Deep Learning of Ultrasound Imaging for Evaluating Ambulatory Function of Individuals with Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
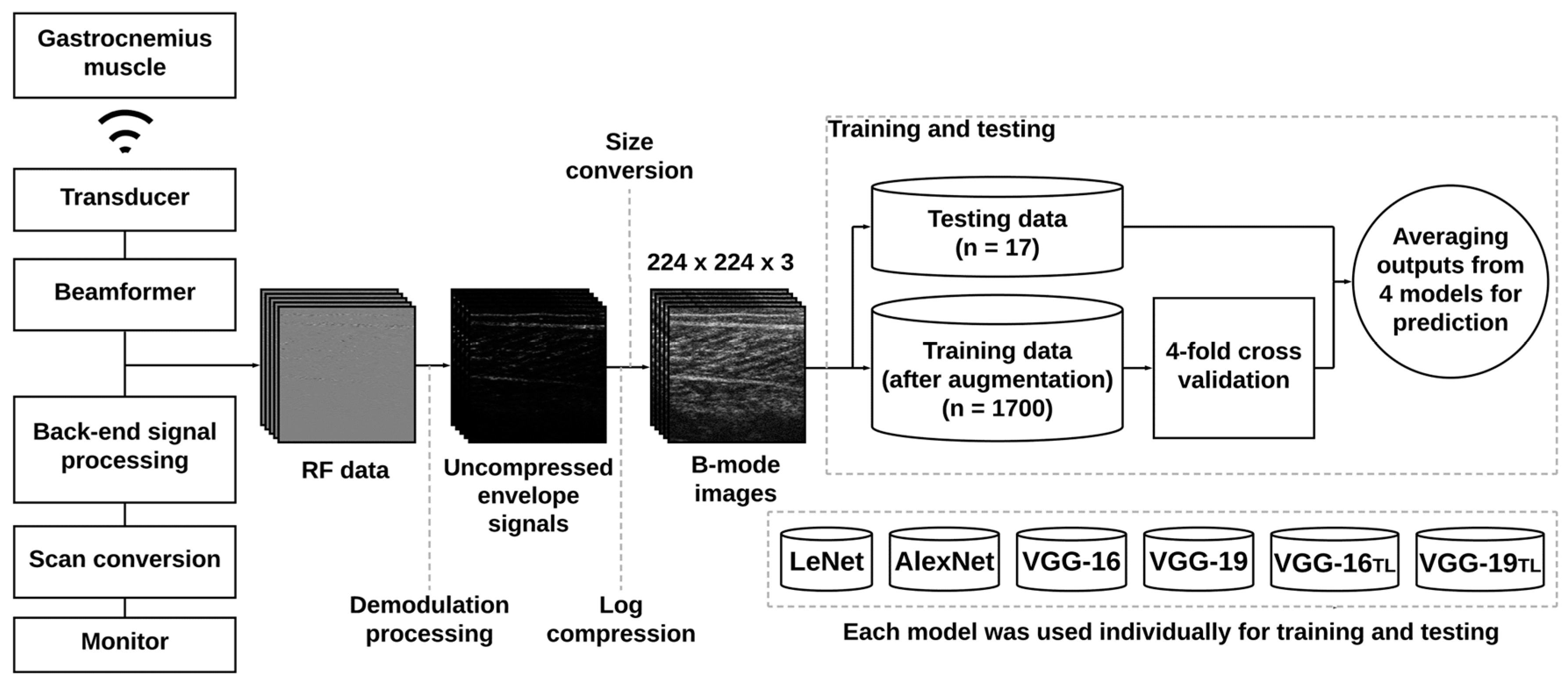
2.2. Ultrasound Data Acquisition
2.3. Data Augmentation
2.4. Deep Learning Approaches
2.5. Statistical Analysis
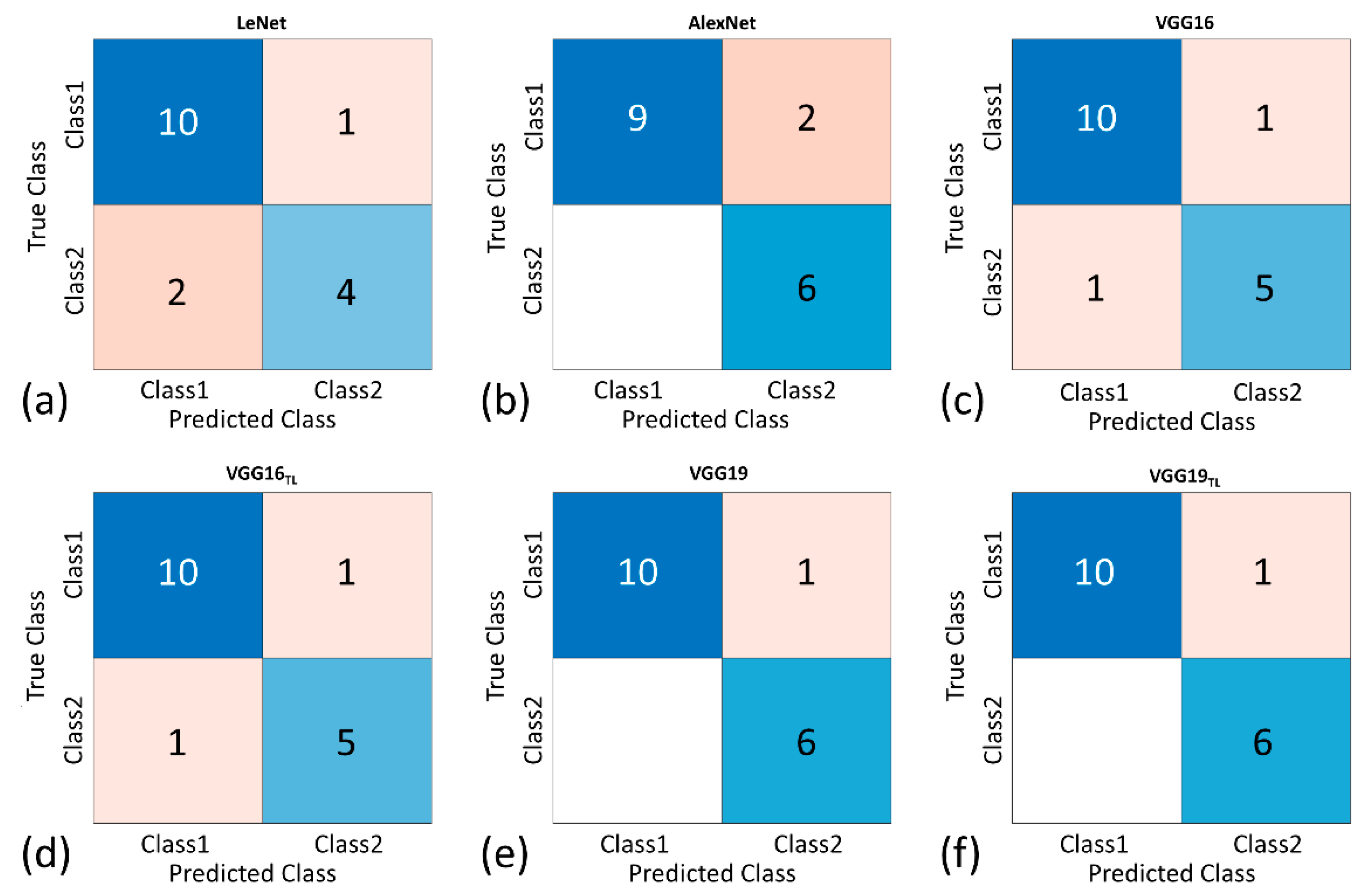
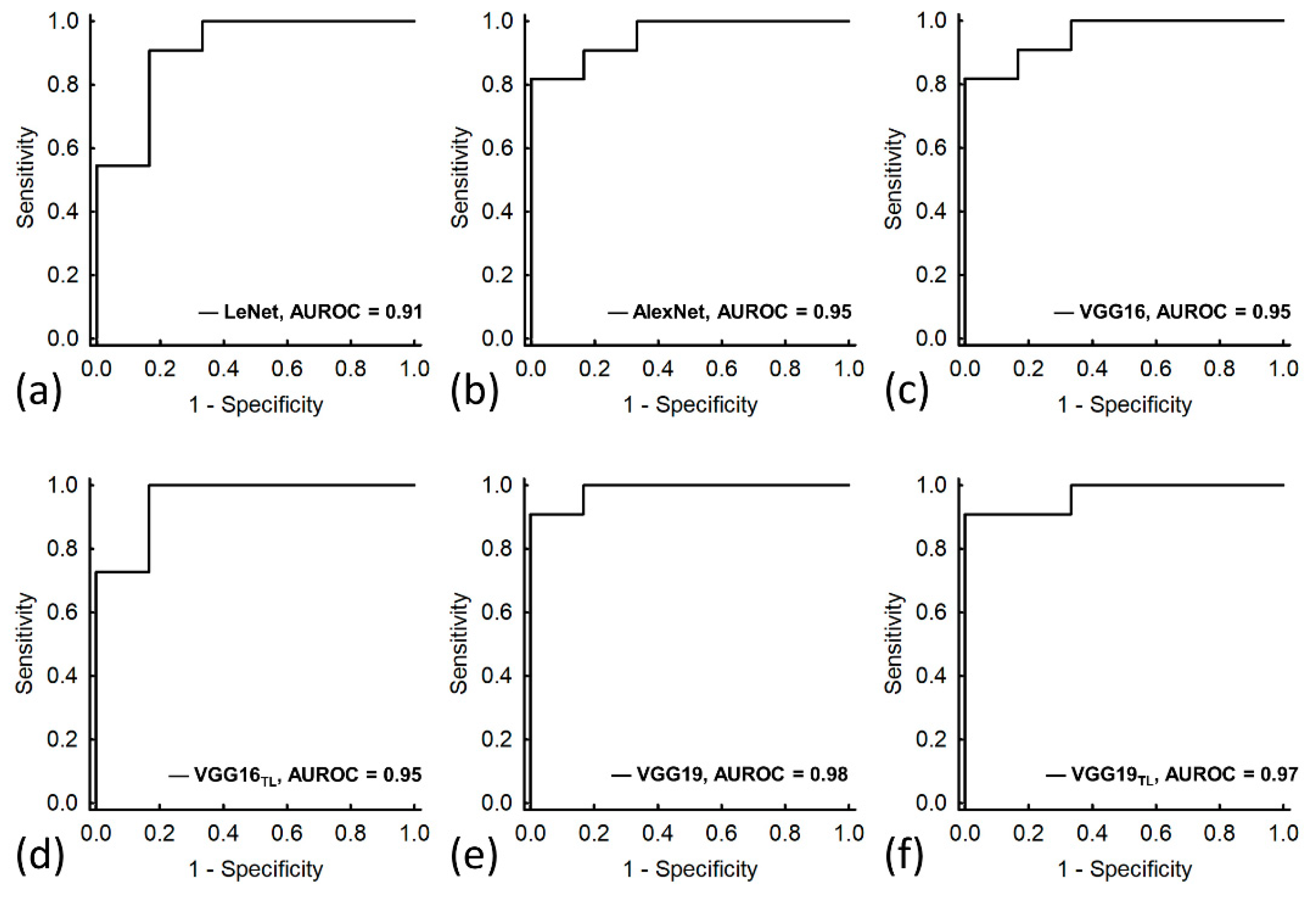
3. Results
4. Discussion
4.1. The Significance of This Study
4.2. Considerations on Ultrasound Evaluations of DMD
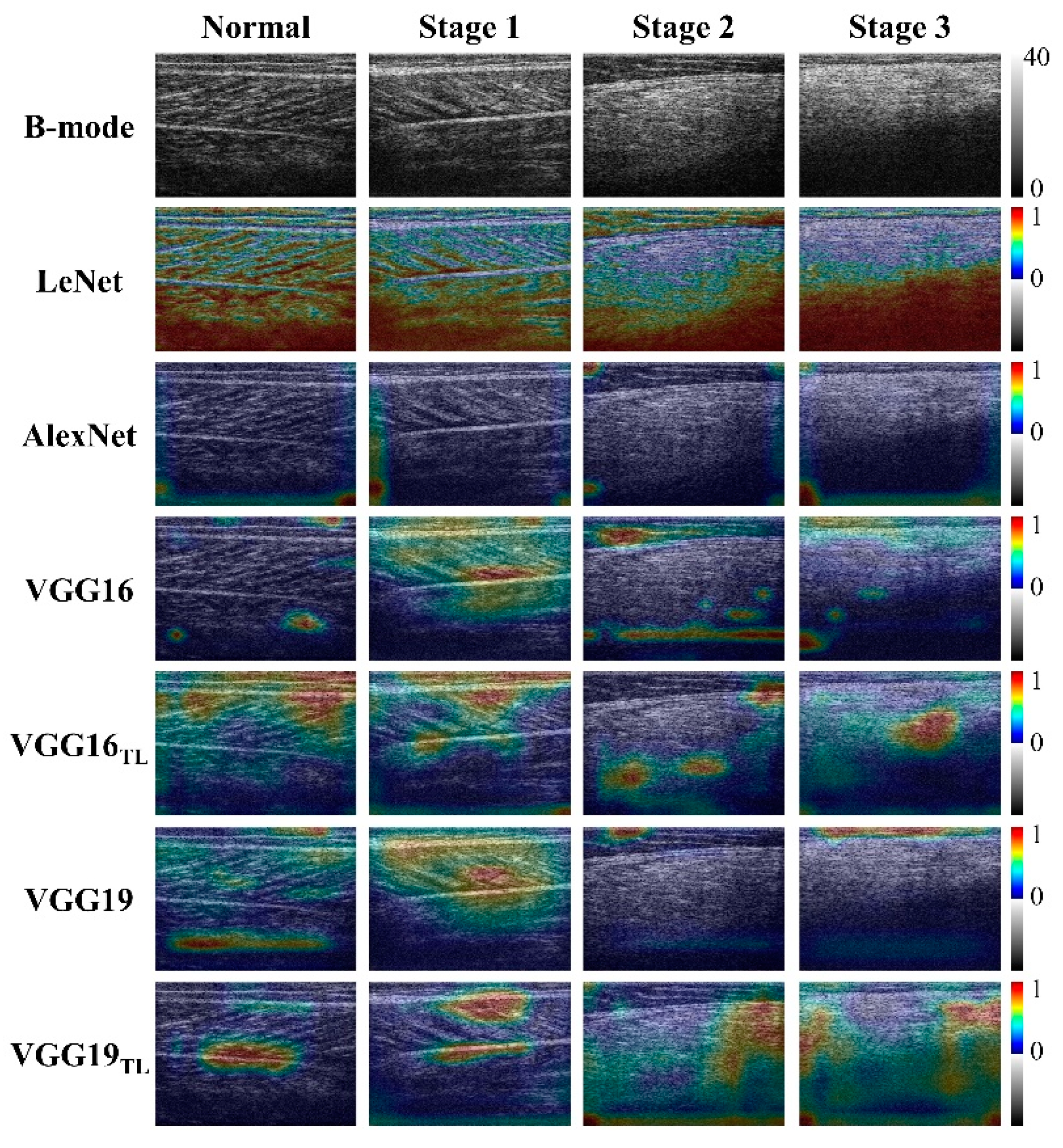
4.3. Physical Interpretations of Deep Learning in Ultrasound Imaging of DMD
4.4. Comparisons with the Proposed Models
4.5. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef]
- Mendell, J.R.; Lloyd-Puryear, M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013, 48, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Clarke, A.J.; Bradley, D.M. Developmental progress in Duchenne muscular dystrophy: Lessons for earlier detection. Eur. J. Paediatr. Neurol. 2004, 8, 145–153. [Google Scholar] [CrossRef]
- Verhaart, I.E.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef]
- Bach, J.R.; O’Brien, J.; Krotenberg, R.; Alba, A.S. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle Nerve 1987, 10, 177–182. [Google Scholar] [CrossRef]
- Guiraud, S.; Davies, K.E. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr. Opin. Pharmacol. 2017, 34, 36–48. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Han, J.J.; Abresch, R.T.; Nicorici, A.; Elfring, G.L.; Atkinson, L.; Reha, A.; Hirawat, S.; Miller, L.L. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2010, 41, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.S.; Messina, S.; Vasco, G.; Main, M.; Eagle, M.; D’Amico, A.; Doglio, L.; Politano, L.; Cavallaro, F.; Frosini, S.; et al. Reliability of the North star ambulatory assessment in a multicentric setting. Neuromuscul. Disord. 