Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Pneumatically Driven Measuring System
2.3. Setting and Procedure
- (a)
- Pre-MVIC series
- (b)
- MVICpri-con series (performed at first in n = 7)
- (c)
- AF series (performed at first in n = 6)
- (d)
- Post-MVIC series
2.4. Data Processing
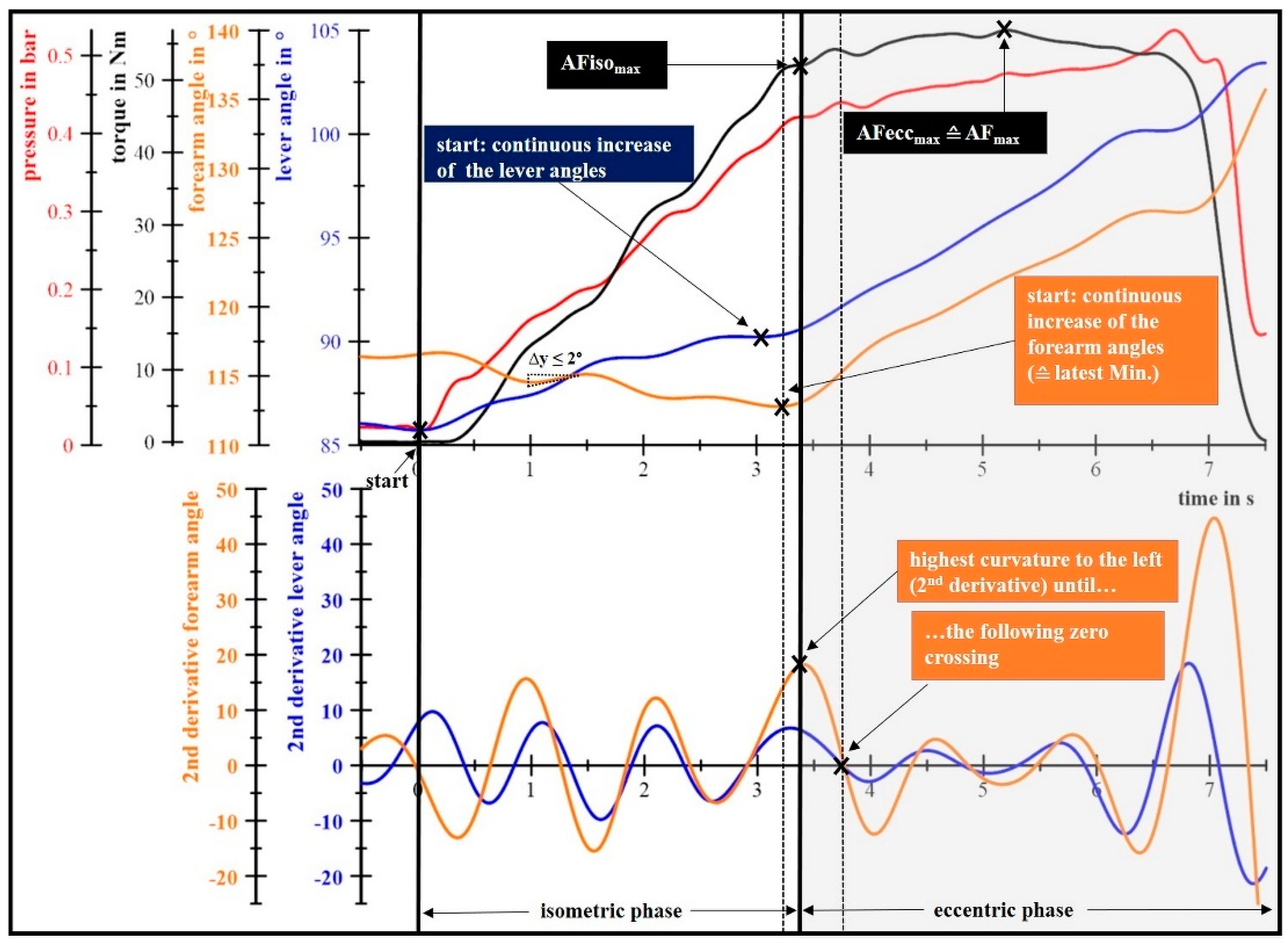
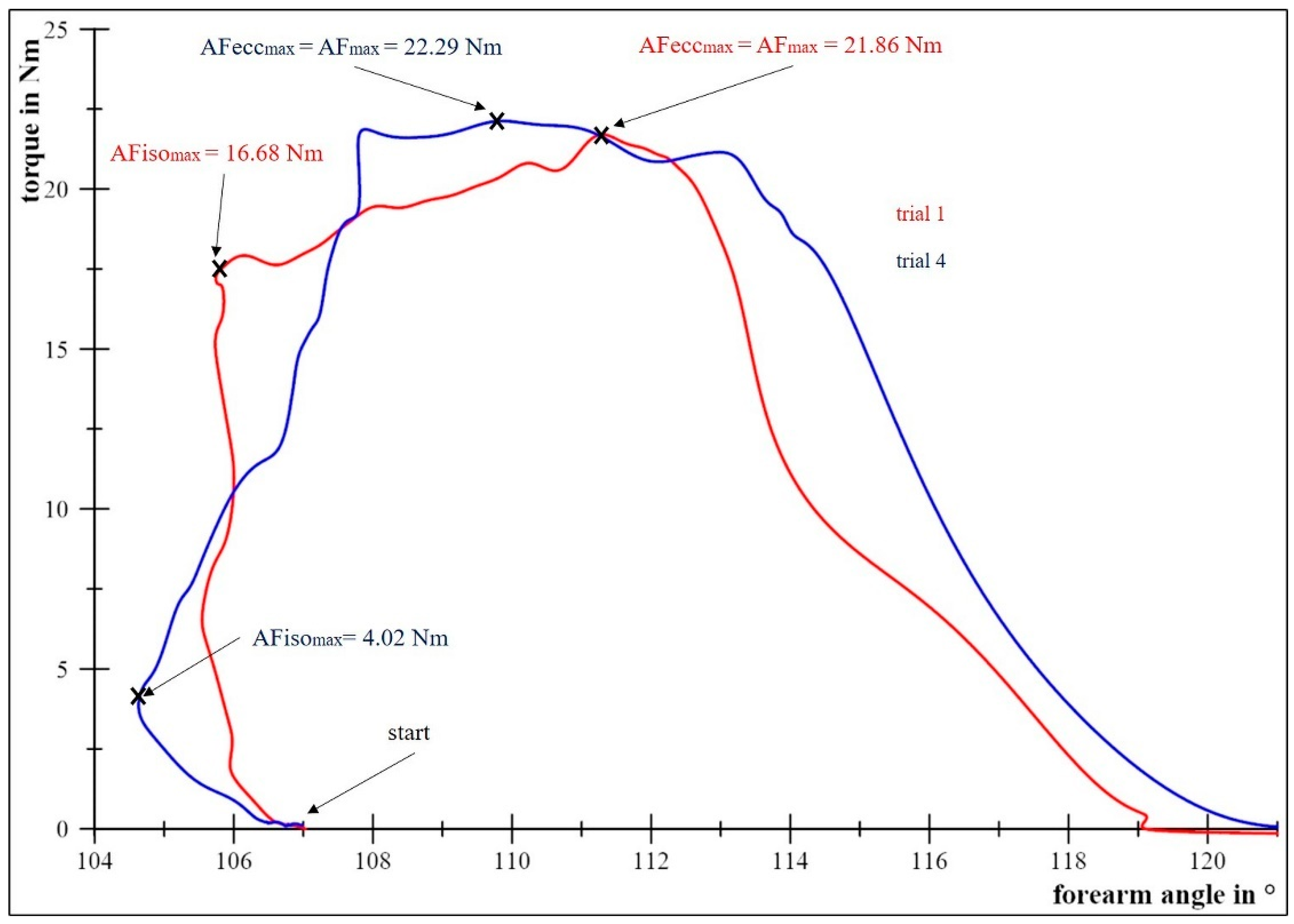
2.4.1. Determination of the Maximal Voluntary Isometric Contractions and the Maximal Adaptive Force
2.4.2. Determination of the Maximal Isometric Adaptive Force
2.4.3. Determination of the Maximal Eccentric Adaptive Force
2.4.4. Calculation of Mean and Maximal Torques and Elapsed Times
2.5. Statistical Analyses
2.5.1. Reliability
2.5.2. Discriminant Validity of the Maximal Isometric Adaptive Force
2.5.3. Analyses Regarding the MVIC with a Prior Concentric Contraction and the Post-MVIC
3. Results
3.1. Gender Comparison
3.2. Description of AF-Measurements
3.3. Test–Retest Reliability
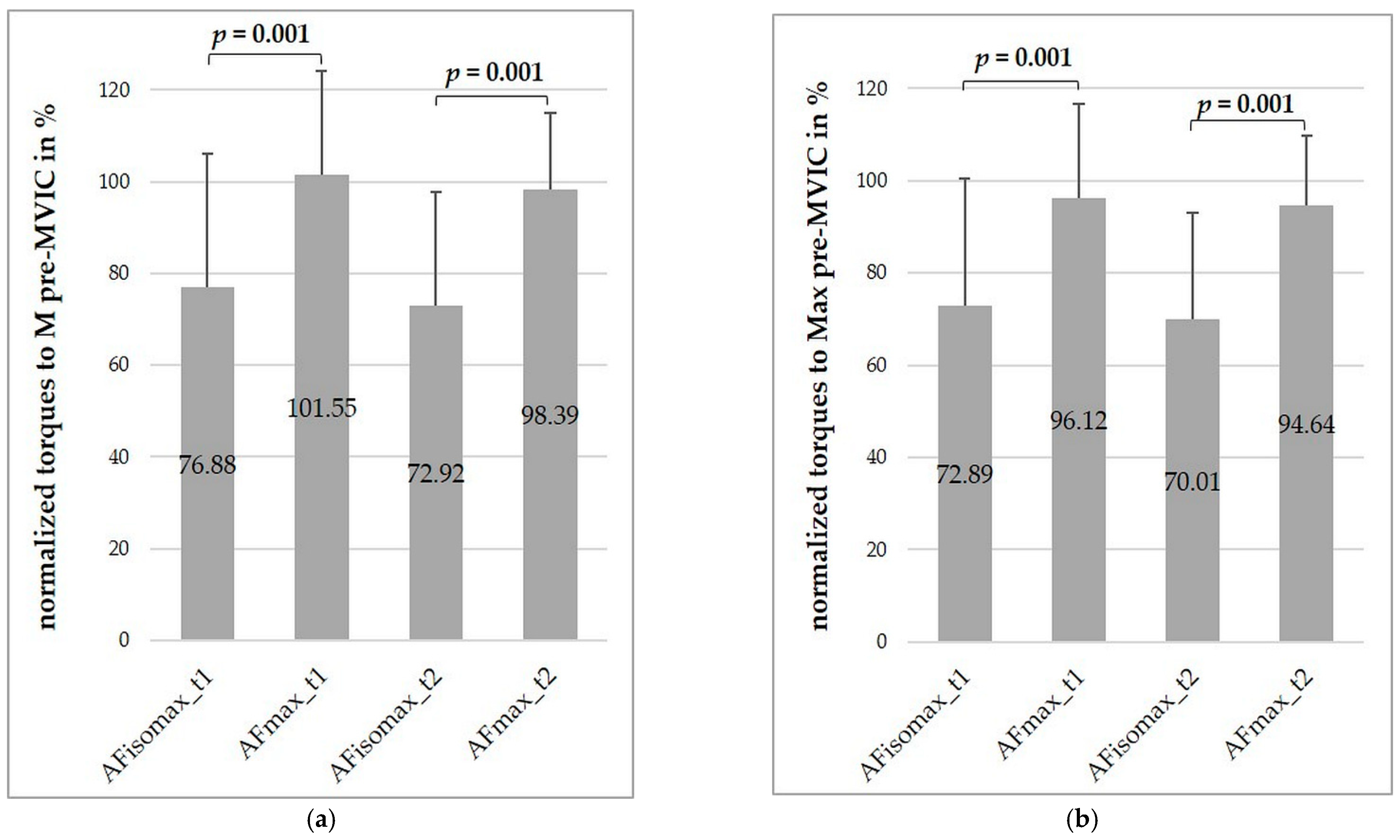
3.4. Comparisons of Force Types
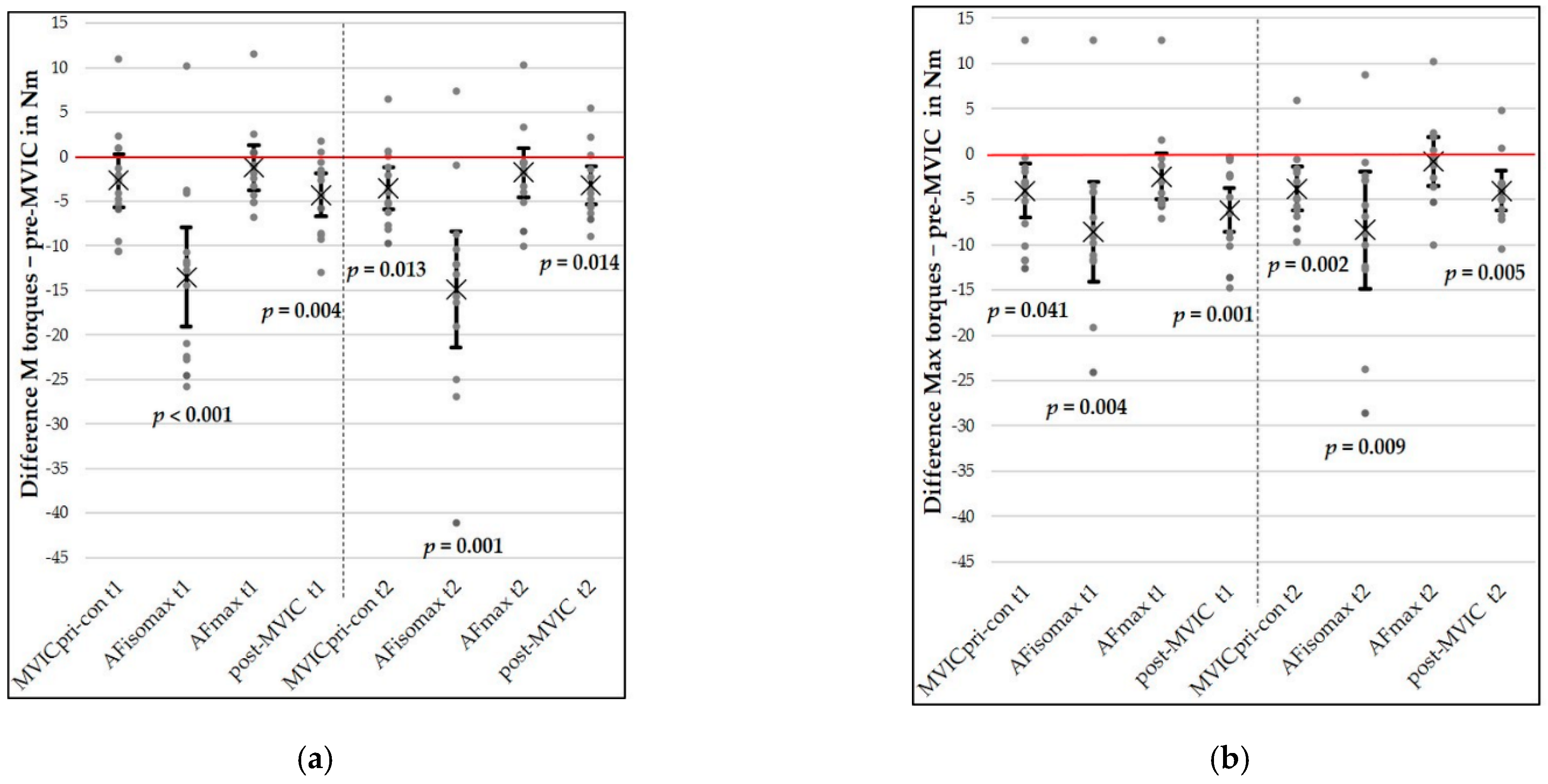
3.4.1. Comparison between AF-Parameters and the Maximal Voluntary Isometric Contraction
3.4.2. Comparisons between Measurement Series Including a Maximal Voluntary Isometric Contraction
4. Discussion
4.1. Reliability of the Measured Forces
4.2. Advantages and Limitations of the New AF-Measurement Procedure
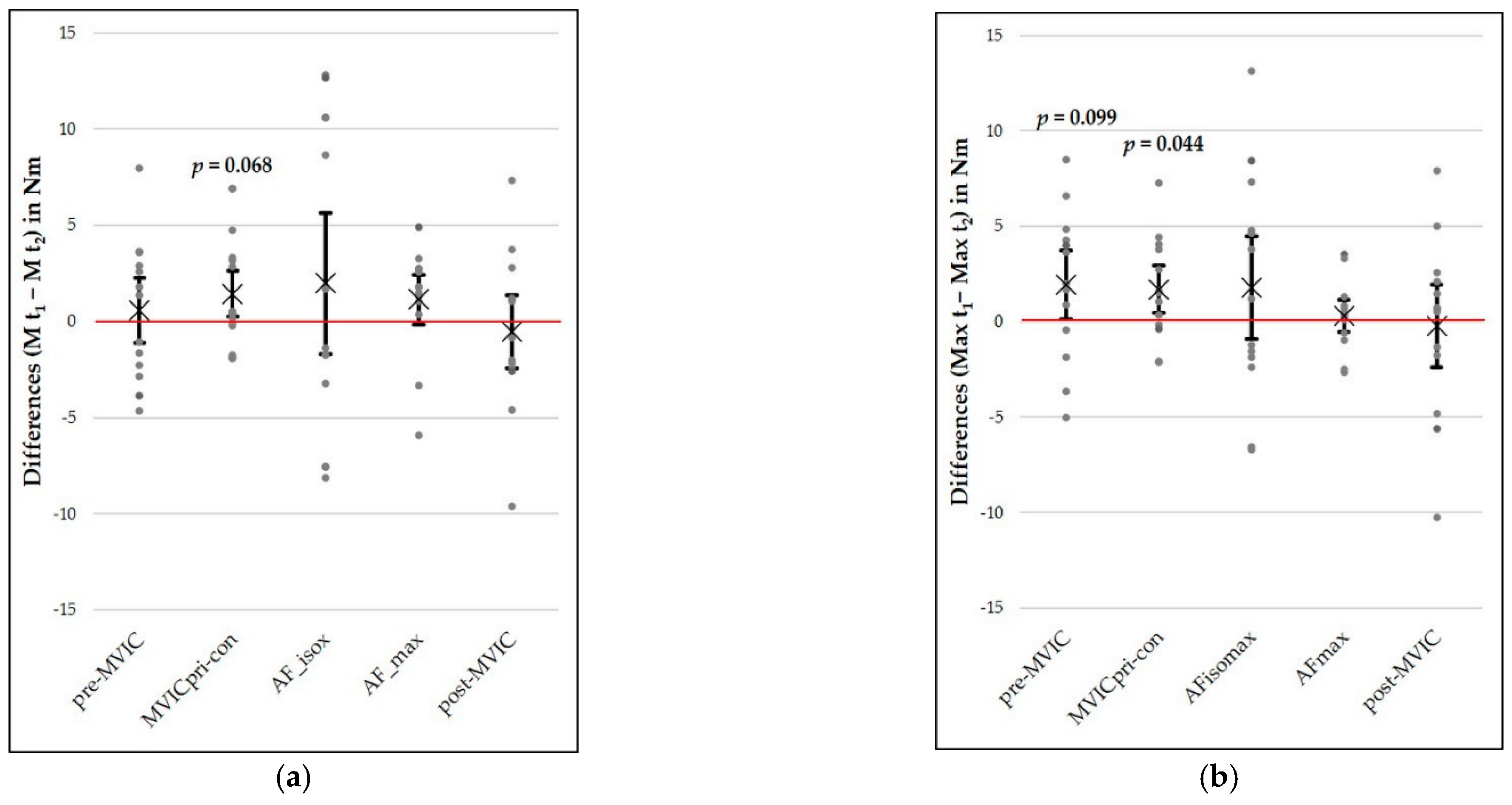
4.3. Influence of a Concentric Muscle Action Prior to a Maximal Voluntary Isometric Contraction
4.4. Comparison of MVICs at the Beginning and at the End of Each Measurement Session
4.5. Integration of the Adaptive Force in Current Concepts of Strength
4.6. Specialty of the Maximal Isometric Adaptive Force
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Adaptive Force |
| AFeccmax | maximal eccentric AF |
| AFisomax | maximal isometric AF |
| AFmax | maximal AF |
| CV | coefficient of variation |
| HIMA | holding isometric muscle action |
| M | mean |
| Max | maximum |
| MD | mean difference |
| MDC95% | minimal detectable change covering 95% of repeated measurements |
| MVIC | maximal voluntary isometric contraction |
| pre-MVIC | MVIC at the beginning of the measurement series |
| post-MVIC | MVIC at the end of the measurement series |
| MVICpri-con | MVIC with a prior concentric contraction |
| NI | National Instruments |
| PIMA | pushing isometric muscle action |
| SD | standard deviation |
| SDMD | standard deviation of mean differences |
| SEM | standard error of measurements (random error) |
| SEM% | random percentage error |
| SEM95% | random percentage error covering 95% of repeated measurements |
| t1 | measuring session at day 1 |
| t2 | measuring session at day 2 |
References
- Verkhoshansky, Y.; Siff, M.C. Supertraining, 6th ed.; Ultimate Athlete Concepts: Rome, Italy, 2009; ISBN 978-88-904038-0-4. [Google Scholar]
