Usefulness of the Electrocardiogram in a Patient Presenting with Right-Sided Pneumothorax and Presyncope
Abstract
1. Introduction
2. Case Presentation
2.1. Patient Information and Timeline
2.2. Clinical Findings
2.3. Diagnostic Assessment and Therapeutic Intervention
2.4. Final Diagnosis
3. Discussion
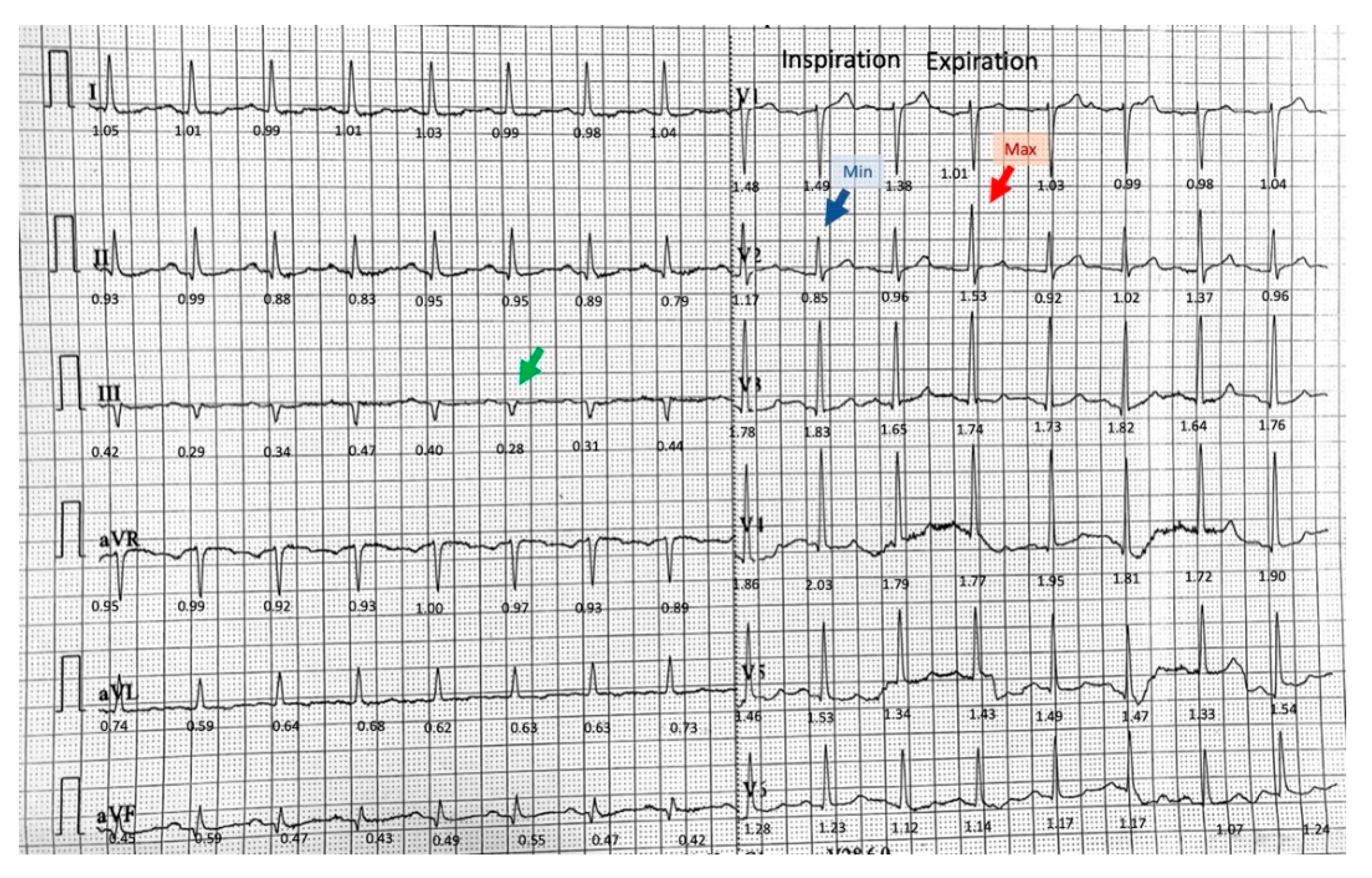
3.1. Phasic Variations in the QRS Complex Amplitude
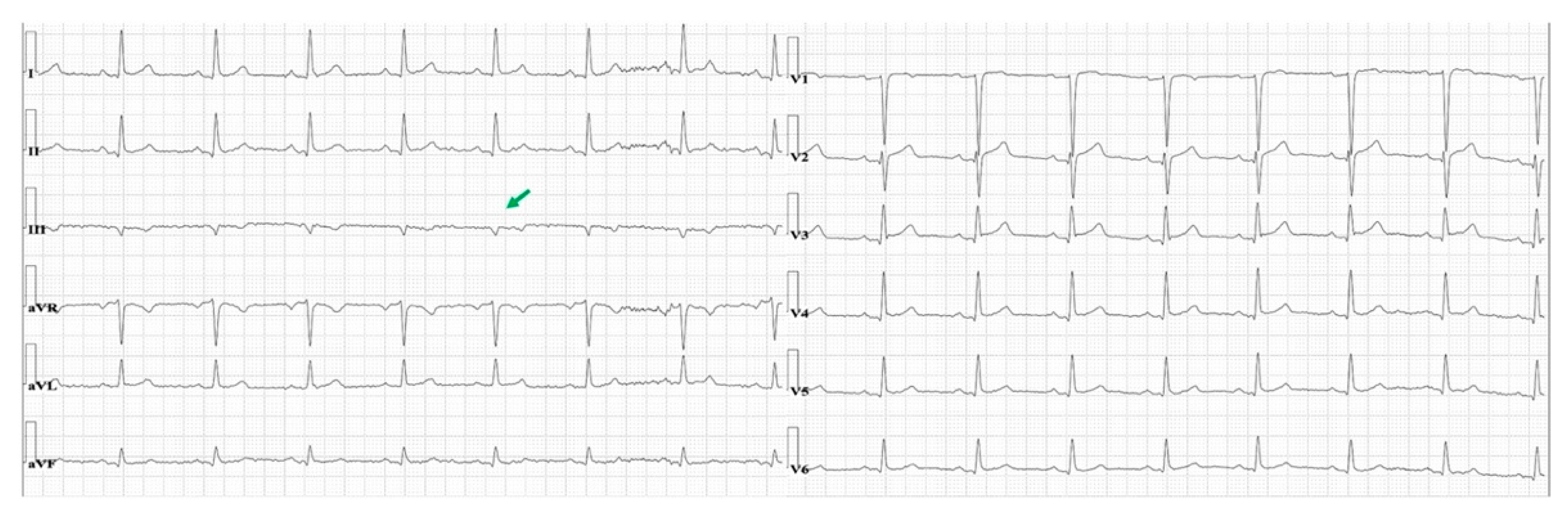
3.2. Isolated Very Low Amplitude QRS Complex in Frontal Leads
3.3. Limitations
3.4. Summary and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melton, L.J., 3rd; Hepper, N.G.; Offord, K.P. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am. Rev. Respir. Dis. 1979, 120, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Bense, L.; Eklund, G.; Wiman, L.G. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987, 92, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.C.; Andersson, C.; Schultz, H.H. ECG with alternating electric axis in relation to left-sided tension pneumothorax: A case report and review of the literature. Eur. Clin. Respir. J. 2018, 5, 1495982. [Google Scholar] [CrossRef]
- Ogunbayo, G.O.; Charnigo, R.; Darrat, Y.; Morales, G.; Kotter, J.; Olorunfemi, O.; Elbadawi, A.; Sorrell, V.L.; Smyth, S.S.; Elayi, C.S. Incidence, predictors, and outcomes associated with pneumothorax during cardiac electronic device implantation: A 16-year review in over 3.7 million patients. Heart Rhythm 2017, 14, 1764–1770. [Google Scholar] [CrossRef]
- Burgard, C.; Stahl, R.; de Figueiredo, G.N.; Dinkel, J.; Liebig, T.; Cioni, D.; Neri, E.; Trumm, C.G. Percutaneous CT Fluoroscopy-Guided Core Needle Biopsy of Mediastinal Masses: Technical Outcome and Complications of 155 Procedures during a 10-Year Period. Diagnostics 2021, 11, 781. [Google Scholar] [CrossRef]
- Aramini, B.; Ruggiero, C.; Stefani, A.; Morandi, U. Giant bulla or pneumothorax: How to distinguish. Int. J. Surg. Case Rep. 2019, 62, 21–23. [Google Scholar] [CrossRef]
- Huang, S.C.; Lin, G.M.; Li, Y.H.; Lin, C.S.; Kao, H.W.; Han, C.L. Abnormal Changes of a 12-Lead Electrocardiogram in Male Patients with Left Primary Spontaneous Pneumothorax. Acta Cardiol. Sin. 2014, 30, 157–164. [Google Scholar]