2009, 19, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Van Alfen, N.; Gijsbertse, K.; de Korte, C.L. How useful is muscle ultrasound in the diagnostic workup of neuromuscular diseases? Curr. Opin. Neurol. 2018, 31, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Mul, K.; Horlings, C.G.; Vincenten, S.C.; Voermans, N.C.; van Engelen, B.G.; van Alfen, N. Quantitative muscle MRI and ultrasound for facioscapulohumeral muscular dystrophy: Complementary imaging biomarkers. J. Neurol. 2018, 265, 2646–2655. [Google Scholar] [CrossRef]
- Shklyar, I.; Geisbush, T.R.; Mijialovic, A.S.; Pasternak, A.; Darras, B.T.; Wu, J.S.; Rutkove, S.B.; Zaidman, C.M. Quantitative muscle ultrasound in Duchenne muscular dystrophy: A comparison of techniques. Muscle Nerve 2015, 51, 207–213. [Google Scholar] [CrossRef]
- Jansen, M.; van Alfen, N.; van der Sanden, M.W.N.; van Dijk, J.P.; Pillen, S.; de Groot, I.J.M. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscul. Disord. 2012, 22, 306–317. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Connolly, A.M.; Malkus, E.C.; Florence, J.M.; Pestronk, A. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 2010, 20, 805–809. [Google Scholar] [CrossRef]
- Weng, W.C.; Lin, C.W.; Shen, H.C.; Chang, C.C.; Tsui, P.H. Instantaneous frequency as a new approach for evaluating the clinical severity of Duchenne muscular dystrophy through ultrasound imaging. Ultrasonics 2019, 94, 235–241. [Google Scholar] [CrossRef]
