Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. CMR Protocol
2.3. Statistical Analysis
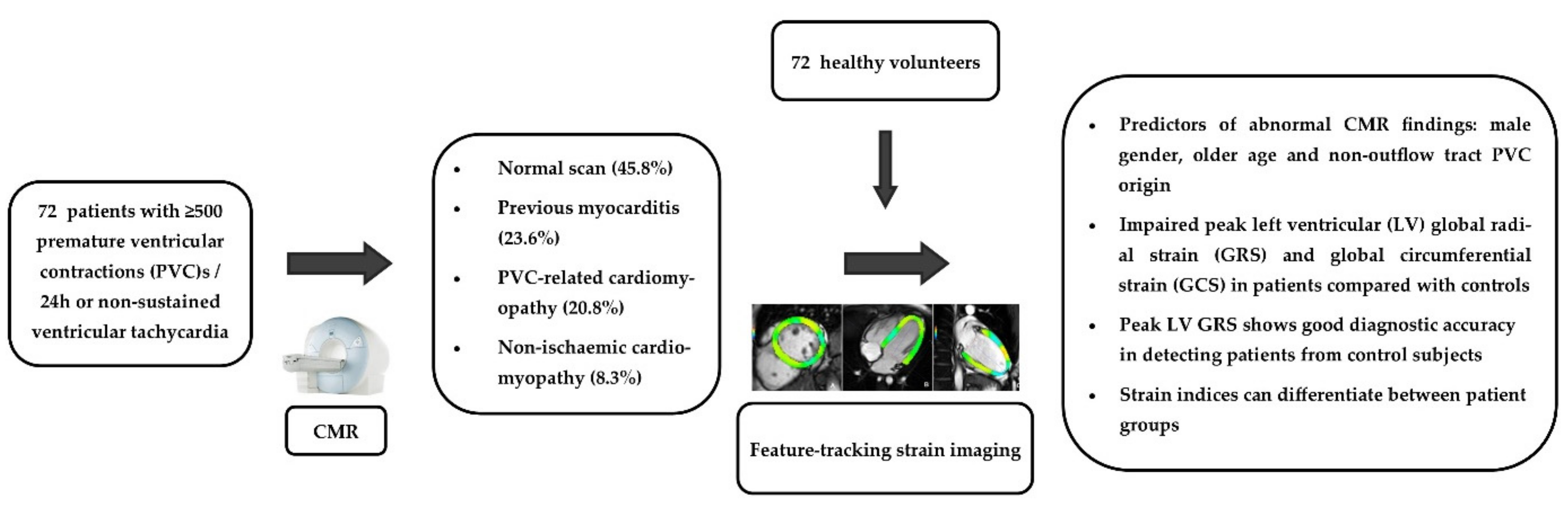
3. Results
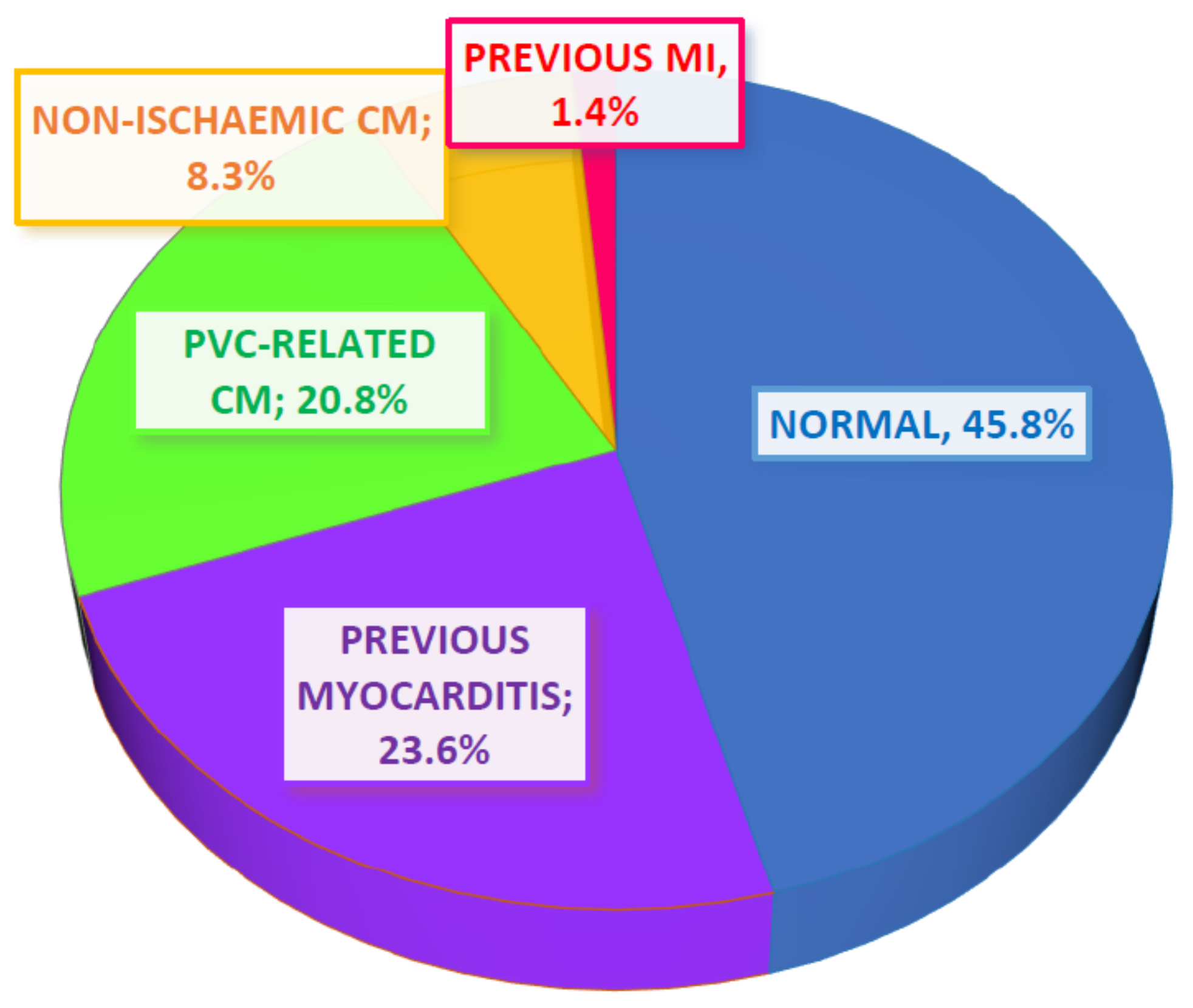
3.1. Patient Characteristics and CMR Findings
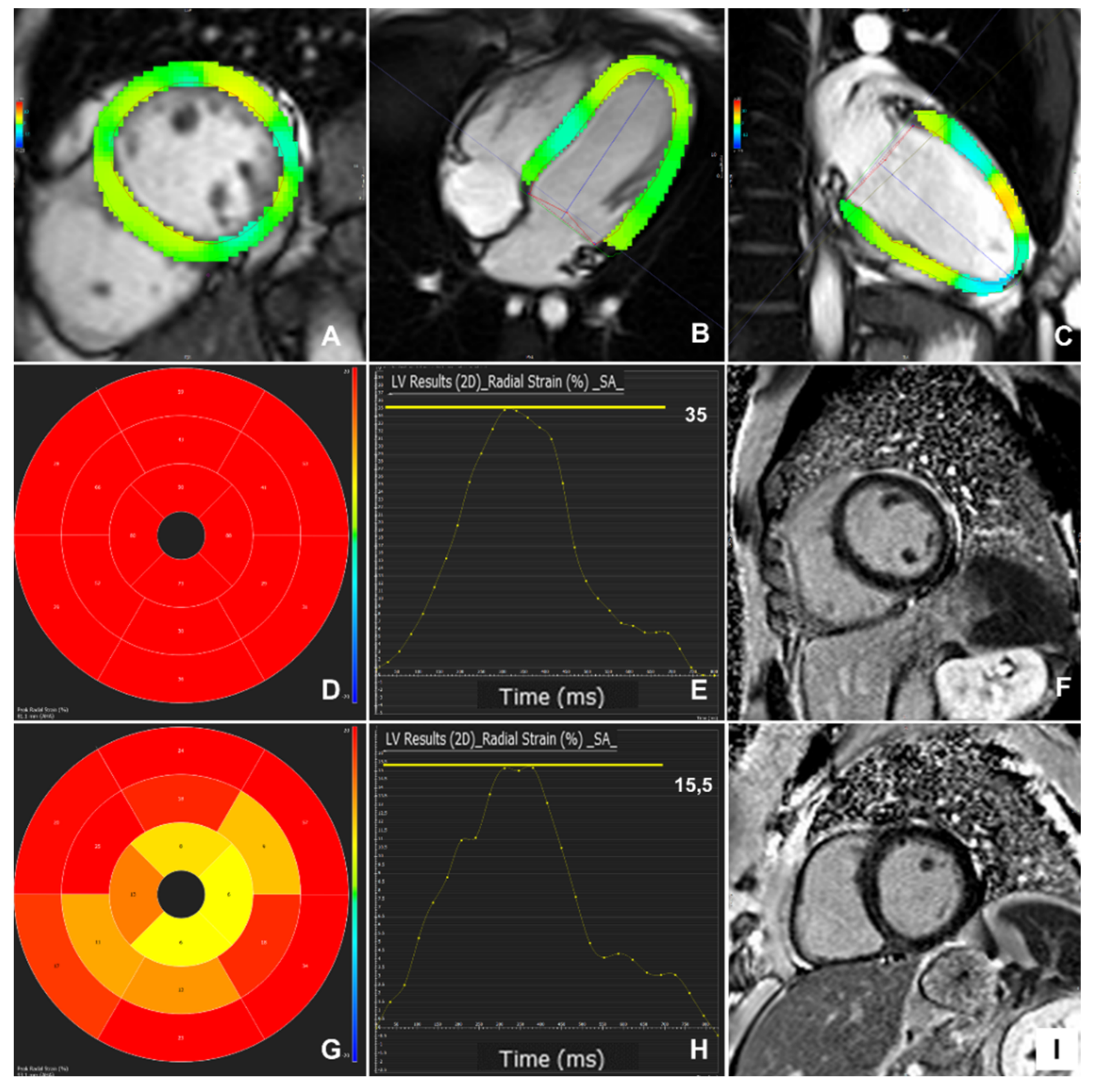
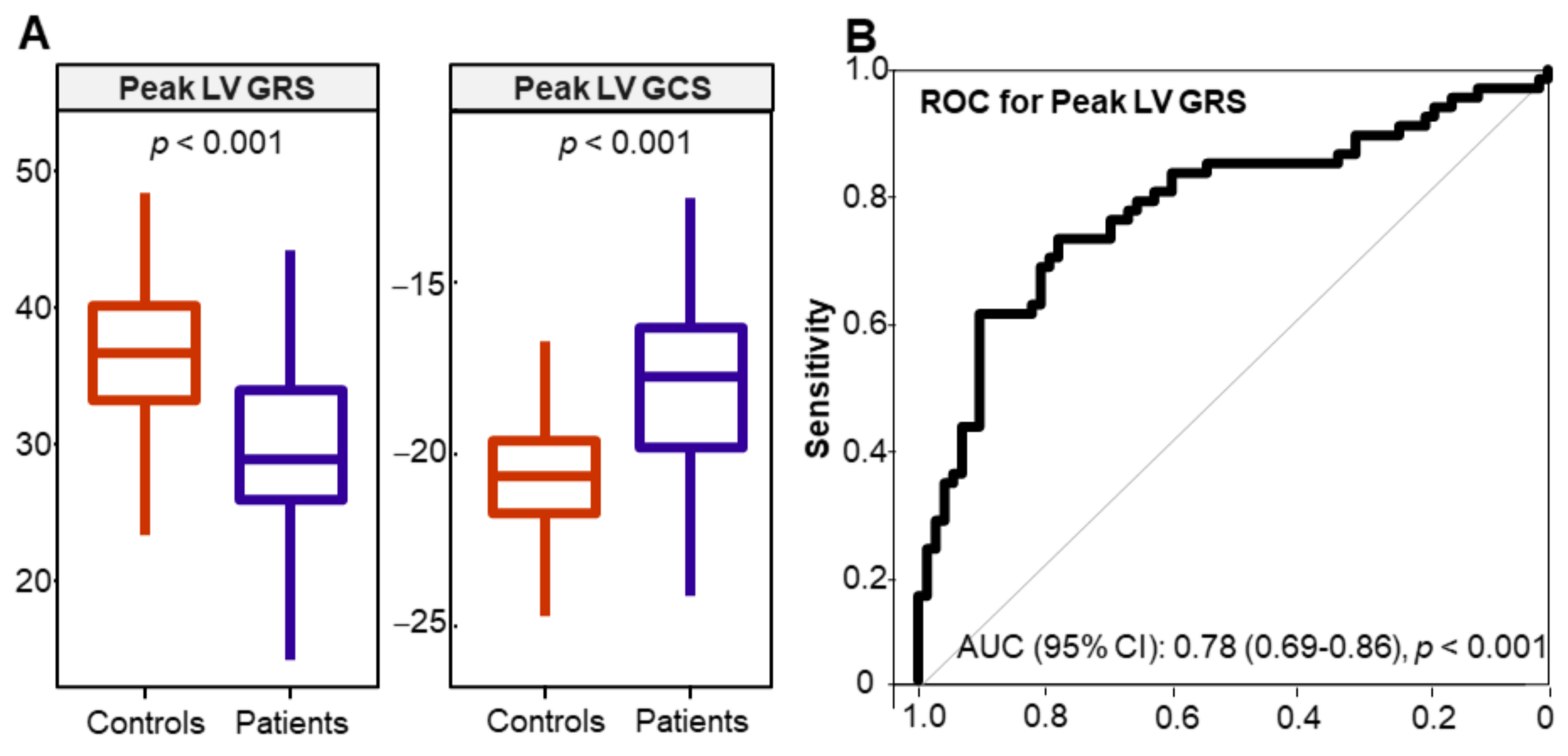
3.2. Myocardial Strain Findings
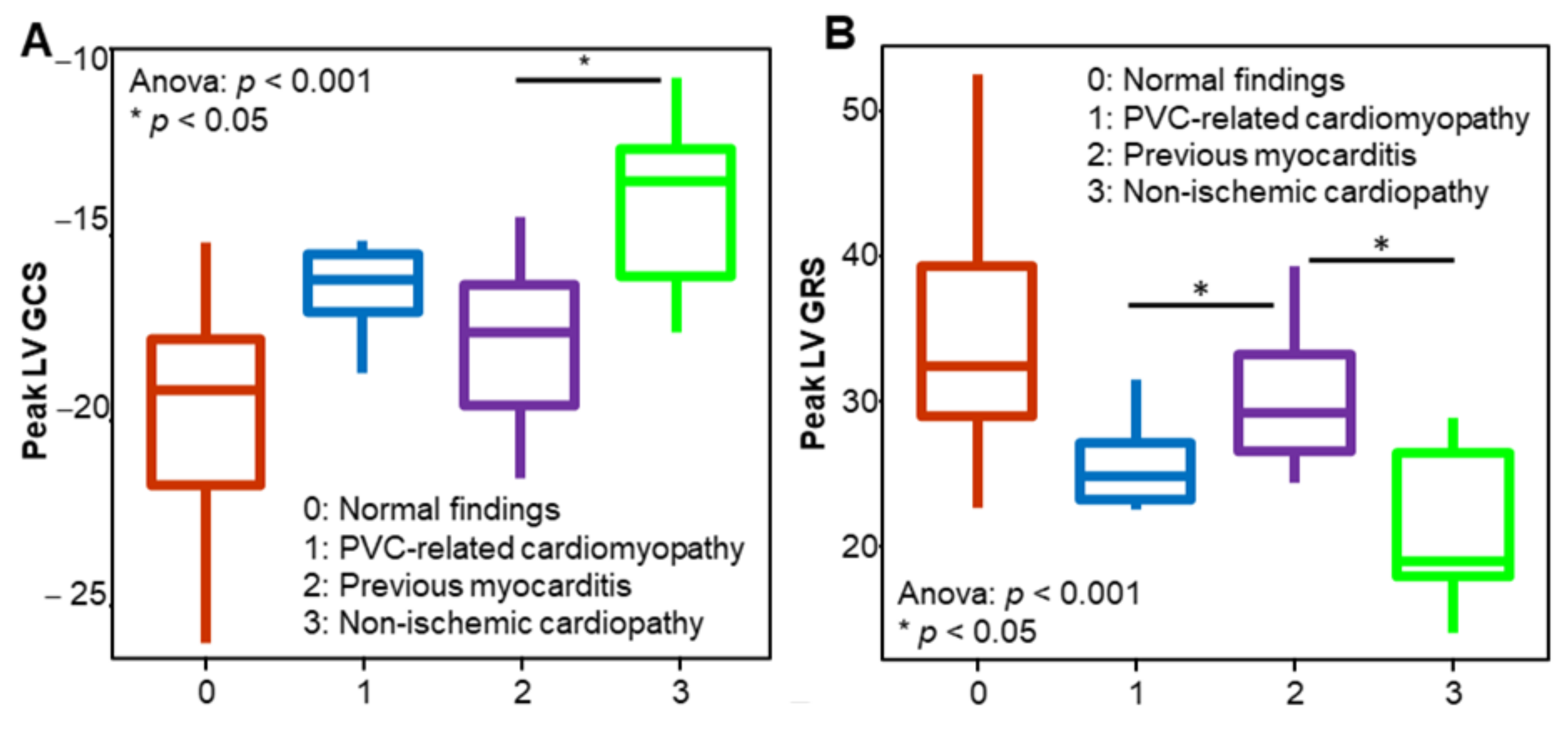
3.3. Myocardial Strain in Different Patient Groups
3.4. Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.H.; Knight, B.; et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014, 16, 1257–1283. [Google Scholar] [CrossRef]
- Ahn, M.S. Current Concepts of Premature Ventricular Contractions. J. Lifestyle Med. 2013, 3, 26–33. [Google Scholar]
