Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission
Abstract
1. Introduction
2. Materials and Methods
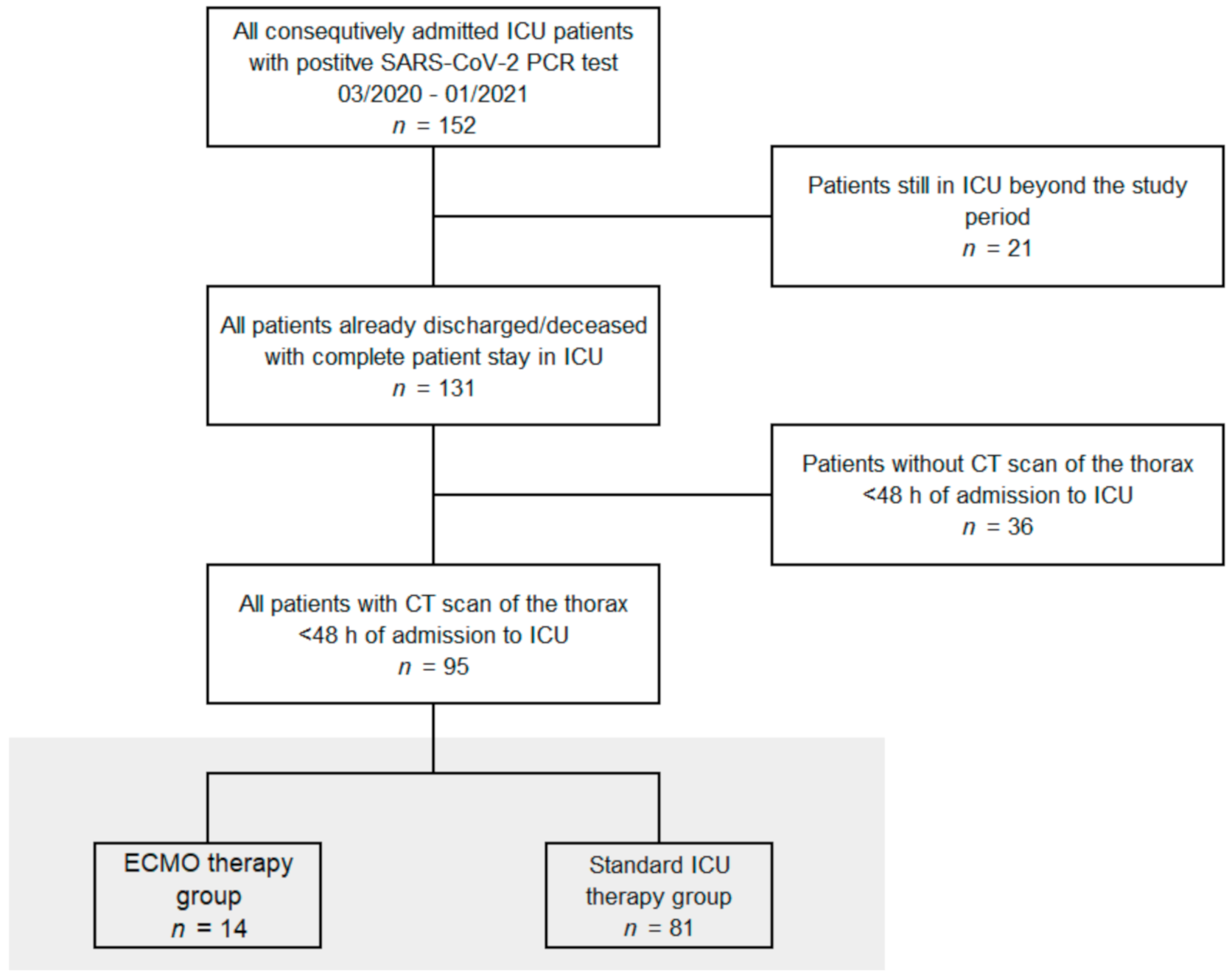
2.1. Patient Data
2.2. Image Acquisition
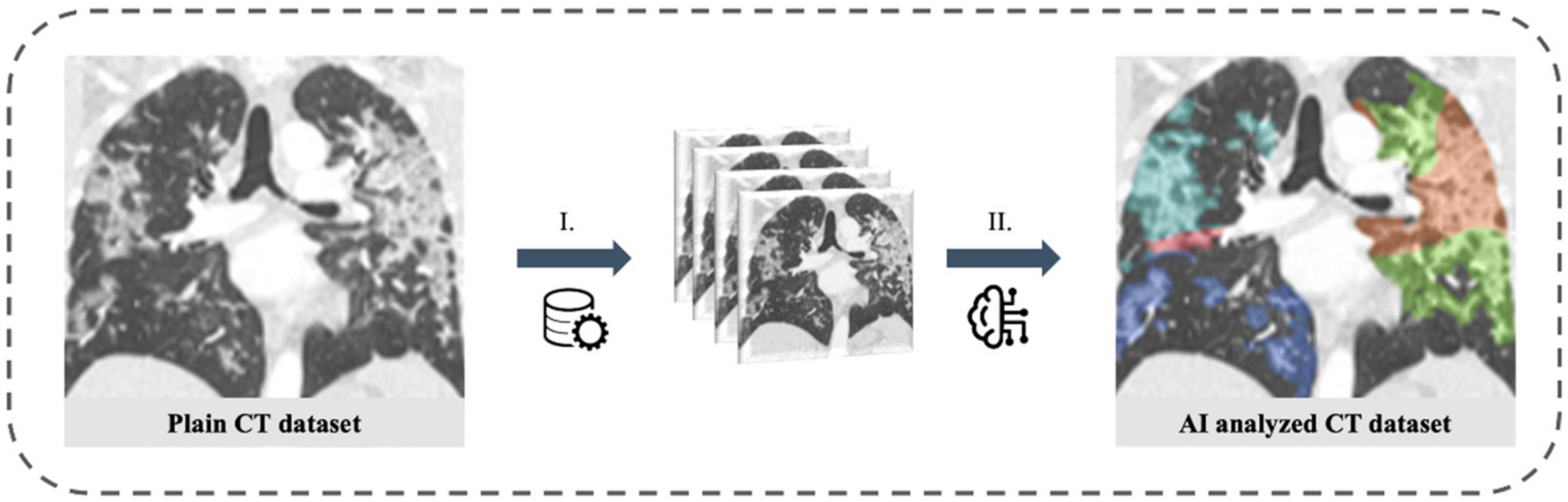
2.3. Artificial Intelligence Based Quantification of Lung Involvement
2.4. Prediction Parameters for the Regression Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics and Demographic Data
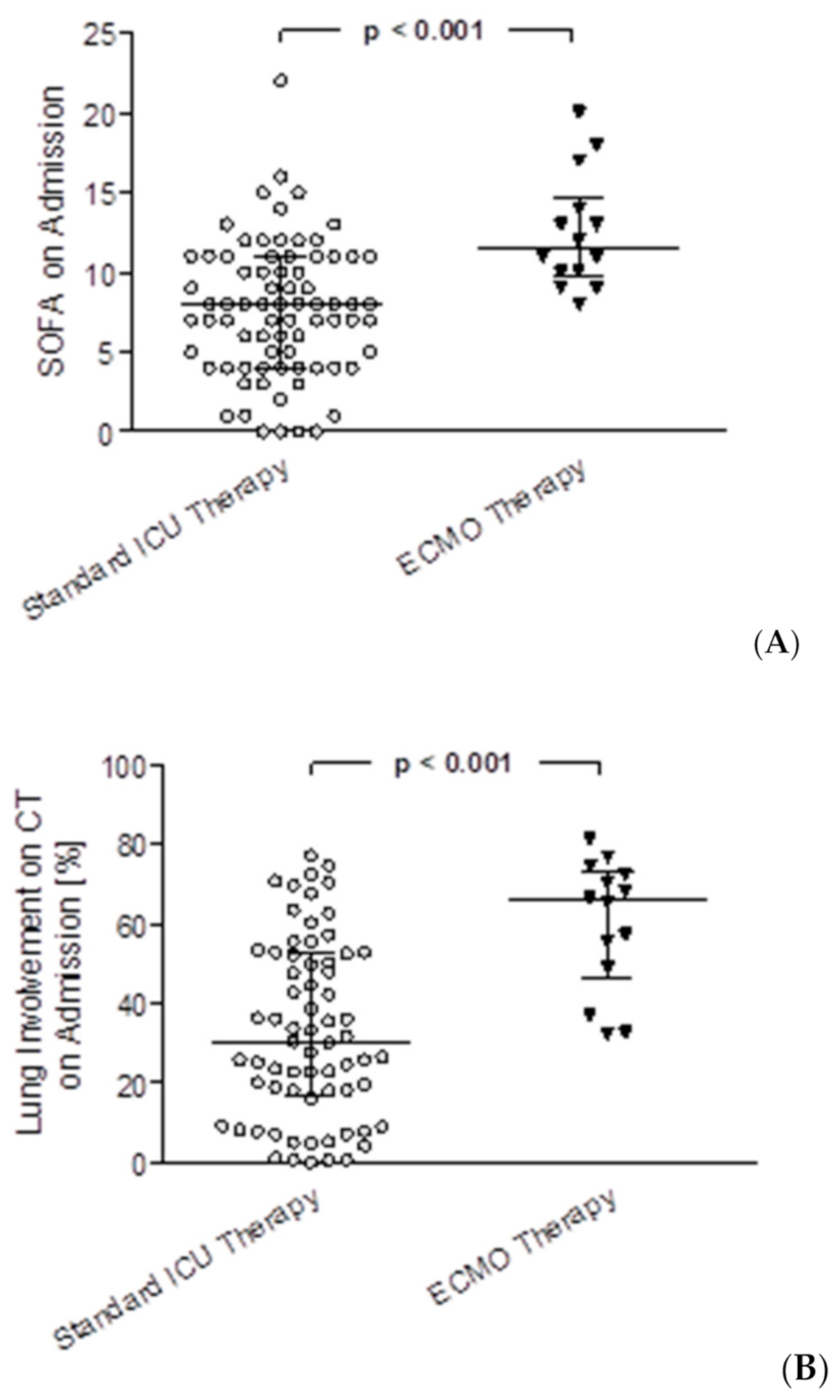
3.2. Differences between the ECMO Group and ICU Standard Therapy Group
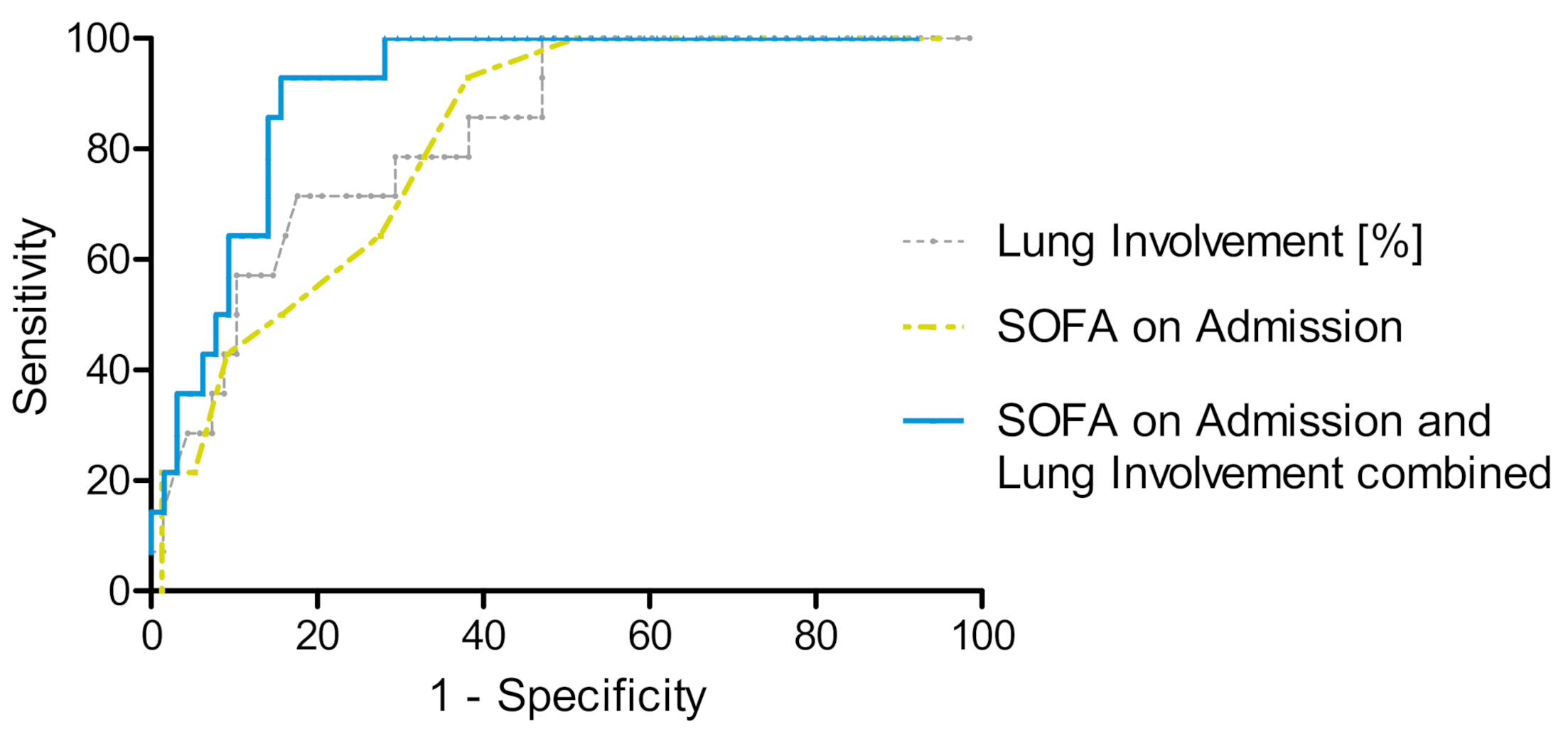
3.3. Risk Stratification for ECMO Therapy
