Prognostic Value of Combing Primary Tumor and Nodal Glycolytic–Volumetric Parameters of 18F-FDG PET in Patients with Non-Small Cell Lung Cancer and Regional Lymph Node Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Protocol and Analysis of 18F-FDG PET Scan
2.3. Follow-Up of Study Participants
2.4. Data Analysis
2.5. Survival Model Validation and Comparison
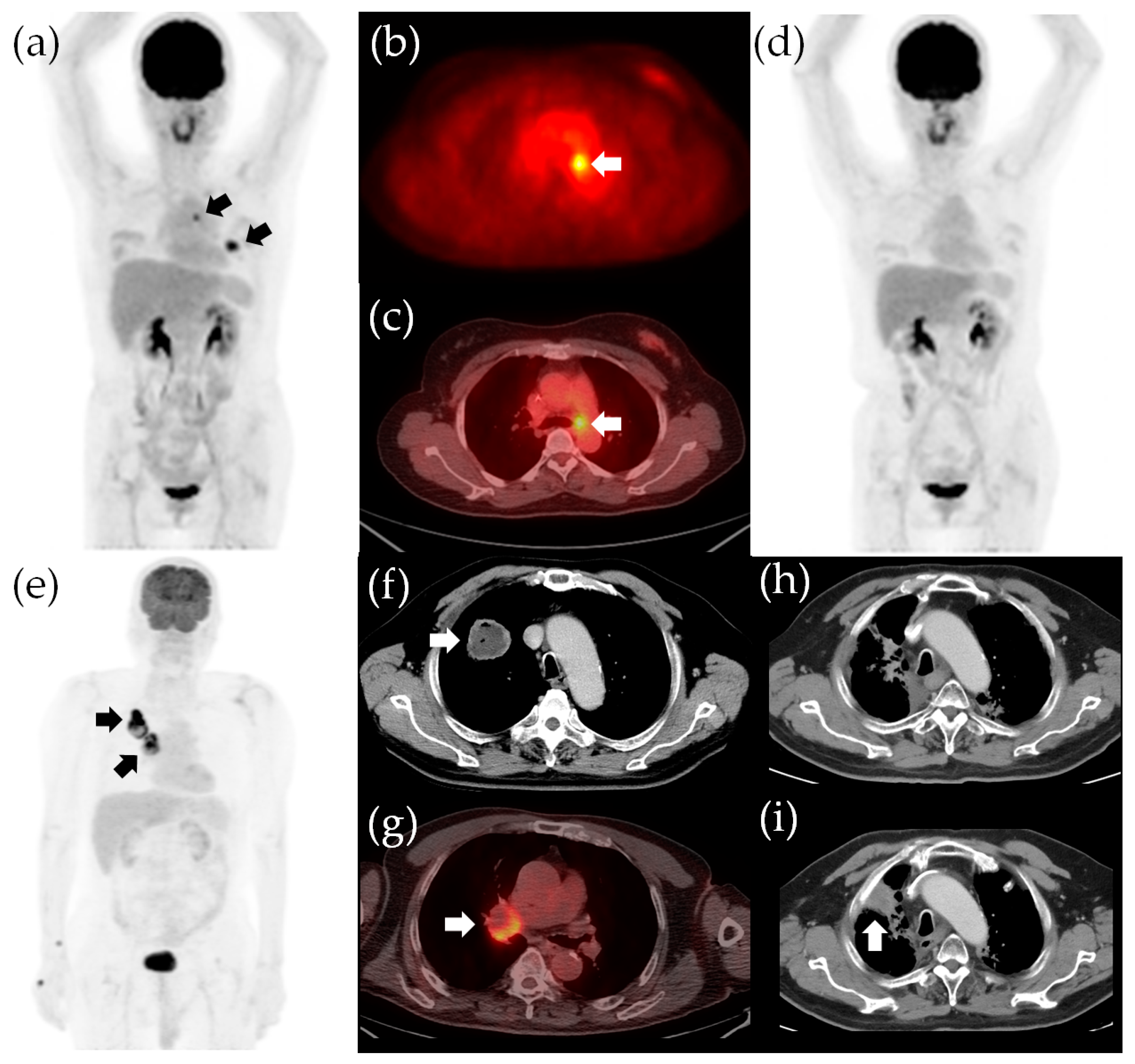
3. Results
3.1. Patient Characteristics
3.2. Univariate and Multivariate Survival Analyses
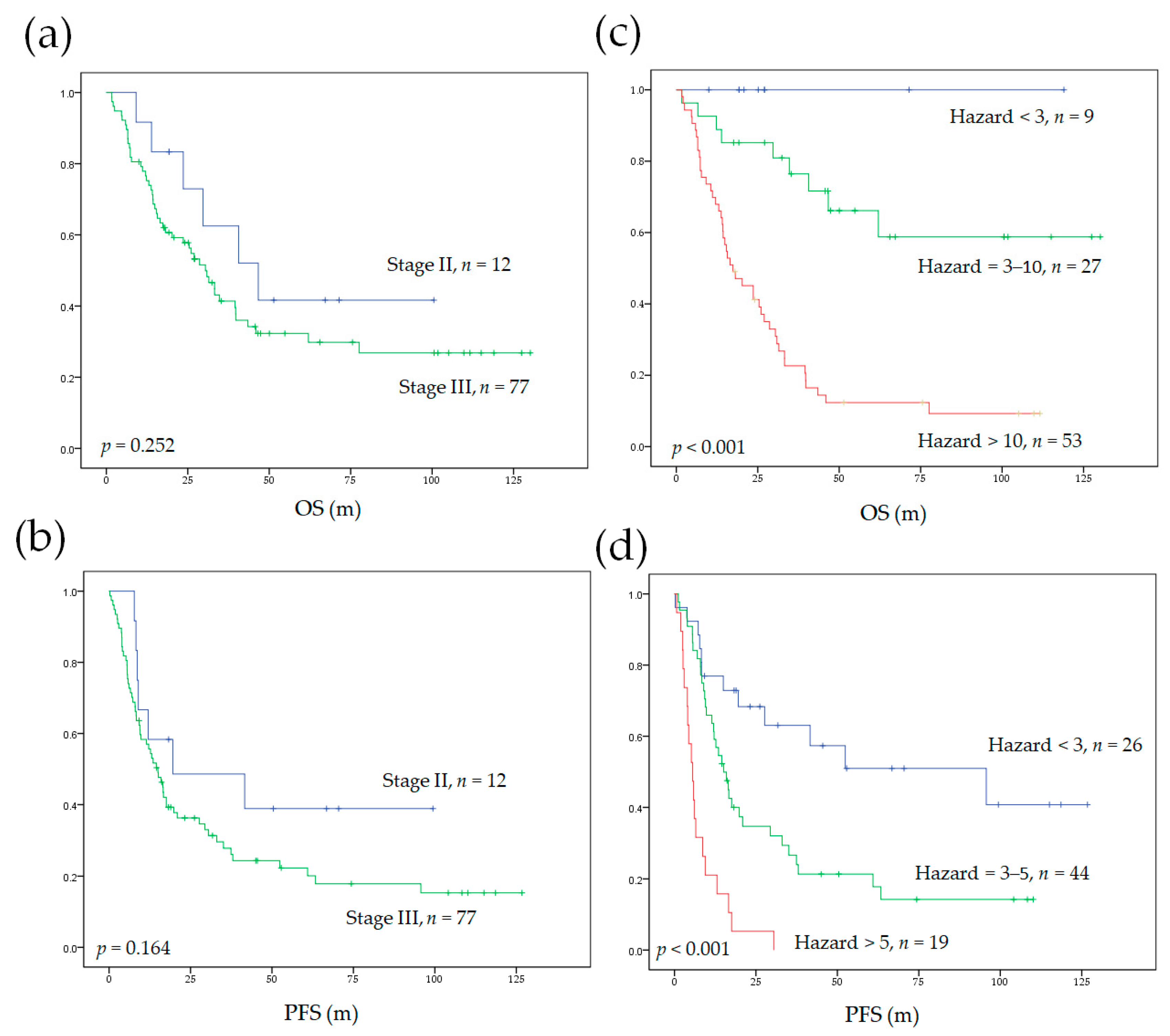
3.3. Survival Model Construction and Validation
3.4. Model Performance and Comparison to AJCC Staging System
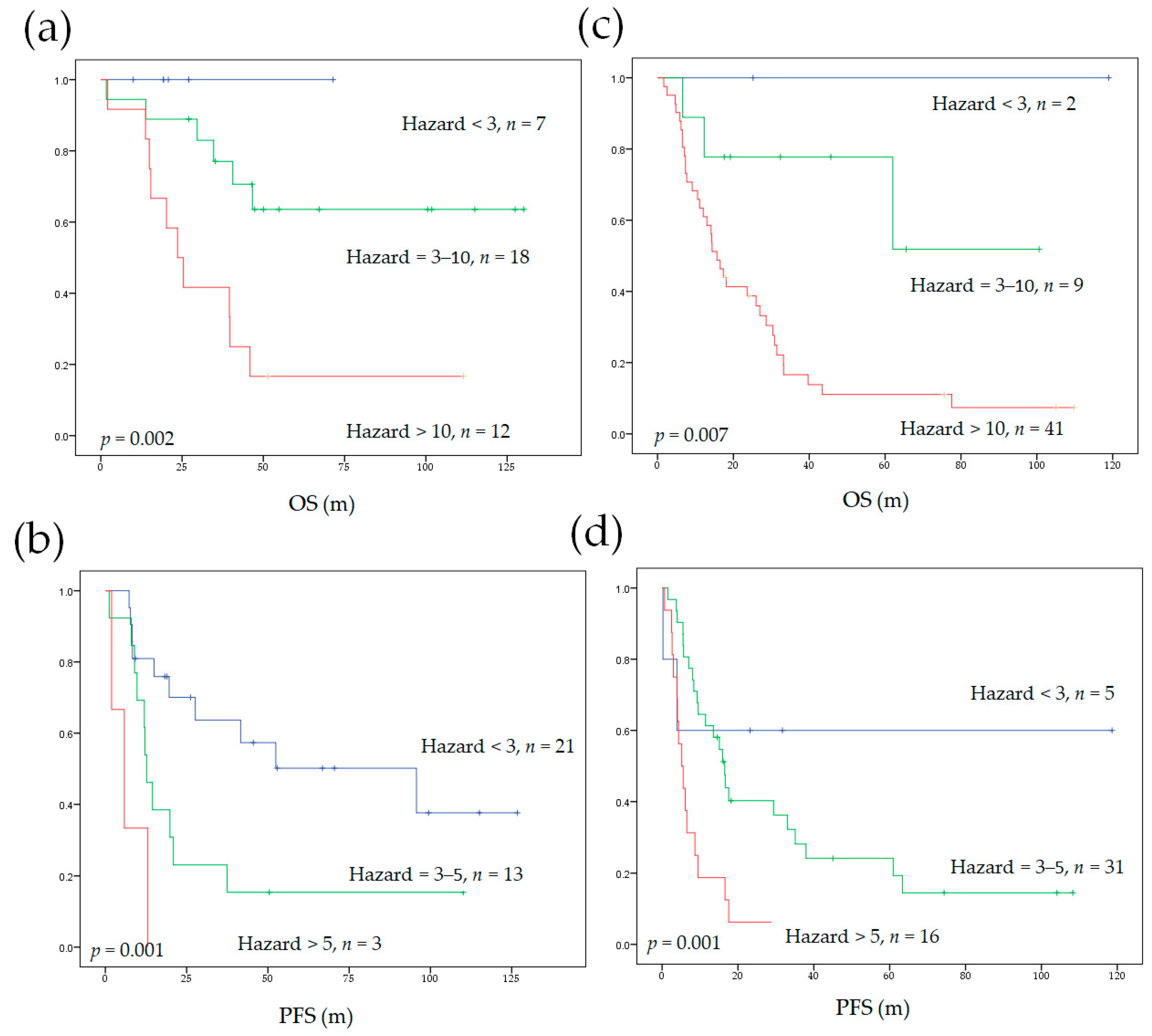
3.5. Model Performance in Subgroups of Different Initial Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K.M. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Jin, F.; Qu, B.; Fu, Z.; Zhang, Y.; Han, A.; Kong, L.; Yu, J. Prognostic Value of Metabolic Parameters of Metastatic Lymph Nodes on (18)F-FDG PET/CT in Patients with Limited-stage Small-cell Lung Cancer with Lymph Node Involvement. Clin. Lung Cancer 2018, 19, e101–e108. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Chai, Z.D.; Tan, L.L.; Wang, Z.Y.; Chen, Z.J.; Le, H.B.; Zhu, W.Y. Association of lymph node involvement with the prognosis of pathological T1 invasive non-small cell lung cancer. World J. Surg Oncol. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, S.; Aokage, K.; Ishii, G.; Nakasone, S.; Sakai, T.; Okada, S.; Miyoshi, T.; Tane, K.; Tsuboi, M. Prognostic Impact of the Number of Metastatic Lymph Nodes on the Eighth Edition of the TNM Classification of NSCLC. J. Thorac. Oncol. 2019, 14, 1408–1418. [Google Scholar] [CrossRef]
- Kim, H.C.; Ji, W.; Lee, J.C.; Kim, H.R.; Song, S.Y.; Choi, C.M.; Korean Association for Lung, C.; Korea Central Cancer, R. Prognostic Factor and Clinical Outcome in Stage III Non-Small Cell Lung Cancer: A Study Based on Real-World Clinical Data in the Korean Population. Cancer Res. Treat. 2021. [Google Scholar] [CrossRef]
- Aragaki, M.; Kato, T.; Fujiwara-Kuroda, A.; Hida, Y.; Kaga, K.; Wakasa, S. Preoperative identification of clinicopathological prognostic factors for relapse-free survival in clinical N1 non-small cell lung cancer: A retrospective single center-based study. J. Cardiothorac. Surg. 2020, 15, 229. [Google Scholar] [CrossRef]
- Xu, L.; Su, H.; She, Y.; Dai, C.; Zhao, M.; Gao, J.; Xie, H.; Ren, Y.; Xie, D.; Chen, C. Which N Descriptor Is More Predictive of Prognosis in Resected Non-Small Cell Lung Cancer: The Number of Involved Nodal Stations or the Location-Based Pathological N Stage? Chest 2020. [Google Scholar] [CrossRef]