- Alqarawi, W.A.; Ramirez, F.D.; Nery, P.B.; Redpath, C.J.; Sadek, M.M.; Green, M.S.; Birnie, D.H.; Nair, G.M. Identifying and Managing Premature Ventricular Contraction-Induced Cardiomyopathy: What, Why, and How? Can. J. Cardiol. 2017, 33, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Santangeli, P.; Selvanayagam, J.B.; Nucifora, G. Role of Cardiac Magnetic Resonance Imaging in Patients with Idiopathic Ventricular Arrhythmias. Curr. Cardiol. Rev. 2019, 15, 12–23. [Google Scholar] [CrossRef]
- Andreini, D.; Dello Russo, A.; Pontone, G.; Mushtaq, S.; Conte, E.; Perchinunno, M.; Guglielmo, M.; Coutinho Santos, A.; Magatelli, M.; Baggiano, A.; et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients with Normal Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 410–421. [Google Scholar] [CrossRef]
- Cabanelas, N.; Vidigal Ferreira, M.J.; Donato, P.; Gaspar, A.; Pinto, J.; Caseiro-Alves, F.; Providência, L.A. Added value of cardiac magnetic resonance in etiological diagnosis of ventricular arrhythmias. Rev. Port. Cardiol. 2013, 32, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; De Stéfano, L.; Pérez de Arenaza, D.; Maid, G.; Falconi, M.; Pietrani, M.; Dragonetti, L.; Pérez Etchepare, R.; García-Mónaco, R.; Belziti, C. Diagnostic Value of Cardiac Magnetic Resonance in Patients with Frequent Ventricular Arrhythmia and Normal Doppler Echocardiography. Argent. J. Cardiol. N. Am. 2013, 81. Available online: http://ppct.caicyt.gov.ar/index.php/rac/article/view/2005 (accessed on 2 February 2021).
- Weisser-Thomas, J.; Ferrari, V.A.; Lakghomi, A.; Lickfett, L.M.; Nickenig, G.; Schild, H.H.; Thomas, D. Prevalence and clinical relevance of the morphological substrate of ventricular arrhythmias in patients without known cardiac conditions detected by cardiovascular MR. Br. J. Radiol. 