herz 2016, 41, 498–502. [Google Scholar] [CrossRef]
- Kelbaek, H.; Fløistrup, S.; Gjørup, T.; Christensen, N.J.; Hartling, O.J.; Godtfredsen, J. Central and peripheral haemodynamic changes after alcohol ingestion. Alcohol Alcohol. 1988, 23, 211–216. [Google Scholar]
- Schulte, T.; Warzel, H.; Westphal, S.; Müller-Oehring, E.M.; Strasburger, H.; Dierkes, J.; Sabel, B.A. Acute moderate alcohol consumption affects cardiovascular responses in healthy males with different tolerance levels. Neuropsychobiology 2002, 45, 191–198. [Google Scholar] [CrossRef]
- Greenspon, A.J.; Schaal, S.F. The “holiday heart”: Electrophysiologic study of alcohol effects in alcoholics. Ann. Intern. Med. 1983, 98, 135–139. [Google Scholar] [CrossRef]
- Mandayam, S.; Jamal, M.M.; Morgan, T.R. Epidemiology of alcoholic liver disease. Semin. Liver Dis. 2004, 24, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.S.; Cook, R.T.; Jerrells, T.R.; Kolls, J.K.; Nagy, L.E.; Szabo, G.; Wands, J.R.; Kovacs, E.J. Acute and Chronic Alcohol Abuse Modulate Immunity. Alcohol. Clin. Exp. Res. 2006, 30, 1624–1631. [Google Scholar] [CrossRef]
- Szabo, G. CONSEQUENCES OF ALCOHOL CONSUMPTION ON HOST DEFENCE. Alcohol Alcohol. 1999, 34, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Anand, B.S. Recent trends in the epidemiology of alcoholic liver disease. Clin. Liver Dis. 2013, 2, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Senzolo, M.; Adam, R.; Delvart, V.; Karam, V.; Germani, G.; Neuberger, J. Liver Transplantation for Alcoholic Liver Disease in Europe: A Study from the ELTR (European Liver Transplant Registry). Am. J. Transplant. 2010, 10, 138–148. [Google Scholar] [CrossRef]
- Kupari, M.; Koskinen, P. Alcohol, cardiac arrhythmias and sudden death. Novartis Found. Symp. 1998, 68–85. [Google Scholar] [CrossRef]
- Pruthi, J.; Medkiff, K.A.; Esrason, K.T.; Donovan, J.A.; Yoshida, E.M.; Erb, S.R.; Steinbrecher, U.P.; Fong, T.L. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transplant. 2001, 7, 811–815. [Google Scholar] [CrossRef]
- Polsky, S.; Akturk, H.K. Alcohol Consumption, Diabetes Risk, and Cardiovascular Disease Within Diabetes. Curr. Diabetes Rep. 2017, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Rico, H. Alcohol and bone disease. Alcohol Alcohol. 1990, 25, 345–352. [Google Scholar] [PubMed]
- Vamvakas, S.; Teschner, M.; Bahner, U.; Heidland, A. Alcohol abuse: Potential role in electrolyte disturbances and kidney diseases. Clin. Nephrol. 1998, 49, 205–213. [Google Scholar]
- Cittadini, F.; De Giovanni, N.; Alcalde, M.; Partemi, S.; Campuzano, O.; Brugada, R.; Oliva, A. Genetic and toxicologic investigation of Sudden Cardiac Death in a patient with Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) under cocaine and alcohol effects. Int. J. Leg. Med. 2014, 129, 89–96. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Kaneku, H.; Jennings, L.W.; Bañuelos, N.; Susskind, B.M.; Terasaki, P.I.; Klintmalm, G.B. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transplant. 