Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How?
Abstract
:1. Introduction
2. Why?
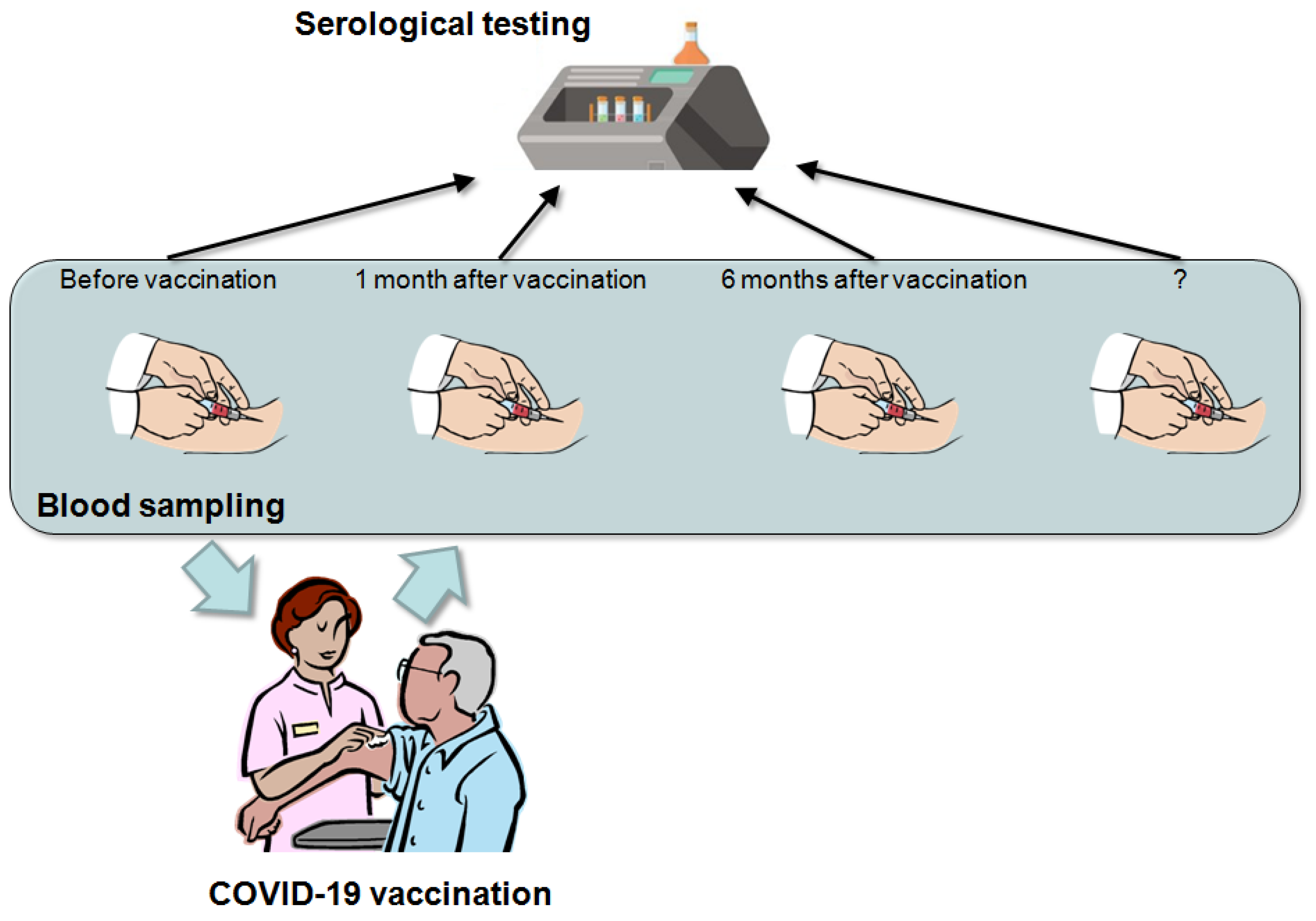
3. When?
4. How?
5. Potential Drawbacks
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johns Hopkins University. Coronavirus Resource Center. Global Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 October 2020).