- Sakao, Y.; Okumura, S.; Mun, M.; Uehara, H.; Ishikawa, Y.; Nakagawa, K. Prognostic heterogeneity in multilevel N2 non-small cell lung cancer patients: Importance of lymphadenopathy and occult intrapulmonary metastases. Ann. Thorac. Surg. 2010, 89, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Chansky, K.; Groome, P.; Bolejack, V.; Crowley, J.; Shemanski, L.; Kennedy, C.; Krasnik, M.; Peake, M.; Rami-Porta, R.; et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1433–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinert, H.C. PET in lung cancer. Chang. Gung Med. J. 2005, 28, 296–305. [Google Scholar]
- Cuaron, J.; Dunphy, M.; Rimner, A. Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front. Oncol. 2012, 2, 208. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Ma, F.; Wang, Y.; Hao, L.; Zeng, H.; Jia, C.; Wang, Y.; Liu, P.; Ong, I.M.; Li, B.; et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 2017, 19, 1358–1370. [Google Scholar] [CrossRef]
- Yu, M.; Chen, S.; Hong, W.; Gu, Y.; Huang, B.; Lin, Y.; Zhou, Y.; Jin, H.; Deng, Y.; Tu, L.; et al. Prognostic role of glycolysis for cancer outcome: Evidence from 86 studies. J. Cancer Res. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Carvalho, S.; Leijenaar, R.T.H.; Troost, E.G.C.; van Timmeren, J.E.; Oberije, C.; van Elmpt, W.; de Geus-Oei, L.F.; Bussink, J.; Lambin, P. 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET)-Radiomics of metastatic lymph nodes and primary tumor in non-small cell lung cancer (NSCLC)—A prospective externally validated study. PLoS ONE 2018, 13, e0192859. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Wang, T.F.; Chu, S.C.; Lin, C.B.; Wang, L.Y.; Lue, K.H.; Liu, S.H.; Chan, S.C. Incorporating radiomic feature of pretreatment 18F-FDG PET improves survival stratification in patients with EGFR-mutated lung adenocarcinoma. PLoS ONE 2020, 15, e0244502. [Google Scholar] [CrossRef]
- Roengvoraphoj, O.; Kasmann, L.; Eze, C.; Taugner, J.; Gjika, A.; Tufman, A.; Hadi, I.; Li, M.; Mille, E.; Gennen, K.; et al. Maximum standardized uptake value of primary tumor (SUVmax_PT) and horizontal range between two most distant PET-positive lymph nodes predict patient outcome in inoperable stage III NSCLC patients after chemoradiotherapy. Transl. Lung Cancer Res. 2020, 9, 541–548. [Google Scholar] [CrossRef]