- Strizik, B.; Forman, R. New ECG changes associated with a tension pneumothorax: A case report. Chest 1999, 115, 1742–1744. [Google Scholar] [CrossRef]
- Fei, J.; Marill, K.A. ECG phasic voltage changes associated with spontaneous pneumothorax in a patient with vanishing lung syndrome. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Barcos, J.C.; Tello Santacruz, I.A.; Monie, C.C.; Fernandez Recalde, M.L.; Humphreys, J.D. Brugada phenocopy induced by severe pneumothorax. J. Electrocardiol. 2018, 51, 343–345. [Google Scholar] [CrossRef]
- Goldstein, L.N.; Wells, M.; So, S.K. Abnormal P and QRS Axes in a Young Male With a Stab Wound to the Chest. Ann. Emerg. Med. 2018, 71, 477–479. [Google Scholar] [CrossRef]
- Lee, W.; Lee, Y.; Kim, C.; Choi, H.J.; Kang, B.; Lim, T.H.; Oh, J.; Kang, H.; Shin, J. Changes in electrocardiographic findings after closed thoracostomy in patients with spontaneous pneumothorax. Clin. Exp. Emerg. Med. 2017, 4, 38–47. [Google Scholar] [CrossRef]
- Arao, K.; Mase, T.; Nakai, M.; Sekiguchi, H.; Abe, Y.; Kuroudu, N.; Oobayashi, O. Concomitant Spontaneous Tension Pneumothorax and Acute Myocardial Infarction. Intern. Med. 2019, 58, 1131–1135. [Google Scholar] [CrossRef]
- Liu, P.Y.; Lin, Y.P.; Li, Y.H.; Su, Z.W.; Han, C.L.; Huang, S.C.; Lin, C.S.; Meng, F.C.; Wu, H.T.; Lin, G.M. Electrocardiographic characteristics in young male patients with left primary spontaneous pneumothorax estimated by the collins equation. Indian Heart J. 2017, 69, 720–724. [Google Scholar] [CrossRef]
- Yamamoto, H.; Satomi, K.; Aizawa, Y. Electrocardiographic manifestations in a large right-sided pneumothorax. BMC Pulm. Med. 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Blendea, D.; McPherson, C.A.; Pop, S.; Anton, F.P.; Crisan, S.; Ruskin, J.N. Isolated very low QRS voltage predicts response to tilt-table testing in patients with neurally mediated syncope. Pacing Clin. Electrophysiol. 2019, 42, 1558–1565. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Yousuf, A.; Jones, H.E.; Corcoran, J.P.; Psallidas, I.; Rahman, N.M. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: A systematic review. Thorax 2017, 72, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, M.; Biddison, J.H. Electrocardiographic changes with right-sided pneumothorax. South. Med. J. 1998, 91, 677–680. [Google Scholar] [CrossRef]