- Weng, W.C.; Tsui, P.H.; Lin, C.W.; Lu, C.H.; Lin, C.Y.; Shieh, J.Y.; Lu, F.L.; Ee, T.W.; Wu, K.W.; Lee, W.T. Evaluation of muscular changes by ultrasound Nakagami imaging in Duchenne muscular dystrophy. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Q.; Lin, C.W.; Shieh, J.Y.; Weng, W.C.; Tsui, P.H. Clinical evaluation of duchenne muscular dystrophy severity using ultrasound small-window entropy imaging. Entropy 2020, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; Boon, A.; Van Alfen, N. Muscle ultrasound. Handb. Clin. Neurol. 2016, 136, 843–853. [Google Scholar] [PubMed]
- Chen, J.R.; Chao, Y.P.; Tsai, Y.W.; Chan, H.J.; Wan, Y.L.; Tai, D.I.; Tsui, P.H. Clinical value of information entropy compared with deep learning for ultrasound grading of hepatic steatosis. Entropy 2020, 22, 1006. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, K.; Zhang, K. Current status and future trends of clinical diagnoses via image-based deep learning. Theranostics 2019, 9, 7556–7565. [Google Scholar] [CrossRef]
- Burlina, P.; Billings, S.; Joshi, N.; Albayda, J. Automated diagnosis of myositis from muscle ultrasound: Exploring the use of machine learning and deep learning methods. PLoS ONE 2017, 12, e0184059. [Google Scholar] [CrossRef]
- Shin, Y.; Yang, J.; Lee, Y.H.; Kim, S. Artificial intelligence in musculoskeletal ultrasound imaging. Ultrasonography 2021, 40, 30–44. [Google Scholar] [CrossRef]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Khan, S.; Yong, S.P. A Deep Learning Architecture for Classifying Medical Images of Anatomy Object. In Proceedings of the 2017 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA ASC), Kuala Lumpur, Malaysia, 12–15 December 2017. [Google Scholar]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sanchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-based Localization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, CD003725. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef]