2013, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Hietanen, S.; Herajärvi, J.; Junttila, J.; Pakanen, L.; Huikuri, H.V.; Liisanantti, J. Characteristics of subjects with alcoholic cardiomyopathy and sudden cardiac death. Heart 2020, 106, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.; Wingren, C.J.; Ottosson, A.; Andersson, L.; Wangefjord, S.; Acosta, S. Necessity of including medico-legal autopsy data in epidemiological surveys of individuals with major trauma. Injury 2015, 46, 1515–1519. [Google Scholar] [CrossRef]
- World Health Organisation. Global Status Report on Alcohol and Health 2014; World Health Organisation: Geneva, Switzerland, 2014; pp. 1–392. [Google Scholar]
- Heidelbaugh, J.J.; Bruderly, M. Cirrhosis and chronic liver failure: Part I. Diagnosis and evaluation. Am. Fam. Physician 2006, 74, 756–762. [Google Scholar]
- Meade, T.; Clayton, T.; Chamberlain, D. Distinguishing between those dying suddenly or not suddenly from coronary heart disease: Long-term prospective results from the Northwick Park Heart Study. Open Heart 2016, 3, 440. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Ginès, P.; Gerbes, A.L.; Dudley, F.J.; Gentilini, P.; Laffi, G.; Reynolds, T.B.; Ring-Larsen, H.; Schölmerich, J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology 1996, 23, 164–176. [Google Scholar] [CrossRef]
- Blei, A.T.; Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar]
- Zipprich, A.; Garcia-Tsao, G.; Rogowski, S.; Fleig, W.E.; Seufferlein, T.; Dollinger, M.M. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012, 32, 1407–1414. [Google Scholar] [CrossRef]
- Cao, G.; Yi, T.; Liu, Q.; Wang, M.; Tang, S. Alcohol consumption and risk of fatty liver disease: A meta-analysis. PeerJ 2016, 2016, e2633. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.J.; Huang, H.C. Management of ascites in patients with liver cirrhosis: Recent evidence and controversies. J. Chin. Med. Assoc. 2013, 76, 123–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poordad, F.F. Presentation and complications associated with cirrhosis of the liver. Curr. Med. Res. Opin. 2015, 31, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Groszmann, R.J.; Fisher, R.L.; Conn, H.O.; Atterbury, C.E.; Glickman, M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985, 5, 419–424. [Google Scholar] [CrossRef]
- Andreu, M.; Sola, R.; Sitges-Serra, A.; Alia, C.; Gallen, M.; Vila, M.C.; Coll, S.; Oliver, M.I. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology 1993, 104, 1133–1138. [Google Scholar] [CrossRef]
- Namba, M.; Hiramatsu, A.; Aikata, H.; Kodama, K.; Uchikawa, S.; Ohya, K.; Morio, K.; Fujino, H.; Nakahara, T.; Murakami, E.; et al. Management of refractory ascites attenuates muscle mass reduction and improves survival in patients with decompensated cirrhosis. J. Gastroenterol. 2020, 55, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Angeli, P.; Lenz, K.; Møller, S.; Moore, K.; Moreau, R.; Merkel, C.; Larsen, H.R.; Bernardi, M.; Garcia-Tsao, G.; et al. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar]