- Hoff, M.; Schaefer, L.; Heinke, N.; Bittmann, F. Report on adaptive force, a specific neuromuscular function. Eur. J. Transl. Myol. 2015, 25, 183–189. [Google Scholar] [CrossRef]
- Schaefer, L.; Hoff, M.; Bittmann, F. Measuring system and method of determining the adaptive force. Eur. J. Transl. Myol. 2017, 27. [Google Scholar] [CrossRef]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual muscle testing—Force Profiles and their reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Muscular pre-activation can boost the maximal explosive eccentric adaptive force. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Rudroff, T.; Barry, B.K.; Stone, A.L.; Barry, C.J.; Enoka, R.M. Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J. Appl. Physiol. 2007, 102, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Justice, J.N.; Holmes, M.R.; Matthews, S.D.; Enoka, R.M. Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J. Appl. Physiol. 2010, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.C.; Blackburn, T.; Weimar, W.; Campbell, B. Comparison of electromyographic activity during eccentrically versus concentrically loaded isometric contractions. J. Electromyogr. Kinesiol. 2008, 18, 466–471. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Are there two forms of isometric muscle action? Results of the experimental study support a distinction between a holding and a pushing isometric muscle function. BMC Sports Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Bittmann, F. Mechanotendography in achillodynia shows reduced oscillation variability of pre-loaded Achilles tendon: A pilot study. Eur. J. Transl. Myol. 2020, 30, 247–257. [Google Scholar] [CrossRef]
- Bittmann, F.; Hoff, M.; Knöchel, M.; Heinke, N.; Stolle, A. Adaptive kraft bei epikondylopathie humeri lateralis. Dtsch. Z. Sportmed. 2011, 7–8, 246. [Google Scholar]
- Oranchuk, D.J.; Nelson, A.R.; Storey, A.G.; Diewald, S.N.; Cronin, J.B. Short-term neuromuscular, morphological, and architectural responses to eccentric quasi-isometric muscle actions. Eur. J. Appl. Physiol. 2021, 121, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N. A web-based sample size calculator for reliability studies. EIMJ 2018, 10, 67–76. [Google Scholar] [CrossRef]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef]
- Meldrum, D.; Cahalane, E.; Keogan, F.; Hardiman, O. Maximum voluntary isometric contraction: Investigation of reliability and learning effect. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003, 4, 36–44. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Laurence Erlbaum Associates Inc.: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roebroeck, M.E.; Harlaar, J.; Lankhorst, G.J. The application of generalizability theory to reliability assessment: An illustration using isometric force measurements. Phys. Ther. 1993, 73, 386–395. [Google Scholar] [CrossRef]