- Pellegrino, S.; Fonti, R.; Pulcrano, A.; Del Vecchio, S. PET-Based Volumetric Biomarkers for Risk Stratification of Non-Small Cell Lung Cancer Patients. Diagnostics 2021, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.W.; Wang, W.X.; Wu, M.J.; Zhu, Y.C.; Zhuang, W.; Lin, G.; Du, K.Q.; Huang, Y.J.; Chen, Y.P.; Chen, G.; et al. Comparison of the c-MET gene amplification between primary tumor and metastatic lymph nodes in non-small cell lung cancer. Thorac. Cancer 2017, 8, 417–422. [Google Scholar] [CrossRef]
- Daniele, L.; Cassoni, P.; Bacillo, E.; Cappia, S.; Righi, L.; Volante, M.; Tondat, F.; Inghirami, G.; Sapino, A.; Scagliotti, G.V.; et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Hess, L.M.; Smith, D.; Cui, Z.L.; Montejano, L.; Liepa, A.M.; Schelman, W.; Bowman, L. The relationship between Eastern Cooperative Oncology Group performance status and healthcare resource utilization among patients with advanced or metastatic colorectal, lung or gastric cancer. J. Drug Assess. 2020, 10, 10–17. [Google Scholar] [CrossRef]
- Albain, K.S.; Crowley, J.J.; Turrisi, A.T., 3rd; Gandara, D.R.; Farrar, W.B.; Clark, J.I.; Beasley, K.R.; Livingston, R.B. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J. Clin. Oncol. 2002, 20, 3454–3460. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez Aguillo, M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Okour, M.; Jacobson, P.A.; Israni, A.; Brundage, R.C. Comparative Evaluation of Median Versus Youden Index Dichotomization Methods: Exposure-Response Analysis of Mycophenolic Acid and Acyl-Glucuronide Metabolite. Eur. J. Drug Metab. Pharm. 2019, 44, 629–638. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lue, K.H.; Chu, S.C.; Chang, B.S.; Wang, L.Y.; Liu, D.W.; Liu, S.H.; Chao, Y.K.; Chan, S.C. Combining the radiomic features and traditional parameters of (18)F-FDG PET with clinical profiles to improve prognostic stratification in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy and surgery. Ann. Nucl. Med. 2019, 33, 657–670. [Google Scholar] [CrossRef]
- Lue, K.H.; Wu, Y.F.; Lin, H.H.; Hsieh, T.C.; Liu, S.H.; Chan, S.C.; Chen, Y.H. Prognostic Value of Baseline Radiomic Features of (18)F-FDG PET in Patients with Diffuse Large B-Cell Lymphoma. Diagnostics 2020, 11, 36. [Google Scholar] [CrossRef]
- Kang, L.; Chen, W.; Petrick, N.A.; Gallas, B.D. Comparing two correlated C indices with right-censored survival outcome: A one-shot nonparametric approach. Stat. Med. 2015, 34, 685–703. [Google Scholar] [CrossRef] [Green Version]