2014, 87, 20140059. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Santangeli, P.; Castro, S.A.; Casado Arroyo, R.; Maeda, S.; Benhayon, D.A.; Liuba, I.; Liang, J.J.; Sadek, M.M.; Chahal, A.; et al. Risk Stratification of Patients with Apparently Idiopathic Premature Ventricular Contractions: A Multicenter International CMR Registry. JACC Clin. Electrophysiol. 2020, 6, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Muser, D.; Masci, P.G.; Barison, A.; Rebellato, L.; Piccoli, G.; Daleffe, E.; Toniolo, M.; Zanuttini, D.; Facchin, D.; et al. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: A magnetic resonance imaging study. Circ. Arrhythm. Electrophysiol. 2014, 7, 456–462. [Google Scholar] [CrossRef]
- Muser, D.; Nucifora, G.; Pieroni, M.; Nucifora, G.; Pieroni, M.; Castro, S.A.; Casado Arroyo, R.; Maeda, S.; Benhayon, D.A.; Liuba, I.; et al. Prognostic Value of Non-Ischemic Ring-Like Left Ventricular Scar in Patients with Apparently Idiopathic Non-Sustained Ventricular Arrhythmias. Circulation 2021, 143, 1359–1373. [Google Scholar] [PubMed]
- Nikolaidou, C.; Kouskouras, K.; Fragakis, N.; Vassilikos, V.P.; Karvounis, H.; Karamitsos, T.D. Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging. Diagnostics 2021, 11, 178. [Google Scholar] [CrossRef]
- Giardina, E.G. Procainamide: Clinical Pharmacology and Efficacy against Ventricular Arrhythmias. Ann. N. Y. Acad. Sci. 1984, 432, 177–188. [Google Scholar] [CrossRef]
- Pritchard, B.; Thompson, H. StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020; Procainamide. [Updated 24 May 2020]. Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 20 December 2020).