- Eliasziw, M.; Young, S.L.; Woodbury, M.G.; Fryday-Field, K. Statistical methodology for the concurrent assessment of interrater and intrarater reliability: Using goniometric measurements as an example. Phys. Ther. 1994, 74, 777–788. [Google Scholar] [CrossRef]
- Stratford, P.W.; Goldsmith, C.H. Use of the standard error as a reliability index of interest: An applied example using elbow flexor strength data. Phys. Ther. 1997, 77, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.W.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kropmans, T.J.B.; Dijkstra, P.U.; Stegenga, B.; Stewart, R.; de Bont, L.G.M. Smallest detectable difference in outcome variables related to painful restriction of the temporomandibular joint. J. Dent. Res. 1999, 78, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Nevill, A.M.; Atkinson, G. Assessing agreement between measurements recorded on a ratio scale in sports medicine and sports science. Br. J. Sports Med. 1997, 31, 314–318. [Google Scholar] [CrossRef]
- Bland, J.; Altman, D. Statistics notes: Measurement error proportional to the mean. BMJ 1996, 313, 106. [Google Scholar] [CrossRef]
- Stokes, M. Reliability and repeatability of methods for measuring muscle in physiotherapy. Physiother. Pract. 1985, 1, 71–76. [Google Scholar] [CrossRef]
- Ashley, C.D.; Weiss, L.W. Vertical jump performance and selected physiological characteristics of women. J. Strength Cond. Res. 1994, 8, 5–11. [Google Scholar]
- Brady, C.; Harrison, A.; Comyns, T. A review of the reliability of biomechanical variables produced during the isometric mid-thigh pull and isometric squat and the reporting of normative data. Sports Biomech. 2018, 19, 1–25. [Google Scholar] [CrossRef]
- Zaki, R.; Bulgiba, A.; Nordin, N.; Ismail, N.A. A systematic review of statistical methods used to test for reliability of medical instruments measuring continuous variables. Iran. J. Basic Med. Sci. 2013, 16, 803–807. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Chapman, D.; Newton, M.; Nosaka, K. Eccentric torque-velocity relationship of the elbow flexors. Isokinet. Exerc. Sci. 2005, 13, 139–145. [Google Scholar] [CrossRef]
- Dartnall, T.J.; Rogasch, N.C.; Nordstrom, M.A.; Semmler, J.G. Eccentric muscle damage has variable effects on motor unit recruitment thresholds and discharge patterns in elbow flexor muscles. J. Neurophysiol. 2009, 102, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Prasartwuth, O.; Taylor, J.L.; Gandevia, S.C. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J. Physiol. 2005, 567, 337–348. [Google Scholar] [CrossRef]
- Marmon, A.R.; Pozzi, F.; Alnahdi, A.H.; Zeni, J.A. The validity of plantarflexor strength measures obtained through hand-held dynamometry measurements of force. Int. J. Sports Phys. Ther. 2013, 8, 820–827. [Google Scholar] [PubMed]