- Iruzubieta, P.; Crespo, J.; Fábrega, E. Long-term survival after liver transplantation for alcoholic liver disease. World J. Gastroenterol. 2013, 19, 9198–9208. [Google Scholar] [CrossRef]
- Chaulk, J.; Carbonneau, M.; Qamar, H.; Keough, A.; Chang, H.J.; Ma, M.; Kumar, D.; Tandon, P. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: A single-centre experience and summary of existing studies. Can. J. Gastroenterol. Hepatol. 2014, 28, 83–88. [Google Scholar] [CrossRef]
- Peng, Y.; Qi, X.; Guo, X. Child–Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine 2016, 95. [Google Scholar] [CrossRef]
- Morentin, B.; Callado, L.F. Sudden cardiac death associated to substances of abuse and psychotropic drugs consumed by young people: A population study based on forensic autopsies. Drug Alcohol Depend. 2019, 201, 23–28. [Google Scholar] [CrossRef]
- McPherson, S.; Lucey, M.R.; Moriarty, K.J. Decompensated alcohol related liver disease: Acute management. BMJ 2016, 352, i124. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.F.; Carithers, R.L. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005, 41, 1407–1432. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.-A. Ascites in patients with cirrhosis. Can. Fam. Physician 2013, 59, 1297–1299. [Google Scholar]
- RETH. Registro Español 2013 RETH; Organización Nacional de Trasplantes: Madrid, Spain, 2013. [Google Scholar]
- Su, F.; Yu, L.; Berry, K.; Liou, I.W.; Landis, C.S.; Rayhill, S.C.; Reyes, J.D.; Ioannou, G.N. Aging of Liver Transplant Registrants and Recipients: Trends and Impact on Waitlist Outcomes, Post-Transplantation Outcomes, and Transplant-Related Survival Benefit. Gastroenterology 2016, 150, 441–453.e6. [Google Scholar] [CrossRef]
- Durand, F.; Levitsky, J.; Cauchy, F.; Gilgenkrantz, H.; Soubrane, O.; Francoz, C. Age and liver transplantation. J. Hepatol. 2019, 70, 745–758. [Google Scholar] [CrossRef]
- Cakaloglu, Y.; Devlin, J.; O’Grady, J.; Sutherland, S.; Portmann, B.C.; Heaton, N.; Tan, K.C.; Williams, R. Importance of concomitant viral infection during late acute liver allograft rejection. Transplantation 1995, 59, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Shaper, A.G. Alcohol and sudden cardiac death. Heart 1992, 68, 443–448. [Google Scholar] [CrossRef]
- SHAPER, A.G. Alcohol and mortality: A review of prospective studies. Br. J. Addict. 1990, 85, 837–847. [Google Scholar] [CrossRef]
- Gustot, T. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef]
- Jalan, R.; Fernandez, J.; Wiest, R.; Schnabl, B.; Moreau, R.; Angeli, P.; Stadlbauer, V.; Gustot, T.; Bernardi, M.; Canton, R.; et al. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J. Hepatol. 2014, 60, 1310–1324. [Google Scholar] [CrossRef]
- Kovacs, E.J.; Messingham, K.A.N. Influence of alcohol and gender on immune response. Alcohol Res. Health 2002, 26, 257–263. [Google Scholar]
- Szabo, G.; Mandrekar, P. A Recent Perspective on Alcohol, Immunity, and Host Defense. Alcohol. Clin. Exp. Res. 2009, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Saha, B. Alcohol’s effect on host defense. Alcohol Res. Curr. Rev. 2015, 37, 159. [Google Scholar]