- Somani, R.; Krahn, A.D.; Healey, J.S.; Chauhan, V.S.; Birnie, D.H.; Champagne, J.; Sanatani, S.; Angaran, P.; Gow, R.M.; Chakrabarti, S.; et al. Procainamide infusion in the evaluation of unexplained cardiac arrest: From the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER). Heart Rhythm. 2014, 11, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Giardina, E.G.; Heissenbuttel, R.H.; Bigger, J.T., Jr. Intermittent Intravenous Procaine Amide to Treat Ventricular Arrhythmias. Correlation of plasma concentration with effect on arrhythmia, electrocardiogram, and blood pressure. Ann. Intern. Med. 1973, 78, 183–193. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2007, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.P.; Charron, P.; Gimeno-Blanes, J.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: Bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2012, 34, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Neubauer, S. Cardiovascular Magnetic Resonance in Heart Failure. Curr. Cardiol. Rep. 2011, 13, 210–219. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Panizo, J.G.; Barra, S.; Mellor, G.; Heck, P.; Agarwal, S. Premature Ventricular Complex-induced Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2018, 7, 128–134. [Google Scholar] [CrossRef]
- Erley, J.; Genovese, D.; Tapaskar, N.; Alvi, N.; Rashedi, N.; Besser, S.A.; Kawaji, K.; Goyal, N.; Kelle, S.; Lang, R.M.; et al. Echocardiography and cardiovascular magnetic resonance based evaluation of myocardial strain and relationship with late gadolinium enhancement. J. Cardiovasc. Magn. Reson. 2019, 21, 46. [Google Scholar] [CrossRef]
- Erley, J.; Tanacli, R.; Genovese, D.; Tapaskar, N.; Rashedi, N.; Bucius, P.; Kawaji, K.; Karagodin, I.; Lang, R.M.; Kelle, S.; et al. Myocardial strain analysis of the right ventricle: Comparison of different cardiovascular magnetic resonance and echocardiographic techniques. J. Cardiovasc. Magn. Reson. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dardeer, A.M.; Moody, W.E.; Edwards, N.C.; Hudsmith, L.E.; Steeds, R.P. Normal values for myocardial deformation within the right heart measured by feature-tracking cardiovascular magnetic resonance imaging. Int. J. Cardiol. 2018, 252, 220–223. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.Q.; Marwick, T.H.; Negishi, K. MRI-Derived Myocardial Strain Measures in Normal Subjects. JACC Cardiovasc. Imaging 2018, 11, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Antiochos, P.; Qamar, I.; Seno, A.; Steigner, M.L.; Aghayev, A.; Blankstein, R.; Stevenson, W.; Jerosch-Herold, M.; Kwong, R.Y. Diagnostic Impact and Prognostic Value of Cardiac Mri in Patients with Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2020, 75 (Suppl. 1), 3665. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships with Myocardial Inflammation. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef]
- Ghannam, M.; Siontis, K.C.; Kim, M.H.; Cochet, H.; Jais, P.; Eng, M.J.; Attili, A.; Sharaf-Dabbagh, G.; Latchamsetty, R.; Jongnarangsin, K.; et al. Risk stratification in patients with frequent premature ventricular complexes in the absence of known heart disease. Heart Rhythm. 2020, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, C.; Zorzi, A.; Vessella, T.; Martino, A.; Panattoni, G.; Cipriani, A.; De Lazzari, M.; Perazzolo Marra, M.; Fusco, A.; Sciarra, L.; et al. Predictors of Left Ventricular Scar at Cardiac Magnetic Resonance in Athletes with Apparently Idiopathic Ventricular Arrhythmias. J. Am. Heart Assoc. 2021, 10, e018206. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Drezner, J.A.; D’Ascenzi, F.; Zorzi, A. How to evaluate premature ventricular beats in the athlete: Critical review and proposal of a diagnostic algorithm. Br. J. Sports Med. 2020, 54, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Pingitore, A.; Strata, E.; Di Bella, G.; Molinaro, S.; Lombardi, M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J. Am. Coll. Cardiol. 2010, 56, 1235–1243. [Google Scholar] [CrossRef]