- Dehing-Oberije, C.; De Ruysscher, D.; van der Weide, H.; Hochstenbag, M.; Bootsma, G.; Geraedts, W.; Pitz, C.; Simons, J.; Teule, J.; Rahmy, A.; et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1039–1044. [Google Scholar] [CrossRef]
- Roengvoraphoj, O.; Wijaya, C.; Eze, C.; Li, M.; Dantes, M.; Taugner, J.; Tufman, A.; Huber, R.M.; Belka, C.; Manapov, F. Analysis of primary tumor metabolic volume during chemoradiotherapy in locally advanced non-small cell lung cancer. Strahlenther Onkol. 2018, 194, 107–115. [Google Scholar] [CrossRef]
- Kanyilmaz, G.; Benli Yavuz, B.; Aktan, M.; Sahin, O. Prognostic importance of (18)F-fluorodeoxyglucose uptake by positron emission tomography for stage III non-small cell lung cancer treated with definitive chemoradiotherapy. Rev. Esp. Med. Nucl. Imagen Mol. 2020, 39, 20–26. [Google Scholar]
- Chen, H.H.; Chiu, N.T.; Su, W.C.; Guo, H.R.; Lee, B.F. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 2012, 264, 559–566. [Google Scholar] [CrossRef]
- Pellegrino, S.; Fonti, R.; Mazziotti, E.; Piccin, L.; Mozzillo, E.; Damiano, V.; Matano, E.; De Placido, S.; Del Vecchio, S. Total metabolic tumor volume by 18F-FDG PET/CT for the prediction of outcome in patients with non-small cell lung cancer. Ann. Nucl. Med. 2019, 33, 937–944. [Google Scholar] [CrossRef]
- Lapa, P.; Oliveiros, B.; Marques, M.; Isidoro, J.; Alves, F.C.; Costa, J.M.N.; Costa, G.; de Lima, J.P. Metabolic tumor burden quantified on [(18)F]FDG PET/CT improves TNM staging of lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2169–2178. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.X.; Liu, H.; Appelbaum, D.; Meng, J.; Penney, B.C. Developing and validating a novel metabolic tumor volume risk stratification system for supplementing non-small cell lung cancer staging. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yoon, J.K.; Park, K.J.; Lee, S.J. Prediction of occult lymph node metastasis using volume-based PET parameters in small-sized peripheral non-small cell lung cancer. Cancer Imaging 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Song, B.I.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Metabolic parameters using (1)(8)F-FDG PET/CT correlate with occult lymph node metastasis in squamous cell lung carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Torresano, L.; Nuevo-Tapioles, C.; Santacatterina, F.; Cuezva, J.M. Metabolic reprogramming and disease progression in cancer patients. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165721. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Arshad, M.; Butnariu, M.; Menzel, A.; Bjorklund, G. Krebs cycle: Activators, inhibitors and their roles in the modulation of carcinogenesis. Arch. Toxicol. 2021, 95, 1161–1178. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Fujimoto, D.; Morimoto, T.; Kumagai, T.; Tamiya, A.; Taniguchi, Y.; Yokoyama, T.; Ishida, T.; Matsumoto, H.; Hirano, K.; et al. Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: A retrospective multicenter study. BMC Cancer 2021, 21, 346. [Google Scholar] [CrossRef]
- Mallen, A.; Todd, S.; Robertson, S.E.; Kim, J.; Sehovic, M.; Wenham, R.M.; Extermann, M.; Chon, H.S. Impact of age, comorbidity, and treatment characteristics on survival in older women with advanced high grade epithelial ovarian cancer. Gynecol. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chang, K.P.; Chu, S.C.; Yen, T.C.; Wang, L.Y.; Chang, J.T.; Hsu, C.L.; Ng, S.H.; Liu, S.H.; Chan, S.C. Value of early evaluation of treatment response using (18)F-FDG PET/CT parameters and the Epstein-Barr virus DNA load for prediction of outcome in patients with primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Driessen, E.; Detillon, D.; Bootsma, G.; De Ruysscher, D.; Veen, E.; Aarts, M.; Janssen-Heijnen, M. Population-based patterns of treatment and survival for patients with stage I and II non-small cell lung cancer aged 65-74years and 75years or older. J. Geriatr. Oncol. 2019, 10, 547–554. [Google Scholar] [CrossRef]
- Tew, W.P. Ovarian cancer in the older woman. J. Geriatr. Oncol. 2016, 7, 354–361. [Google Scholar] [CrossRef]
- Dorshkind, K.; Montecino-Rodriguez, E.; Signer, R.A. The ageing immune system: Is it ever too old to become young again? Nat. Rev. Immunol. 2009, 9, 57–62. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J. Aging of the T cell compartment in mice and humans: From no naive expectations to foggy memories. J. Immunol. 2014, 193, 2622–2629. [Google Scholar] [CrossRef]
- Briceno, O.; Lissina, A.; Wanke, K.; Afonso, G.; von Braun, A.; Ragon, K.; Miquel, T.; Gostick, E.; Papagno, L.; Stiasny, K.; et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell 2016, 15, 14–21. [Google Scholar] [CrossRef]
- Tubin, S.; Khan, M.K.; Gupta, S.; Jeremic, B. Biology of NSCLC: Interplay between Cancer Cells, Radiation and Tumor Immune Microenvironment. Cancers 2021, 13, 775. [Google Scholar] [CrossRef]
- Dickhoff, C.; Dahele, M.; Paul, M.A.; van de Ven, P.M.; de Langen, A.J.; Senan, S.; Smit, E.F.; Hartemink, K.J. Salvage surgery for locoregional recurrence or persistent tumor after high dose chemoradiotherapy for locally advanced non-small cell lung cancer. Lung Cancer 2016, 94, 108–113. [Google Scholar] [CrossRef]