- Hallifax, R.J.; McKeown, E.; Sivakumar, P.; Fairbairn, I.; Peter, C.; Leitch, A.; Knight, M.; Stanton, A.; Ijaz, A.; Marciniak, S.; et al. Ambulatory management of primary spontaneous pneumothorax: An open-label, randomised controlled trial. Lancet 2020, 396, 39–49. [Google Scholar] [CrossRef]
- Blendea, D.; McPherson, C.A.; Pop, S.; Ruskin, J.N. Isolated very low QRS voltage in the frontal leads predicts recurrence of neurally mediated syncope. Heart Rhythm 2019, 16, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Tentea, C.-P.; Chiorescu, R.; Crisan, S.; Pop, S.; Ruskin, J.N.; Blendea, D. A Risk Score That Predicts Recurrence of Neurally Mediated Syncope Using Electrocardiographic and Vectorcardiographic Parameters. Circulation 2020, 142, A16337. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florescu, L.M.; Țentea, C.-P.; Eötvös, C.-A.; Lazar, R.-D.; Zehan, I.-G.; Sabha, W.; Pop, S.; Todea, D.A.; Blendea, D. Usefulness of the Electrocardiogram in a Patient Presenting with Right-Sided Pneumothorax and Presyncope. Diagnostics 2021, 11, 1069. https://doi.org/10.3390/diagnostics11061069
Florescu LM, Țentea C-P, Eötvös C-A, Lazar R-D, Zehan I-G, Sabha W, Pop S, Todea DA, Blendea D. Usefulness of the Electrocardiogram in a Patient Presenting with Right-Sided Pneumothorax and Presyncope. Diagnostics. 2021; 11(6):1069. https://doi.org/10.3390/diagnostics11061069
Chicago/Turabian StyleFlorescu, Lavinia Maria, Călina-Patricia Țentea, Csilla-Andrea Eötvös, Roxana-Daiana Lazar, Iulia-Georgiana Zehan, Wissam Sabha, Sorin Pop, Doina Adina Todea, and Dan Blendea. 2021. "Usefulness of the Electrocardiogram in a Patient Presenting with Right-Sided Pneumothorax and Presyncope" Diagnostics 11, no. 6: 1069. https://doi.org/10.3390/diagnostics11061069
APA StyleFlorescu, L. M., Țentea, C.-P., Eötvös, C.-A., Lazar, R.-D., Zehan, I.-G., Sabha, W., Pop, S., Todea, D. A., & Blendea, D. (2021). Usefulness of the Electrocardiogram in a Patient Presenting with Right-Sided Pneumothorax and Presyncope. Diagnostics, 11(6), 1069. https://doi.org/10.3390/diagnostics11061069