- Valeriano, V.; Funaro, S.; Lionetti, R.; Riggio, O.; Pulcinelli, G.; Fiore, P.; Masini, A.; De Castro, S.; Merli, M. Modification of cardiac function in cirrhotic patients with and without ascites. Am. J. Gastroenterol. 2000, 95, 3200–3205. [Google Scholar] [CrossRef]
- Pozzi, M.; Carugo, S.; Boari, G.; Pecci, V.; de Ceglia, S.; Maggiolini, S.; Bolla, G.B.; Roffi, L.; Failla, M.; Grassi, G.; et al. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology 1997, 26, 1131–1137. [Google Scholar]
- Naidoo, D.P.; Bramdev, A.; Cooper, K. Autopsy prevalence of Wernicke’s encephalopathy in alcohol-related disease. S. Afr. Med. J. 1996, 86, 1110–1112. [Google Scholar]

| Total AC Patients N = 122, n (%) | |
|---|---|
| Mean age (mean years ± SD) | 53.1 ± 8.9 |
| Sex (male/female) | 113 (92.6)/9 (7.4) |
| Pre-transplantation complications | |
| Ascites +, n = 41 Mean age * | 41/50 (82.0) 54.2 ± 1.3 |
| Grade | |
| I | 8 (19.5) |
| II | 18 (43.9) |
| III | 15 (36.6) |
| Encephalopathy +, n = 21 Mean age * | 21/52 (40.4) 56.7 ± 2.0 |
| Grade | |
| I | 9 (42.8) |
| II | 10 (47.7) |
| III | 2 (9.5) |
| Viral infection a +, n = 122 Mean age * | 33/122 (27.0); 51.3 ± 1.9 |
| Biochemical parameters | |
| Creatinine (mg/dL) | 1.12 ± 0.35 |
| Albumin (g/dL) | 3.23 ± 0.07 |
| Total bilirubin (mg/dL) | 3.01 ± 0.19 |
| GOT (U/L) | 97.23 ± 12.67 |
| GPT (U/L) | 70.8 ± 9.56 |
| GGT (U/L) | 98.96 ± 7.10 |
| AP (U/L) | 172.09 ± 8.32 |
| Prothrombin activity (%) | 59.08 ± 0.97 |
| INR | 1.47 ± 0.22 |
| MELD | 14.4 ± 0.7 |
| Child–Pugh % (A/B/C) | 18.4/52.4/29.5 |
| AC Patients by Sex | AC Patients by Viral Infection | |||||||
|---|---|---|---|---|---|---|---|---|
| Total AC Patients * N = 122, n (%) Mean ± SD | Men N = 113, n (%) Mean ± SD | Women N = 9, n (%) Mean ± SD | p ** | OR (95% CI) | Virus *** N = 33, n (%) Mean ± SD | Non-virus N = 89, n (%) Mean ± SD | p ** | |
| Mean age (years) | 53.1 ± 8.9 | 53.2 ± 9.1 | 50.7 ± 6.2 | 0.274 | - | 54.2 ± 0.9 | 49.9 ± 1.6 | 0.019 |
| Pre-transplant complications | ||||||||
| Ascites + | 41/50 (82.0) a 54.2 ± 1.3 | 38/47 (80.9) b 54.6 ± 1.4 | 3/3 (100) 50.0 ± 4.6 | 1.000 0.369 | 1.079 (0.990–1.176) - | 15/41 (36.6) c 50.4 ± 2.0 | 26/41 (63.4) d 56.4 ± 1.6 | 0.715 0.024 |
| Encephalopathy + | 21/52 (40.4) 56.7 ± 2.0 | 21/49 (42.9) 57.0 ± 1.9 | 0/3 (0) - | 0.264 - | 0.903 (0.805–1.014) - | 5/21 (23.8) 47.2 ± 2.8 | 16/21 (76.2) 60.1 ± 1.8 | 0.149 0.002 |
| Viral infection + | 33/122 (27.0) 51.3 ± 1.9 | 30/113 (26.5) 50.0 ± 1.7 | 3/9 (33.3) 49.0 ± 6.3 | 0.702 0.854 | 1.383 (0.325–5.882) - | - - | - - | - - |
| Post-transplant complications | ||||||||
| Acute rejection | 25/122 (20.5) 54.3 ± 3.0 | 23/113 (20.4) 51.3 ± 2.1 | 2/9 (22.2) 54.5 ± 0.5 | 1.000 0.664 | 1.118 (0.218–5.745) - | 8/25 (32.0) 45.4 ± 3.6 | 17/25 (68.0) e 54.4 ± 2.1 | 0.615 0.029 |
| Chronic rejection | 13/122 (10.7) 52.8 ± 2.4 | 13/113 (11.5) 50.7 ± 2.3 | 0/9 (0) - | 0.595 - | 0.917 (0.867–0.971) - | 0/13 (0) - | 13/13 (100) 50.7 ± 2.3 | 0.019 - |
| Cause of death | ||||||||