- Hamada, A.; Soh, J.; Mitsudomi, T. Salvage surgery after definitive chemoradiotherapy for patients with non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 555–562. [Google Scholar] [CrossRef]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Wei, Y.C.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Yin, R.; et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial. J. Clin. Oncol. 2021, 39, 713–722. [Google Scholar] [CrossRef]
- Liang, H.; Deng, H.; Liang, W.; Guo, K.; Gao, Z.; Wiesel, O.; Flores, R.M.; Song, K.; Redwan, B.; Migliore, M.; et al. Perioperative chemoimmunotherapy in a patient with stage IIIB non-small cell lung cancer. Ann. Transl. Med. 2020, 8, 245. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; Garcia-Campelo, M.R.; Casal-Rubio, J.; Domine, M.; Majem, M.; Rodriguez-Abreu, D.; Martinez-Marti, A.; De Castro Carpeno, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. 2020, 15, 1281–1297. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, J.; Cai, X.; Pan, C.; Long, Y.; Chen, J.; Zhou, C.; Yin, X. Prognostic value of epidermal growth factor receptor mutations in resected non-small cell lung cancer: A systematic review with meta-analysis. PLoS ONE 2014, 9, e106053. [Google Scholar] [CrossRef]
- Collins, G.S.; Ogundimu, E.O.; Altman, D.G. Sample size considerations for the external validation of a multivariable prognostic model: A resampling study. Stat. Med. 2016, 35, 214–226. [Google Scholar] [CrossRef]

| Characteristics | Value |
|---|---|
| Age, years, mean ± SD | 67 ± 11.4 |
| Sex, n (%) | |
| Male | 59 (66.3) |
| Female | 30 (33.7) |
| Histology | |
| Adenocarcinoma | 43 (48.3) |
| Squamous cell carcinoma | 45 (50.6) |
| NSCLC—otherwise specified | 1 (1.1) |
| T classification, n (%) a | |
| T1b | 2 (2.2) |
| T1c | 16 (18.0) |
| T2a | 10 (11.2) |
| T2b | 11 (12.3) |
| T3 | 25 (28.1) |
| T4 | 25 (28.1) |
| N classification, n (%) a | |
| N1 | 15 (16.9) |
| N2 | 53 (59.6) |
| N3 | 21 (23.6) |
| Overall stage, n (%) a | |
| Stage IIB | 12 (13.5) |
| Stage IIIA | 23 (25.8) |
| Stage IIIB | 41 (46.1) |
| Stage IIIC | 13 (14.6) |
| ECOG, n (%) | |
| 0 | 20 (22.5) |
| 1 | 59 (66.3) |
| 2 | 9 (10.1) |
| 3 | 1 (1.1) |
| Initial treatment, n (%) | |
| Surgery | 30 (33.7) |
| Neoadjuvant CCRT and surgery | 7 (7.9) |
| Definitive CCRT | 33 (37.0) |
| Definitive Radiotherapy | 19 (21.4) |
| Time from 18F-FDG PET to initial treatment, d, median (IQR) | 14 (12) |