- Muser, D.; Piccoli, G.; Puppato, M.; Proclemer, A.; Nucifora, G. Incremental value of cardiac magnetic resonance imaging in the diagnostic work-up of patients with apparently idiopathic ventricular arrhythmias of left ventricular origin. Int. J. Cardiol. 2015, 180, 142–144.17. [Google Scholar] [CrossRef]
- Muser, D.; Puppato, M.; Proclemer, A.; Nucifora, G. Value of cardiac magnetic resonance imaging in the setting of familiar cardiomyopathy: A step toward preclinical diagnosis. Int. J. Cardiol. 2016, 203, 43–45. [Google Scholar] [CrossRef]
- Oebel, S.; Dinov, B.; Arya, A.; Hilbert, S.; Sommer, P.; Bollmann, A.; Hindricks, G.; Paetsch, I.; Jahnke, C. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J. Cardiovasc. Electrophysiol. 2017, 28, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.M.; Weinsaft, J.W.; Waldman, L.; Petashnick, M.; Liu, C.F.; Cheung, J.W.; Thomas, G.; Ip, J.E.; Lerman, B.B. Reappraisal of cardiac magnetic resonance imaging in idiopathic outflow tract arrhythmias. J. Cardiovasc. Electrophysiol. 2014, 25, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Senior, R.; Pennell, D.J. Assessing left ventricular systolic function: From ejection fraction to strain analysis. Eur. Heart J. 2020, ehaa587. [Google Scholar] [CrossRef]
- Rosen, B.D.; Lima, J.A.C. The prognostic value of global circumferential strain in patients with suspected myocardial disease. JACC Cardiovasc. Imaging 2015, 8, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.J.; Campbell, T.; Stefani, L.; Kumar, S.; Thomas, L. Speckle-Tracking Strain Echocardiography in the Assessment of Myocardial Mechanics in Patients With Idiopathic Ventricular Arrhythmias. Circulation: Arrhythmia Electrophysiol. 2020, 13, e008748. [Google Scholar]
- Yao, J.; Yang, R.; Xu, D.; Zhuang, Y.; Yong, Y.; Cao, K. Circumferential myocardial contraction patterns in patients with idiopathic frequent premature ventricular 18 complexes from the right ventricular outflow tract. Int. J. Cardiol. 2013, 166, 166–172. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.; Lewandowski, A.J.; Lazdam, M.; Rai, A.; Francis, J.; Myerson, S.; Noble, A.; Becher, H.; Neubauer, S.; Petersen, S.E.; et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: Comparison with tagging and relevance of gender. J. Cardiovasc. Magn. Reson. 2013, 15, 8. [Google Scholar] [CrossRef]
- Mangion, K.; Burke, N.M.M.; McComb, C.; Carrick, D.; Woodward, R.; Berry, C. Feature-tracking myocardial strain in healthy adults—A magnetic resonance study at 3.0 tesla. Sci. Rep. 2019, 9, 3239. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yu, S.; Yu, Y.; Ren, H.; Li, S.; Zhou, L.; Yang, Z.; Wu, H.; Zhou, W.; Gong, L. Left ventricular myocardial strain in ventricular arrhythmia without structural heart disease using cardiac magnetic resonance. Am. J. Transl. Res. 2017, 9, 3006–3016. [Google Scholar]