- Rodrigues, F.M.; Demeyer, H.; Hornikx, M.; Camillo, C.A.; Calik-Kutukcu, E.; Burtin, C.; Janssens, W.; Troosters, T.; Osadnik, C. Validity and reliability of strain gauge measurement of volitional quadriceps force in patients with COPD. Chron. Respir. Dis. 2017, 14, 289–297. [Google Scholar] [CrossRef]
- Schlumberger, A.; Schmidtbleicher, D. Grundlagen der kraftdiagnostik in prävention und rehabilitation. Man. Med. 2000, 38, 223–231. [Google Scholar] [CrossRef]
- Martin, H.J.; Yule, V.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometry. GER 2006, 52, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Čeklić, U.; Šarabon, N. Comparison between Gymnasts and Non-Gymnasts in Isometric Strength of the Lower Limbs. Eur. J. Transl. Myol. 2021, 31. [Google Scholar] [CrossRef]
- Doss, W.S.; Karpovich, P.V. A comparison of concentric, eccentric, and isometric strength of elbow flexors. J. Appl. Physiol. 1965, 20, 351–353. [Google Scholar] [CrossRef]
- Griffin, J.W. Differences in elbow flexion torque measured concentrically, eccentrically, and isometrically. Phys. Ther. 1987, 67, 1205–1208. [Google Scholar] [CrossRef]
- Seliger, V.; Dolejš, L.; Karas, V. A dynamometric comparison of maximum eccentric, concentric, and isometric contractions using EMG and energy expenditure measurements. Eur. J. Appl. Physiol. 1980, 45, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Westing, S.H.; Seger, J.Y.; Karlson, E.; Ekblom, B. Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur. J. Appl. Physiol. 1988, 58, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Westing, S.H.; Seger, J.Y.; Thorstensson, A. Effects of electrical stimulation on eccentric and concentric torque-velocity relationships during knee extension in man. Acta Physiol. Scand. 1990, 140, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Babault, N.; Pousson, M.; Ballay, Y.; van Hoecke, J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J. Appl. Physiol. 2001, 91, 2628–2634. [Google Scholar] [CrossRef]
- Amiridis, I.G.; Martin, A.; Morlon, B.; Martin, L.; Cometti, G.; Pousson, M.; van Hoecke, J. Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur. J. Appl. Physiol. 1996, 73, 149–156. [Google Scholar] [CrossRef]
- Singh, M.; Karpovich, P.V. Isotonic and isometric forces of forearm flexors and extensors. J. Appl. Physiol. 1966, 21, 1435–1437. [Google Scholar] [CrossRef]
- Olson, V.L.; Smidt, G.L.; Johnston, R.C. The maximum torque generated by the eccentric, isometric, and concentric contractionsof the hip abductor muscles. Phys. Ther. 1972, 52, 149–158. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Katch, F.I. Eccentric and concentric torque-velocity relationships during arm flexion and extension. Eur. J. Appl. Physiol. 1990, 60, 395–401. [Google Scholar] [CrossRef]
- Hunter, S.K.; Ryan, D.L.; Ortega, J.D.; Enoka, R.M. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J. Neurophysiol. 2002, 88, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, T.; Katch, F.I.; LaChance, P.F. Interrelationships among various measures of upper body strength assessed by different contraction modes. Eur. J. Appl. Physiol. 1989, 58, 749–755. [Google Scholar] [CrossRef]