| Quantitative analysis of 18F-FDG PET, mean ± SD | |
| Primary tumor SUVmax | 11.3 ± 5.22 |
| Primary tumor TLG | 292.1 ± 420.86 |
| Nodal SUVmax | 7.0 ± 4.96 |
| NTSUVR | 0.68 ± 0.415 |
| Nodal TLG | 67.4 ± 161.46 |
| NTTLGR | 0.79 ± 2.023 |
| total TLG | 359.6 ± 489.10 |
| TNSUVproduct | 89.7 ± 89.74 |
| Variable | No. | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age | <0.001 | 0.001 | 0.001 | <0.001 | |||||
| >75.5 | 24 | 2.8 (1.6–4.9) | 2.6 (1.5–4.6) | 2.5 (1.5–4.2) | 2.7 (1.6–4.7) | ||||
| ≤75.5 | 65 | Reference | Reference | Reference | Reference | ||||
| Histopathology | 0.012 | 0.126 | 0.175 | NA | |||||
| Squamous cell | 45 | 2.0 (1.2–3.5) | 1.4 (0.9–2.3) | ||||||
| Others | 44 | Reference | Reference | ||||||
| At least T2 disease | 0.045 | 0.590 | 0.053 | NA | |||||
| Yes | 71 | 2.3 (1.0–5.0) | 2.0 (1.0–3.9) | ||||||
| No | 18 | Reference | Reference | ||||||
| N3 disease | 0.303 | NA | 0.408 | NA | |||||
| Yes | 21 | 1.4 (0.8–2.5) | 1.3 (0.7–2.2) | ||||||
| No | 68 | Reference | Reference | ||||||
| Staging | 0.257 | NA | 0.169 | NA | |||||
| Stage III | 77 | 1.6 (0.7–3.8) | 1.7 (0.8–3.8) | ||||||
| Stage II | 12 | Reference | Reference | ||||||
| ECOG status | 0.007 | 0.022 | 0.084 | NA | |||||
| ECOG > 0 | 69 | 4.1 (1.5–11.4) | 3.3 (1.2–9.4) | 1.8 (0.9–3.6) | |||||
| ECOG = 0 | 20 | Reference | Reference | Reference | |||||
| Received surgery a | 0.001 | 0.240 | 0.030 | 0.938 | |||||
| Absence | 52 | 2.6 (1.5–4.7) | 1.8 (1.1–2.9) | ||||||
| Presence | 37 | Reference | Reference | ||||||
| Radiotherapy only b | <0.001 | 0.338 | 0.002 | 0.642 | |||||
| Yes | 19 | 3.2 (1.8–5.6) | 2.4 (1.4–4.2) | ||||||
| No | 70 | Reference | Reference | ||||||
| Primary tumor SUVmax | <0.001 | 0.135 | <0.001 | 0.238 | |||||
| >8.05 | 62 | 4.9 (2.2–10.9) | 3.0 (1.6–5.6) | ||||||
| ≤8.05 | 27 | Reference | Reference | ||||||
| Primary tumor TLG | 0.001 | 0.873 | 0.024 | 0.064 | |||||
| >42.5 | 63 | 3.7 (1.7–8.3) | 2.0 (1.1–3.6) | ||||||
| ≤42.5 | 26 | Reference | Reference | ||||||
| Nodal SUVmax | 0.012 | 0.114 | 0.204 | NA | |||||
| >2.94 | 70 | 3.0 (1.3–6.9) | 1.5 (0.8–2.8) | ||||||
| ≤2.94 | 19 | Reference | Reference | ||||||
| Nodal TLG | 0.014 | 0.454 | 0.104 | NA | |||||
| >18.3 | 40 | 2.0 (1.1–3.3) | 1.5 (0.9–2.4) | ||||||
| ≤18.3 | 49 | Reference | Reference | ||||||
| total TLG | <0.001 | <0.001 | 0.001 | <0.001 | |||||
| >81 | 63 | 5.2 (2.2–12.2) | 5.1 (2.2–12.0) | 3.0 (1.6–5.7) | 3.3 (1.7–6.2) | ||||
| ≤81 | 26 | Reference | Reference | Reference | Reference | ||||