- Pradhan, D.; Biswasroy, P.; Naik, P.K.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S. The story behind COVID-19 vaccines. Science 2021, 372, 109. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19: Unravelling the clinical progression of nature’s virtually perfect biological weapon. Ann. Transl. Med. 2020, 8, 693. [Google Scholar] [CrossRef]
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Topol, E.J. Messenger RNA vaccines against SARS-CoV-2. Cell 2021, 184, 1401. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; de Oliveira, T. New SARS-CoV-2 Variants—Clinical, Public Health, and Vaccine Implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Lippi, G.; Sciacovelli, L.; Trenti, T.; Plebani, M. Kinetics and biological characteristics of humoral response developing after SARS-CoV-2 infection: Implications for vaccination. Clin. Chem. Lab. Med. 2021. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Cornelius, A.R.; Wilson, J.P.; Karmacharya, T.; Gray-Gaillard, S.L.; Allen, J.R.; Hyman, S.W.; Moritz, G.; Ali, M.; Koralov, S.B.; et al. Poor antigen-specific responses to the second BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. medRxiv 2021. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Padoan, A.; Dall’Olmo, L.; Della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Syangtan, G.; Bista, S.; Dawadi, P.; Rayamajhee, B.; Shrestha, L.B.; Tuladhar, R.; Joshi, D.R. Asymptomatic SARS-CoV-2 Carriers: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 8, 587374. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, B. What the data say about asymptomatic COVID infections. Nat. Cell Biol. 2020, 587, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Baj, A.; Maggi, F. Is a single COVID-19 vaccine dose enough in convalescents? Hum. Vaccines Immunother. 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, S.; Warrener, L.A.; Hoschler, K.; Perry, K.R.; Shute, J.; Whitaker, H.; O’Brien, M.; Baawuah, F.; Moss, P.; Parry, H.; et al. Robust antibody responses in 70–80-year-olds 3 weeks after the first or second doses of Pfizer/BioNTech COVID-19 vaccine, United Kingdom, January to February. Euro Surveill. 2021, 26, 2100329. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Bion-tech/Pfizer BNT162b2 COVID-19 vaccination. MedRxiv 2021. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Obesity may hamper SARS-CoV-2 vaccine immuno-genicity. medRxiv 2021. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. Coronavirus disease 2019 (COVID-19): The portrait of a perfect storm. Ann. Transl. Med. 2020, 8, 497. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lavie, C.J.; Mehra, M.R.; Henry, B.M.; Lippi, G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin. Proc. 2020, 95, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; Liebeskind, M.J.; et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. medRxiv 2021. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021. [Google Scholar] [CrossRef]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Villain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation 2021. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021. [Google Scholar] [CrossRef]
- Ben Zadok, O.I.; Shaul, A.A.; Ben-Avraham, B.; Yaari, V.; Ben Zvi, H.; Shostak, Y.; Pertzov, B.; Eliakim-Raz, N.; Abed, G.; Abuhazira, M.; et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients—A prospective cohort study. Eur. J. Heart Fail. 2021. [Google Scholar] [CrossRef]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sümbül, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021. [Google Scholar] [CrossRef]
- Palich, R.; Veyri, M.; Marot, S.; Vozy, A.; Gligorov, J.; Maingon, P.; Marcelin, A.-G.; Spano, J.-P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. 2021. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Barrio, I.D.M.D.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Moshiashvili, M.M.; Ziv-Baran, T.; Shorer, Y.; Scarfo, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Walensky, R.P.; Walke, H.T.; Fauci, A.S. SARS-CoV-2 Variants of Concern in the United States-Challenges and Opportunities. JAMA 2021, 325, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: Where are we on vaccines and variants? BMJ 2021, 372, n597. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. How will emerging SARS-CoV-2 variants impact herd immunity? Ann. Transl. Med. 2021, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Arora, P.; Groß, R.; Seidel, A.; Hörnich, B.F.; Hahn, A.S.; Krüger, N.; Graichen, L.; Hofmann-Winkler, H.; Kempf, A.; et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021, 2393. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Zhou, D.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.; Tuekprakhon, A.; Nutalai, R.; et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 2021. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef]