- Bakker, J.P.; De Groot, I.J.; Beelen, A.; Lankhorst, G.J. Predictive factors of cessation of ambulation in patients with Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 2002, 81, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Kinali, M.; Arechavala-Gomeza, V.; Cirak, S.; Glover, A.; Guglieri, M.; Feng, L.; Hollingsworth, K.G.; Hunt, D.; Jungbluth, H.; Roper, H.P.; et al. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 2011, 76, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Riaz, H.; Park, J.; Choi, H.; Kim, H.; Kim, J. Deep and densely connected networks for classification of diabetic retinopathy. Diagnostics 2020, 10, 24. [Google Scholar] [CrossRef]
- Castelvecchi, D. Can we open the black box of AI? Nat. News 2016, 538, 20–23. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.Y.; Verrijp, K.N.; Arts, I.M.P.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.M.; Verrips, A. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Brockmann, K.; Becker, P.; Schreiber, G.; Neubert, K.; Brunner, E.; Bönnemann, C. Sensitivity and specificity of qualitative muscle ultrasound in assessment of suspected neuromuscular disease in childhood. Neuromuscul. Disord. 2007, 17, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Sandford, N.L.; Walsh, P.; Matis, C.; Baddeley, H.; Powell, L.W. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985, 89, 186–191. [Google Scholar] [CrossRef]
- Joseph, A.E.A.; Saverymuttu, S.H. Ultrasound in the assessment of diffuse parenchymal liver disease. Clin. Radiol. 1991, 44, 219–221. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep learning in medical ultrasound analysis: A review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Zhong, G.; Ling, X.; Wang, L.N. From shallow feature learning to deep learning: Benefits from the width and depth of deep architectures. WIREs Data Min. Knowl. Discov. 2018, 9, e1255. [Google Scholar] [CrossRef]