| Sudden death | 34 (27.9) 55.2 ± 9.9 | 31 (27.4) 56.3 ± 9.6 | 3 (33.3) 44.7 ± 6.1 | 0.708 0.051 | 0.756 (0.178–3.211) - | 8/34 (23.5) f 55.5 ± 3.5 | 26/34 (76.5) g 55.2 ± 2.0 | 0.827 0.933 |
| Non-sudden death | 65 (53.2) 51.9 ± 9.0 | 60 (53.1) 51.8 ± 9.3 | 5 (55.5) 53.4 ± 4.2 | 1.000 0.500 | 0.906 (0.231–3.549) - | 19/65 (29.2) 47.3 ± 2.0 | 46/65 (70.8) 53.9 ± 1.3 | 0.827 0.007 |
| Unknown | 23 (18.9) 52.9 ± 6.8 | 22 (19.5) 52.9 ± 7.0 | 1 (11.1) 55.0 ± 0.0 | 1.000 0.768 | 1.934 (0.230–16.281) - | 6/23 (26.1) 50.8 ± 2.8 | 17/23 (73.9) 53.7 ± 1.7 | 0.827 0.389 |
| Time of death | ||||||||
| ≤1 month | 14 (11.8) h 53.1 ± 10.8 | 13 (11.7) i 52.6 ± 11.0 | 1 (12.5) j 60.0 ± 0.0 | 0.305 0.531 | 0.455 (0.085–2.428) - | 5/14 (35.7) k 56.8 ± 3.0 | 9/14 (64.3) l 51.1 ± 4.1 | 0.697 0.364 |
| ˃1–6 months | 44 (37.0) 55.7 ± 9.3 | 39 (35.1) 56.3 ± 9.8 | 5 (62.2) 51.4 ± 2.1 | 0.719 0.012 | 0.659 (0.167–2.595) - | 12/44 (27.3) 49.1 ± 2.6 | 32/44 (72.7) 58.2 ± 1.5 | 0.697 0.003 |
| ≥6 months–10 years | 61 (51.3) 51.4 ± 7.8 | 59 (53.2) 51.7 ± 7.7 | 2 (25.0) 42.0 ± 5.7 | 0.306 0.082 | 2.346 (0.559–9.847) - | 15/61 (24.6) 49.4 ± 2.2 | 46/61 (75.4) 52.0 ± 1.1 | 0.697 0.259 |
| Time of Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤One Month | ≥1–6 Months | 6 Months | 1 Year | 3 Years | 5 Years | 10 Years | |||
| Cause of Death, n (%) | N = 14, n (%) | N = 43, n (%) | N = 57, n (%) | N = 74, n (%) | N = 99, n (%) | N = 110, n (%) | Total * N = 122, n (%) | Men N = 113, n (%) | Women N = 9, n (%) |
| Sudden death | 2 (14.3) | 15 (34.9) | 17 (29.8) | 21 (28.4) | 26 (26.3) | 33 (30.0) | 34 (27.8) | 31 (27.4) | 3 (33.3) |
| Non-liver related | 2 (14.3) | 15 (34.9) | 17 (29.8) | 21 (28.4) | 26 (26.3) | 33 (30.0) | 34 (27.8) | 31 (27.4) | 3 (33.3) |
| Cardiac arrest | - | 1 (6.7) | 5 (29.4) | 2 (9.5) | 3 (11.5) | 6 (18.2) | 6 (17.6) | 4 (13.0) | 2 (66.7) |
| Digestive bleeding | - | 2 (13.3) | 5 (29.4) | 2 (9.5) | 5 (19.3) | 6 (18.2) | 7 (20.5) | 7 (22.5) | - |
| Edema lung | - | - | 2 (11.8) | - | 1 (3.8) | 2 (6.1) | 2 (5.9) | 2 (6.5) | - |
| Multiorgan failure | 2 (100) | 12 (80.0) | 5 (29.4) | 17 (81.0) | 17 (65.4) | 19 (57.5) | 19 (56.0) | 18 (58) | 1(33.3) |
| Non-sudden death | 10 (71.4) | 24 (55.8) | 34 (59.7) | 41 (55.4) | 54 (54.5) | 57 (51.8) | 65 (53.3) | 60 (53.1) | 5 (55.6) |
| Non-liver related | 5 (50.0) | 15 (30.2) | 17 (31.6) | 21 (28.4) | 31(31.3) | 33 (30.0) | 37 (30.4) | 33 (29.1) | 4 (44.5) |
| Metastasis | - | 1 (6.7) | 5 (29.4) | 1 (4.7) | 4 (13.0) | 5 (15.2) | 6 (16.2) | 6 (18.1) | - |
| Neoplasia pharynx | - | - | 1 (5.9) | - | 1 (3.2) | 1 (3.0) | 1 (2.7) | 1 (3.0) | - |
| Pancreatitis | - | 1 (6.7) | - | 1 (4.7) | 1 (3.2) | 1 (3.0) | 1 (2.7) | 1 (3.0) | - |
| Pneumonia | - | 1 (6.7) | 2 (1.8) | 2 (9.6) | 3 (9.6) | 3 (9.1) | 3 (8.1) | 3 (9.1) | - |
| Sepsis | 5 (50.0) | 12 (80.0) | 9 (52.9) | 17 (81.0) | 22 (71.0) | 23 (69.7) | 26 (70.3) | 22 (66.8) | 4 (100) |
| Liver related | 5 (35.7) | 10 (25.6) | 16 (28.1) | 20 (27.0) | 23 (23.2) | 24 (21.8) | 28 (22.9) | 27 (24.0) | 1 (11.1) |