- Abernethy, P.J.; Jürimäe, J. Cross-sectional and longitudinal uses of isoinertial, isometric, and isokinetic dynamomety. Med. Sci. Sports Exerc. 1996, 28, 1180–1187. [Google Scholar] [CrossRef]
- Muehlbauer, T.; Gollhofer, A.; Granacher, U. Association of balance, strength, and power measures in young adults. J. Strength Cond. Res. 2013, 27, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Wilson, G.; Carlyon, B. Generality versus specificity: A comparison of dynamic and isometric measures of strength and speed-strength. Eur. J. Appl. Physiol. 1994, 68, 350–355. [Google Scholar] [CrossRef]
- Liu, H.; Garrett, W.E.; Moorman, C.T.; Yu, B. Injury rate, mechanism, and risk factors of hamstring strain injuries in sports: A review of the literature. J. Sport Health Sci. 2012, 1, 92–101. [Google Scholar] [CrossRef]
- Van Hooren, B.; Bosch, F. Is there really an eccentric action of the hamstrings during the swing phase of high-speed running? Part I: A critical review of the literature. J. Sports Sci. 2017, 35, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Quatman, C.E.; Quatman-Yates, C.C.; Hewett, T.E. A ‘plane’ explanation of anterior cruciate ligament injury mechanisms. Sports Med. 2010, 40, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.T.; Chan, Y.-Y.; Mok, K.-M.; Yung, P.S.; Chan, K.-M. Understanding acute ankle ligamentous sprain injury in sports. BMC Sports Sci. Med. Rehabil. 2009, 1, 14. [Google Scholar] [CrossRef]
- Beeson, M.S. Complications of shoulder dislocation. Am. J. Emerg. Med. 1999, 17, 288–295. [Google Scholar] [CrossRef]
- Macefield, V.G.; Knellwolf, T.P. Functional properties of human muscle spindles. J. Neurophysiol. 2018, 120, 452–467. [Google Scholar] [CrossRef]
- Houk, J.C.; Rymer, W.Z. Neural control of muscle length and tension. In Comprehensive Physiology; American Cancer Society: Atlanta, GA, USA, 2011; pp. 257–323. ISBN 978-0-470-65071-4. [Google Scholar]
- Pruszynski, J.A.; Scott, S.H. Optimal feedback control and the long-latency stretch response. Exp. Brain Res. 2012, 218, 341–359. [Google Scholar] [CrossRef]
- Nijs, J.; Daenen, L.; Cras, P.; Struyf, F.; Roussel, N.; Oostendorp, R.A.B. Nociception affects motor output: A review on sensory-motor interaction with focus on clinical implications. Clin. J. Pain 2012, 28, 175–181. [Google Scholar] [CrossRef]
- Kugelberg, E.; Eklund, K.; Grimby, L. An electromyographic study of the nociceptive reflexes of the lower limb. Mechanism of the plantar responses. Brain 1960, 83, 394–410. [Google Scholar] [CrossRef]