- Huizar, J.F.; Ellenbogen, K.A.; Tan, A.Y.; Kaszala, K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. JACC 2019, 73, 2328–2344. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. JACC 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Sassone, B.; Muser, D.; Casella, M.; Luzi, M.; Virzì, S.; Balla, C.; Nucifora, G.; Task Force on Imaging and Task Force on Ablation of Ventricular Tachycardia of the Italian Association of Arrhythmias and Cardiac Pacing (AIAC). Detection of concealed structural heart disease by imaging in patients with apparently idiopathic premature ventricular complexes: A review of current literature. Clin. Cardiol. 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
| All Patients (n = 72) | Controls (n = 72) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 49.5 (36.8, 58.0) | 48.5 (40.0, 58.0) | 0.701 |
| Males, n (%) | 34 (47.2) | 34 (47.2) | 1.0 |
| BSA (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.809 |
| Smoking, n (%) * | 15 (24.2) | 2 (2.8) | 0.001 |
| Hypertension, n (%) † | 12 (17.4) | 7 (9.7) | 0.277 |
| Hyperlipidaemia, n (%) | 25 (36.2) | 15 (20.8) | 0.066 |
| CMR measurements | |||
| LVEDV index (ml/m2) | 84.0 (75.4, 92.3) | 79.0 (69.0, 87.3) | 0.012 |
| LVESV index (ml/m2) | 33.7 (28.3, 41.8) | 27.0 (24.3, 31.5) | <0.001 |
| LV ejection fraction (%) | 59.5 (56.0, 64.0) | 65.0 (63.0, 67.0) | <0.001 |
| RVEDV index (ml/m2) | 76.9 (68.4, 86.0) | 77.6 (67.0, 85.2) | 0.837 |
| RVESV index (ml/m2) | 28.5 (23.7, 34.5) | 25.6 (21.9, 30.0) | 0.053 |
| RV ejection fraction (%) | 62.6 ± 6.0 | 66.1 ± 4.3 | <0.001 |
| Normal CMR Scan (n = 33, 45.8%) | Abnormal CMR Scan (n = 39, 54.2%) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 46.0 (35.0, 51.0) | 54.0 (42.0, 60.5) | 0.045 |
| Males, n (%) | 9 (27.3) | 25 (64.1) | 0.004 |
| BMI | 27.1(4.7) | 27.3 (5.1) | 0.851 |
| BSA | 1.85 (0.20) | 1.95 (0.21) | 0.039 |
| Smoking, n (%) * | 7 (24.1) | 8 (24.2) | 1 |
| Hypertension, n (%) † | 3 (9.4) | 9 (24.3) | 0.188 |
| Ectopy burden, n | 11,668 (5283, 20,684) | 14,363 (4731, 27,583) | 0.739 |
| NSVT, n (%) | 5 (15.2) | 11 (28.2) | 0.297 |
| Uncommon PVC origin | 4 (12.9) | 13 (38.2) | 0.041 |
| CMR measurements | |||
| LVEDVi (ml/m2) | 78.6 ± 9.0 | 89.8 ± 13.2 | <0.001 |
| LVESVi (ml/m2) | 28.4 (25.3, 31.3) | 39.6 (34.7, 45.6) | <0.001 |
| LVEF (%) | 63.4 ± 3.6 | 55.5 ± 5.8 | <0.001 |
| RVEDVi (ml/m2) | 73.8 (67.3, 85.1) | 79.2 (69.1, 86.8) | 0.369 |
| RVESVi (ml/m2) | 27.3 (8.0) | 30.8 (10.1) | 0.111 |
| RVEF (%) | 64.5 ± 5.5 | 60.9 ± 6.0 | 0.012 |
| LGE, n (%) | 1 (3.0%) | 21 (53.8%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidou, C.; Kotanidis, C.P.; Wijesurendra, R.; Leal-Pelado, J.; Kouskouras, K.; Vassilikos, V.P.; Karvounis, H.; Ntusi, N.; Antoniades, C.; Neubauer, S.; et al. Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias. Diagnostics 2021, 11, 1109. https://doi.org/10.3390/diagnostics11061109
Nikolaidou C, Kotanidis CP, Wijesurendra R, Leal-Pelado J, Kouskouras K, Vassilikos VP, Karvounis H, Ntusi N, Antoniades C, Neubauer S, et al. Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias. Diagnostics. 2021; 11(6):1109. https://doi.org/10.3390/diagnostics11061109
Chicago/Turabian StyleNikolaidou, Chrysovalantou, Christos P. Kotanidis, Rohan Wijesurendra, Joana Leal-Pelado, Konstantinos Kouskouras, Vassilios P. Vassilikos, Haralambos Karvounis, Ntobeko Ntusi, Charalambos Antoniades, Stefan Neubauer, and et al. 2021. "Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias" Diagnostics 11, no. 6: 1109. https://doi.org/10.3390/diagnostics11061109
APA StyleNikolaidou, C., Kotanidis, C. P., Wijesurendra, R., Leal-Pelado, J., Kouskouras, K., Vassilikos, V. P., Karvounis, H., Ntusi, N., Antoniades, C., Neubauer, S., & Karamitsos, T. D. (2021). Cardiac Magnetic Resonance to Detect the Underlying Substrate in Patients with Frequent Idiopathic Ventricular Arrhythmias. Diagnostics, 11(6), 1109. https://doi.org/10.3390/diagnostics11061109