- Bressem, K.K.; Adams, L.C.; Erxleben, C.; Hamm, B.; Niehues, S.M.; Vahldiek, J.L. Comparing different deep learning architectures for classification of chest radiographs. Sci. Rep. 2020, 10, 13590. [Google Scholar] [CrossRef] [PubMed]
| Stage | Clinical Symptoms | Age (Years) (Range) | Number of Subjects |
|---|---|---|---|
| Normal | No weakness: No neuromuscular disorders or weakness | 10.75 ± 4.59 (3–18) | 12 |
| Stage 1 | Presymptomatic: Subtle symptoms of delayed walking or delayed speech (but often unnoticed). Early ambulatory: Showing a Gowers’ sign (patients need to support themselves with hands to get up from the floor), waddling type walking (gait), and walking on their toes. Late ambulatory: Walking becomes increasingly difficult (labored gait) and climbing stairs and getting up from the floor are more problematic. | 7.92 ± 2.33 (2–13) | 41 |
| Stage 2 | Early non-ambulatory: Patients start to need to use a wheelchair; they may be able to wheel the chair themselves and typically their postures can be maintained even scoliosis is possible. | 12.68 ± 2.05 (9–16) | 20 |
| Stage 3 | Late non-ambulatory: Upper limb function and maintenance of good posture are increasingly difficult, and complications are more likely. | 17.08 ± 2.90 (13–24) | 12 |
| Subjects | Number of Subjects | Number of Subjects (Training, Test) | Amount of Training Data (after Augmentation) |
|---|---|---|---|
| Normal control | 12 | (10, 2) | 250 |
| Stage 1 | 41 | (32, 9) | 800 |
| Stage 2 | 20 | (16, 4) | 400 |
| Stage 3 | 12 | (10, 2) | 250 |
| Model | LeNet | AlexNet | VGG-16 | VGG-16TL | VGG-19 | VGG-19TL |