- Sagaspe, P.; Schwartz, S.; Vuilleumier, P. Fear and stop: A role for the amygdala in motor inhibition by emotional signals. NeuroImage 2011, 55, 1825–1835. [Google Scholar] [CrossRef]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, T.; Fulle, S.; Coscia, F.; Gigliotti, P.V.; Fanò-Illic, G. Old muscle in young body: An aphorism describing the Chronic Fatigue Syndrome. Eur. J. Transl. Myol. 2018, 28, 7688. [Google Scholar] [CrossRef]
- Angelini, C.; Siciliano, G. Neuromuscular diseases and Covid-19: Advices from scientific societies and early observations in Italy. Eur. J. Transl. Myol. 2020, 30, 286–290. [Google Scholar] [CrossRef]
- Dalise, S.; Tropea, P.; Galli, L.; Sbrana, A.; Chisari, C. Muscle function impairment in cancer patients in pre-cachexia stage. Eur. J. Transl. Myol. 2020, 30, 258–267. [Google Scholar] [CrossRef]
- Hajjar, K.; Hagenacker, T. Neuromuscular Disorder as Initial Manifestation of Secondary Hyperparathyroidism—A Case Report. Eur. J. Transl. Myol. 2017, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Duyff, R.F.; Bosch, J.V.D.; Laman, D.M.; Van Loon, B.-J.P.; Linssen, W.H.J.P. Neuromuscular findings in thyroid dysfunction: A prospective clinical and electrodiagnostic study. J. Neurol. Neurosurg. Psychiatry 2000, 68, 750–755. [Google Scholar] [CrossRef]
- Carraro, U.; Albertin, G.; Martini, A.; Giuriati, W.; Guidolin, D.; Masiero, S.; Kern, H.; Hofer, C.; Marcante, A.; Ravara, B. To contrast and reverse skeletal muscle weakness by Full-Body In-Bed Gym in chronic COVID-19 pandemic syndrome. Eur. J. Transl. Myol. 2021, 31. [Google Scholar] [CrossRef]
- Barbalho, M.; Coswig, V.S.; Bottaro, M.; De Lira, C.A.B.; Campos, M.H.; Vieira, C.A.; Gentil, P. “NO LOAD” resistance training increases functional capacity and muscle size in hospitalized female patients: A pilot study. Eur. J. Transl. Myol. 2019, 29, 8492. [Google Scholar] [CrossRef] [PubMed]
- Šarabon, N.; Smajla, D.; Kozinc, Ž.; Kern, H. Speed-power based training in the elderly and its potential for daily movement function enhancement. Eur. J. Transl. Myol. 2020, 30, 125–128. [Google Scholar] [CrossRef] [PubMed]
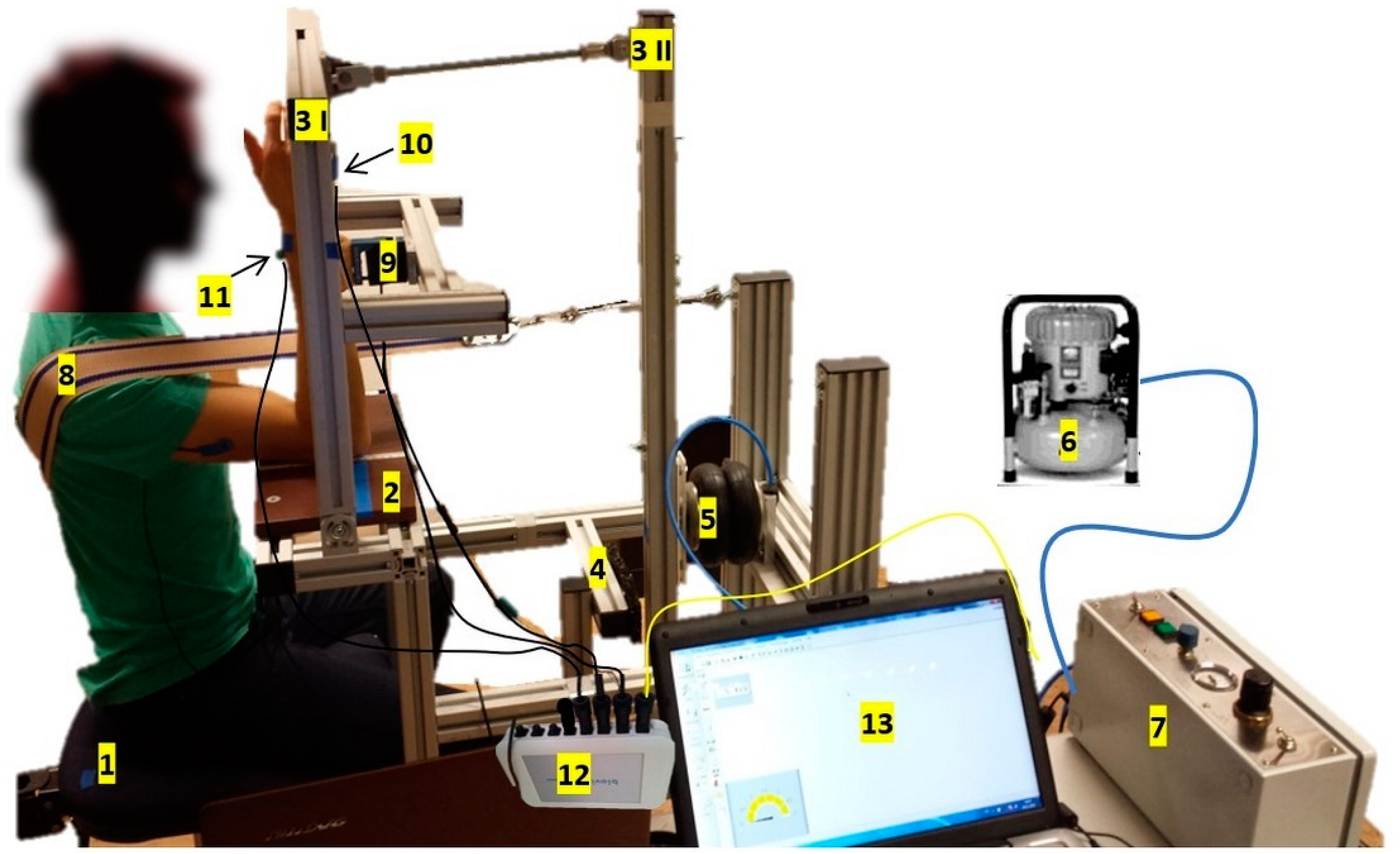
| Section | Components | Technical Specifications |
|---|---|---|
| Basic construction | pivoted and connected levers | range: flexion/extension: 80°–107° |
| Pressure system | compressor (JUN-AIR 700367; Condor MDR2 EN 60947-4-1) | max. system pressure: 8 bar |
| pressure control unit (custom build, Seifert Drucklufttechnik GmbH, Lauter-Bernsbach, Germany) | pressure reduction to max. 2 bar | |
| bellows cylinder (Zitec SP−2 B04, 2−fach) | Ø 165 mm force: max. 9 kN stroke length: 1–110 m (adjustable) rise time: 0.1–30 s (continuously) | |
| Measuring equipment | 1 strain gauge (LMZ 2000N 3006 + amplifier, modified by Biovision, Wehrheim, Germany) | linearly 1 V = 124.74 N |
| 2 accelerometers + amplifier (modified by Biovision, Wehrheim, Germany) | sensitivity 312 mV/g (range ± 2 g) cosinusoidal between 70°–110° approx. linear linearity: ± 0.2% | |
| 1 pressure sensor (Seifert Drucklufttechnik GmbH, Lauter-Bernsbach, Germany) | linear 1 V = 1.05 bar | |