| TNSUVproduct | <0.001 | 0.164 | 0.011 | 0.481 | |||||
| >27 | 67 | 5.3 (2.1–13.3) | 2.3 (1.2–4.3) | ||||||
| ≤27 | 22 | Reference | Reference | ||||||
| Model | c-Index for OS | p-Value d | c-Index for PFS | p-Value d |
|---|---|---|---|---|
| AJCC staging system a | 0.544 | NA | 0.521 | NA |
| Our Cox regression model | 0.732 | <0.001 | 0.672 | <0.001 |
| Model with primary tumor TLG b | 0.696 | 0.002 | 0.639 | 0.012 |
| Model with nodal TLG c | 0.708 | 0.001 | 0.632 | 0.010 |
| Initial Surgery Group (n = 37) a | ||||
| Model | c-Index for OS | p-Value | c-Index for PFS | p-value |
| Our Cox regression model | 0.742 | NA | 0.657 | NA |
| AJCC staging system b | 0.513 | <0.001 | 0.531 | 0.074 |
| Initial non-surgery group (n = 52) | ||||
| Model | c-index for OS | p-value | c-index for PFS | p-Value |
| Our Cox regression model | 0.667 | NA | 0.627 | NA |
| AJCC staging system b | 0.466 | 0.004 | 0.441 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Chu, S.-C.; Wang, L.-Y.; Wang, T.-F.; Lue, K.-H.; Lin, C.-B.; Chang, B.-S.; Liu, D.-W.; Liu, S.-H.; Chan, S.-C. Prognostic Value of Combing Primary Tumor and Nodal Glycolytic–Volumetric Parameters of 18F-FDG PET in Patients with Non-Small Cell Lung Cancer and Regional Lymph Node Metastasis. Diagnostics 2021, 11, 1065. https://doi.org/10.3390/diagnostics11061065
Chen Y-H, Chu S-C, Wang L-Y, Wang T-F, Lue K-H, Lin C-B, Chang B-S, Liu D-W, Liu S-H, Chan S-C. Prognostic Value of Combing Primary Tumor and Nodal Glycolytic–Volumetric Parameters of 18F-FDG PET in Patients with Non-Small Cell Lung Cancer and Regional Lymph Node Metastasis. Diagnostics. 2021; 11(6):1065. https://doi.org/10.3390/diagnostics11061065
Chicago/Turabian StyleChen, Yu-Hung, Sung-Chao Chu, Ling-Yi Wang, Tso-Fu Wang, Kun-Han Lue, Chih-Bin Lin, Bee-Song Chang, Dai-Wei Liu, Shu-Hsin Liu, and Sheng-Chieh Chan. 2021. "Prognostic Value of Combing Primary Tumor and Nodal Glycolytic–Volumetric Parameters of 18F-FDG PET in Patients with Non-Small Cell Lung Cancer and Regional Lymph Node Metastasis" Diagnostics 11, no. 6: 1065. https://doi.org/10.3390/diagnostics11061065
APA StyleChen, Y.-H., Chu, S.-C., Wang, L.-Y., Wang, T.-F., Lue, K.-H., Lin, C.-B., Chang, B.-S., Liu, D.-W., Liu, S.-H., & Chan, S.-C. (2021). Prognostic Value of Combing Primary Tumor and Nodal Glycolytic–Volumetric Parameters of 18F-FDG PET in Patients with Non-Small Cell Lung Cancer and Regional Lymph Node Metastasis. Diagnostics, 11(6), 1065. https://doi.org/10.3390/diagnostics11061065