|---|---|---|---|---|---|---|
| Accuracy, % | 82.35 | 88.24 | 88.24 | 88.24 | 94.18 | 94.18 |
| Precision, % | 80.00 | 75.00 | 83.33 | 83.33 | 85.71 | 85.71 |
| Sensitivity, % | 66.67 | 100.00 | 78.82 | 78.82 | 100.00 | 100.00 |
| Specificity, % | 90.91 | 81.82 | 90.91 | 90.91 | 90.91 | 90.91 |
| F1-score | 0.73 | 0.86 | 0.83 | 0.83 | 0.92 | 0.92 |
| AUROC (95% CI) | 0.91 (0.75–1.00) | 0.95 (0.87–1.00) | 0.95 (0.87–1.00) | 0.95 (0.85–1.00) | 0.98 (0.94–1.00) | 0.97 (0.90–1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, A.-H.; Chen, J.-R.; Liu, S.-H.; Lu, C.-H.; Lin, C.-W.; Shieh, J.-Y.; Weng, W.-C.; Tsui, P.-H. Deep Learning of Ultrasound Imaging for Evaluating Ambulatory Function of Individuals with Duchenne Muscular Dystrophy. Diagnostics 2021, 11, 963. https://doi.org/10.3390/diagnostics11060963
Liao A-H, Chen J-R, Liu S-H, Lu C-H, Lin C-W, Shieh J-Y, Weng W-C, Tsui P-H. Deep Learning of Ultrasound Imaging for Evaluating Ambulatory Function of Individuals with Duchenne Muscular Dystrophy. Diagnostics. 2021; 11(6):963. https://doi.org/10.3390/diagnostics11060963
Chicago/Turabian StyleLiao, Ai-Ho, Jheng-Ru Chen, Shi-Hong Liu, Chun-Hao Lu, Chia-Wei Lin, Jeng-Yi Shieh, Wen-Chin Weng, and Po-Hsiang Tsui. 2021. "Deep Learning of Ultrasound Imaging for Evaluating Ambulatory Function of Individuals with Duchenne Muscular Dystrophy" Diagnostics 11, no. 6: 963. https://doi.org/10.3390/diagnostics11060963
APA StyleLiao, A.-H., Chen, J.-R., Liu, S.-H., Lu, C.-H., Lin, C.-W., Shieh, J.-Y., Weng, W.-C., & Tsui, P.-H. (2021). Deep Learning of Ultrasound Imaging for Evaluating Ambulatory Function of Individuals with Duchenne Muscular Dystrophy. Diagnostics, 11(6), 963. https://doi.org/10.3390/diagnostics11060963