| analog to digital converter (National Instruments, modified by Biovision, Wehrheim, Germany) | 14-bit range:−5 to 5 V | |
| software: NI DIAdem | Version 2012 | |
| Additional measuring equipment | hydrogoniometer (MT.DOK; Desimed GmbH & Co. KG, Müllheim, Germany) | range: 360° with 2°-intervals |
| Type of Force | Total Sample M ± SD n = 13 | Male M ± SD n = 9 | Female M ± SD n = 4 | ||||
|---|---|---|---|---|---|---|---|
| t1 | t2 | t1 | t2 | t1 | t2 | ||
| mean torques | pre-MVIC | 50.70 ± 22.65 | 50.13 ± 22.50 | 63.03 ± 14.30 | 61.89 ± 15.78 | 22.95 ± 4.89 | 23.66 ± 3.51 |
| post-MVIC | 46.38 b ± 21.25 | 46.91 b ± 20.76 | 56.94 ± 16.16 | 56.80 ± 16.91 | 22.62 ± 4.80 | 24.68 ± 2.86 | |
| MVICpri-con | 48.02 a ± 20.50 | 46.58a,b ± 18.98 | 58.07 ± 16.09 | 56.10 ± 14.38 | 25.41 ± 2.51 | 25.17 ± 2.58 | |
| AFisomax | 37.17 b ± 17.39 | 35.20 b ± 17.77 | 45.61 ± 13.40 | 42.23 ± 16.53 | 18.19 ± 6.05 | 19.36 ± 7.12 | |
| AFmax | 49.49 ± 21.01 | 48.35 ± 21.66 | 60.40 ± 14.93 | 58.79 ± 17.17 | 24.95 ± 3.35 | 24.87 ± 5.29 | |
| AFeccmax | 49.39 ± 21.04 | 48.35 ± 21.66 | 60.36 ± 14.86 | 58.79 ± 17.17 | 24.72 ± 3.15 | 24.88 ± 5.30 | |
| maximal torques | pre-MVIC | 53.67 a ± 24.83 | 51.72a ± 22.83 | 66.70 ± 17.13 | 63.41 ± 16.68 | 24.34 ± 5.24 | 25.43 ± 3.61 |
| post-MVIC | 47.47 b ± 21.89 | 47.68 b ± 21.07 | 58.19 ± 16.97 | 57.69 ± 17.22 | 23.34 ± 5.22 | 25.18 ± 3.11 | |
| MVICpri-con | 49.61 a,b ± 21.48 | 47.92a,b ± 19.94 | 59.86 ± 17.47 | 57.71 ± 15.58 | 26.56 ± 2.79 | 25.88 ± 2.86 | |
| AFisomax | 45.09 b ± 19.89 | 43.31 b ± 20.01 | 55.76 ± 12.83 | 52.58 ± 16.35 | 21.08 ± 5.79 | 22.47 ± 7.22 | |
| AFmax | 51.20 ± 21.99 | 50.90 ± 21.93 | 62.29 ± 16.42 | 61.53 ± 17.31 | 26.26 ± 4.03 | 26.97 ± 4.64 | |
| AFeccmax | 51.10 ± 22.10 | 50.90 ± 21.93 | 62.29 ± 16.42 | 61.53 ± 17.31 | 25.93 ± 3.73 | 26.97 ± 4.64 | |
| Type of Force | MD (t1 − t2) (Nm) | SDMD (Nm) | 90%–CI (Nm) | t | df | p | dz | SEM (Nm) | MDC 95% (Nm) | SEM% (%) | SEM 95% (%) | ICC(3,1) [95%–CI] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean torques | pre-MVIC | 0.57 | 3.65 | −1.23–2.38 | 0.57 | 12 | 0.582 | - | 2.58 | 7.15 | 5.12 | 10.03 | 0.987 [0.958–0.996] |
| post-MVIC | −0.54 | 4.19 | −2.61–1.53 | −0.46 | 12 | 0.652 | - | 2.96 | 8.20 | 6.35 | 12.44 | 0.980 [0.936–0.994] | |
| MVICpri-con | 1.44 | 2.59 | 0.16–2.71 | 2.01 | 12 | 0.068 | 0.56 | 1.82 | 5.04 | 3.67 | 7.19 | 0.991 [0.972–0.997] | |
| AFisomax | 1.98 | 8.03 | −1.99–5.95 | 0.89 | 12 | 0.392 | - | 5.68 | 15.74 | 15.70 | 30.77 | 0.896 [0.694–0.967] | |
| AFmax | 1.14 | 2.85 | −0.27–2.55 | 1.44 | 12 | 0.175 | - | 2.01 | 5.57 | 4.11 | 8.05 | 0.991 [0.971–0.997] | |
| maximal torques | pre-MVIC | 1.95 | 3.94 | 0.003–3.90 | 1.79 | 12 | 0.099 | 0.50 | 2.79 | 7.73 | 5.29 | 10.38 | 0.986 [0.956–0.996] |
| post-MVIC | −0.22 | 4.70 | −2.54–2.11 | −0.17 | 12 | 0.869 | - | 3.16 | 8.76 | 6.64 | 13.02 | 0.976 [0.924–0.993] | |