- Ikegame, S.; Siddiquey, M.; Hung, C.T.; Haas, G.; Brambilla, L.; Oguntuyo, K.; Kowdle, S.; Vilardo, A.; Edelstein, A.; Perandones, C.; et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef] [PubMed]
- Ketas, T.J.; Chaturbhuj, D.; Portillo, V.C.; Francomano, E.; Golden, E.; Chandrasekhar, S.; Debnath, G.; Tapia, R.D.; Yasmeen, A.; Leconet, W.; et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Booster dose will be needed in autumn to avoid winter surge, says government adviser. BMJ 2021, 372, n664. [Google Scholar] [CrossRef]
- Voss, W.N.; Hou, Y.J.; Johnson, N.V.; Delidakis, G.; Kim, J.E.; Javanmardi, K.; Horton, A.P.; Bartzoka, F.; Paresi, C.J.; Tanno, Y.; et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science 2021, eabg5268. [Google Scholar] [CrossRef]
- Bohn, M.K.; Loh, T.P.; Wang, C.B.; Mueller, R.; Koch, D.; Sethi, S.; Rawlinson, W.D.; Clementi, M.; Erasmus, R.; Leportier, M.; et al. IFCC Interim Guidelines on Serological Testing of Antibodies against SARS-CoV-2. Clin. Chem. Lab. Med. 2020, 58, 2001–2008. [Google Scholar] [CrossRef]
- Trenti, T.; Pecoraro, V.; Pirotti, T.; Plebani, M. IgM anti-SARS-CoV-2-specific determination: Useful or confusing? Big Data analysis of a real-life scenario. Intern. Emerg. Med. 2021, 1–4. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA Covid-19 vaccination: A three-case series. Clin. Chem. Lab. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. Clinical value of anti-SARS-COV-2 serum IgA titration in patients with COVID-19. J. Med. Virol. 2021, 93, 1210–1211. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. SARS-CoV-2 antibodies titration: A reappraisal. Ann. Transl. Med. 2020, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Gelati, M.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comparison of five commercial anti-SARS-CoV-2 total antibodies and IgG immunoassays after vaccination with BNT162b2 mRNA. J. Med. Biochem. 2021. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Plebani, M. Potential drawbacks of pharmacy-based COVID-19 testing. J. Lab. Precis. Med. 2021, 6, 10. [Google Scholar] [CrossRef]
- Stephens, D.S.; McElrath, M.J. COVID-19 and the Path to Immunity. JAMA 2020, 324, 1279. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; Wang, K.; Röltgen, K.; Nielsen, S.; Roach, J.; Naccache, S.; Yang, F.; Wirz, O.; Yost, K.; Lee, J.; et al. Reinfection with SARS-CoV-2 and Failure of Humoral Immunity: A case report. medRxiv 2020. [Google Scholar] [CrossRef]
- Gómez, C.E.; Perdiguero, B.; Esteban, M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines 2021, 9, 243. [Google Scholar] [CrossRef]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Mazzoni, A.; Di Lauria, N.; Maggi, L.; Salvati, L.; Vanni, A.; Capone, M.; Lamacchia, G.; Mantengoli, E.; Spinicci, M.; Zammarchi, L.; et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in recovered COVID-19 subjects. J. Clin. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and clinical performances of five immu-noassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef] [PubMed]
- Freed, G.L. Actionable lessons for the US COVID vaccine program. Isr. J. Health Policy Res. 2021, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sim, F. Early Covid-19 vaccination rollout: A commentary from England. Isr. J. Health Policy Res. 2021, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Wang, Y.; Zeng, X.; Luo, T.; Liu, Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250602. [Google Scholar] [CrossRef]
- Ledford, H. International COVID-19 trial to restart with focus on immune responses. Nature 2021. [Google Scholar] [CrossRef]
| Variants of Concern | B.1.1.7 | B.1.351 | P.1 | B.1.617 | B.1.427/B.1.429 |
|---|---|---|---|---|---|
| Original emergence | U.K. | South Africa | Brazil | India | USA (California) |
| Decay of neutralization potential * | ~1.7-fold | ~7.9-fold | ~5.0-fold | ~6.8-fold | ~3.6-fold |
|
|
|
|
|
|
|
|
|
|
|
| Advantages | Drawbacks |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, G.; Henry, B.M.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. https://doi.org/10.3390/diagnostics11060941
Lippi G, Henry BM, Plebani M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics. 2021; 11(6):941. https://doi.org/10.3390/diagnostics11060941
Chicago/Turabian StyleLippi, Giuseppe, Brandon Michael Henry, and Mario Plebani. 2021. "Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How?" Diagnostics 11, no. 6: 941. https://doi.org/10.3390/diagnostics11060941
APA StyleLippi, G., Henry, B. M., & Plebani, M. (2021). Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics, 11(6), 941. https://doi.org/10.3390/diagnostics11060941