| Chronic rejection | - | - | 2 (16.7) | - | - | 1 (4.2) | 2 (7.1) | 2 (7.4) | - |
| Graft failure | 4 (80.0) | 9 (90.0) | 9 (75.0) | 18 (90.0) | 21 (91.4) | 21 (87.4) | 23 (82.0) | 22 (81.5) | 1 (100) |
| Primary dysfunction | 1 (20.0) | - | 1 (8.3) | 1 (5.0) | 1 (4.3) | 1 (4.2) | 2 (7.1) | 2 (7.4) | - |
| Viral relapse (HCV) | - | 1 (10.0) | - | 1 (5.0) | 1 (4.3) | 1 (4.2) | 1 (3.8) | 1 (3.7) | - |
| Unknown | 2 (14.3) | 4 (9.3) | 15 (10.5) | 12 (16.2) | 19 (19.2) | 20 (18.2) | 23 (18.9) | 22 (19.5) | 1 (11.1) |
| Pre-Transplant Complications | Post-Transplant Complications | |||||
|---|---|---|---|---|---|---|
| Main Causes Of Death N (%), (Mean Years ± SD) | Total a | Ascites + | Encephalopathy + | Viral Infections + b | AR | CR |
| Multiorgan failure, n (%) | 19 (15.6) 59.2 ± 2.1 c | 7/9 (77.8) 56.1 ± 4.2 | 5/9 (55.6) 62.6 ± 4.3 d | 5/19 (26.3) 57.8 ± 3.4 e | 5/19 (26.3) 59.8 ± 4.5 f | 0/19 (0) - |
| Sepsis, n (%) | 26 (21.3) 52.7 ± 1.5 | 8/11 (72.7) 56.3 ± 2.4 | 4/11 (36.4) 57.3 ± 1.4 | 5/26 (19.2) 53.0 ± 2.4 | 5/26 (19.2) 53.2 ± 4.3 | 4/26 (15.4) 49.0 ± 4.1 |
| Graft failure, n (%) | 23 (18.9) 54.0 ± 2.0 | 12/13 (92.3) 55.0 ± 2.6 | 6/14 (42.9) 57.0 ± 3.7 | 8/23(34.8) 50.9 ± 3.7 | 3/23 (13.0) 44.7 ± 5.8 | 2/23 (8.7) 45.5 ± 4.5 |
| Wald | p | OR | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 0.150 | 0.698 | 1.010 | 0.961 | 1.061 |
| Sex | 1.610 | 0.204 | 2.686 | 0.584 | 12.354 |
| Ascites | 5.880 | 0.015 | 4.487 | 1.334 | 15.099 |
| Encephalopathy | 2.632 | 0.105 | 2.007 | 0.865 | 4.658 |
| Viral infection (HCV/HBV) | 0.151 | 0.698 | 1.206 | 0.468 | 3.105 |
| Acute rejection | 1.368 | 0.242 | 1.822 | 0.667 | 4.977 |
| Chronic rejection | 4.329 | 0.037 | 0.111 | 0.014 | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolarín, J.M.; Pérez-Cárceles, M.D.; Hernández del Rincón, J.P.; Luna, A.; Minguela, A.; Muro, M.; Legaz, I. Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient’s Clinical Variables and Transplant Outcome Complications. Diagnostics 2021, 11, 968. https://doi.org/10.3390/diagnostics11060968
Bolarín JM, Pérez-Cárceles MD, Hernández del Rincón JP, Luna A, Minguela A, Muro M, Legaz I. Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient’s Clinical Variables and Transplant Outcome Complications. Diagnostics. 2021; 11(6):968. https://doi.org/10.3390/diagnostics11060968
Chicago/Turabian StyleBolarín, J. M., M. D. Pérez-Cárceles, J. P. Hernández del Rincón, A. Luna, A. Minguela, M. Muro, and I. Legaz. 2021. "Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient’s Clinical Variables and Transplant Outcome Complications" Diagnostics 11, no. 6: 968. https://doi.org/10.3390/diagnostics11060968
APA StyleBolarín, J. M., Pérez-Cárceles, M. D., Hernández del Rincón, J. P., Luna, A., Minguela, A., Muro, M., & Legaz, I. (2021). Causes of Death and Survival in Alcoholic Cirrhosis Patients Undergoing Liver Transplantation: Influence of the Patient’s Clinical Variables and Transplant Outcome Complications. Diagnostics, 11(6), 968. https://doi.org/10.3390/diagnostics11060968