| MVICpri-con | 1.69 | 2.71 | 0.35–3.03 | 2.25 | 12 | 0.044 | 0.62 | 1.92 | 5.32 | 3.76 | 7.36 | 0.991 [0.972–0.997] | |
| AFisomax | 1.77 | 5.90 | −1.15–4.69 | 1.08 | 12 | 0.300 | - | 4.17 | 11.56 | 9.43 | 18.49 | 0.956 [0.863–0.986] | |
| AFmax | 0.31 | 1.82 | −0.50–1.21 | 0.61 | 12 | 0.552 | - | 1.29 | 3.58 | 2.53 | 4.95 | 0.996 [0.986–0.999] |
| Comparison | MD (Nm) | SDMD (Nm) | 95% CI (Nm) | t | df | p | dz | ||
|---|---|---|---|---|---|---|---|---|---|
| mean torques | t1 | AFisomax−pre-MVIC | −13.52 | 10.23 | −19.70–−7.34 | −4.77 | 12 | <0.001 | 1.32 |
| AFmax−pre-MVIC | −1.21 | 4.66 | −1.61–4.02 | −0.93 | 12 | 0.369 | - | ||
| AFisomax−AFmax | −12.32 | 8.10 | −17.21–−7.42 | −5.48 | 12 | <0.001 | 1.52 | ||
| MVICpri-con−pre-MVIC | −2.68 | 5.47 | −0.63–5.98 | −1.77 | 12 | 0.103 | - | ||
| post-MVIC−pre-MVIC | −4.32 | 4.44 | −7.00–1.64 | −3.51 | 12 | 0.004 | 0.97 | ||
| t2 | AFisomax−pre-MVIC | −14.93 | 11.96 | −22.16–−7.70 | −4.50 | 12 | 0.001 | 1.23 | |
| AFmax−pre-MVIC | −1.77 | 5.06 | −1.28–4.83 | −1.26 | 12 | 0.230 | - | ||
| AFisomax−AFmax | −13.16 | 8.83 | −18.49–−7.82 | −5.37 | 12 | <0.001 | 1.49 | ||
| MVICpri-con−pre-MVIC | −3.54 | 4.37 | −6.18–0.91 | −2.93 | 12 | 0.013 | 0.81 | ||
| post-MVIC−pre-MVIC | −3.21 | 4.01 | −5.63–0.79 | −2.89 | 12 | 0.014 | 0.80 | ||
| maximal torques | t1 | AFisomax−pre-MVIC | −8.59 | 8.65 | −13.81–−3.36 | −3.58 | 12 | 0.004 | 0.99 |
| AFmax−pre-MVIC | −2.47 | 5.14 | −0.64–5.57 | −1.73 | 12 | 0.109 | - | ||
| AFisomax−AFmax | −6.12 | 5.75 | −9.59–−2.64 | −3.84 | 12 | 0.002 | 1.06 | ||
| MVICpri-con−pre-MVIC | −4.06 | 6.38 | −7.92–0.21 | −2.30 | 12 | 0.041 | 0.64 | ||
| post-MVIC−pre-MVIC | −6.20 | 4.91 | −9.17–3.24 | −4.56 | 12 | 0.001 | 1.26 | ||
| t2 | AFisomax−pre-MVIC | −8.41 | 9.73 | −14.29–−2.53 | −3.12 | 12 | 0.009 | 0.86 | |
| AFmax−pre-MVIC | −0.83 | 4.62 | −1.96–3.62 | −0.65 | 12 | 0.531 | - | ||
| AFisomax−AFmax | −7.58 | 6.28 | −11.38–−3.79 | −4.36 | 12 | 0.001 | 1.21 | ||
| MVICpri-con−pre-MVIC | −3.80 | 4.03 | −6.24–1.37 | −3.4 | 12 | 0.005 | 0.94 | ||
| post-MVIC−pre-MVIC | −4.04 | 3.74 | −6.30–1.77 | −3.89 | 12 | 0.02 | 1.08 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dech, S.; Bittmann, F.N.; Schaefer, L.V. Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics 2021, 11, 923. https://doi.org/10.3390/diagnostics11060923
Dech S, Bittmann FN, Schaefer LV. Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics. 2021; 11(6):923. https://doi.org/10.3390/diagnostics11060923
Chicago/Turabian StyleDech, Silas, Frank N. Bittmann, and Laura V. Schaefer. 2021. "Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria" Diagnostics 11, no. 6: 923. https://doi.org/10.3390/diagnostics11060923
APA StyleDech, S., Bittmann, F. N., & Schaefer, L. V. (2021). Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics, 11(6), 923. https://doi.org/10.3390/diagnostics11060923