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, F.B.; Cisewski, J.A.; Miniño, A.; Anderson, R.N. Provisional Mortality Data-United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 519–522. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case Characteristics, Resource Use, and Outcomes of 10 021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Abate, S.M.; Ali, S.A.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit Admission and Outcomes among Patients with Coronavirus: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef]
- Immovilli, P.; Morelli, N.; Antonucci, E.; Radaelli, G.; Barbera, M.; Guidetti, D. COVID-19 Mortality and ICU Admission: The Italian Experience. Crit. Care 2020, 24, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients In-fected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Song, J.; Deane, A.M.; Plummer, M.P. Global Impact of Coronavirus Disease 2019 Infection Requiring Admission to the ICU. Chest 2021, 159, 524–536. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Kane, A.D.; Cook, T.M. Outcomes from Intensive Care in Patients with COVID-19: A Systematic Review and Meta-Analysis of Observational Studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef]
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; McAuley, D.F.; Firmin, R.K.; et al. Referral to an Extracorporeal Membrane Oxy-Genation Center and Mortality Among Patients with Severe 2009 Influenza A(H1N1). JAMA 2011, 306, 1659. [Google Scholar] [CrossRef]
- Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Life Support for Adults with Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Poor survival with Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome (ARDS) due to Coronavirus Disease 2019 (COVID-19): Pooled Analysis of Early Reports. J. Crit. Care 2020, 58, 27–28. [Google Scholar] [CrossRef]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.; et al. Guidelines on the Management of Acute Respiratory Distress Syndrome. BMJ Open Respir. Res. 2019, 6, e000420. [Google Scholar] [CrossRef]
- Laurent, P.; Jean-Marie, F.; Arnaud, G.; Christine, P.R.; Gilles, P.; Anderson, L.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Badulak, J.; Antonini, M.V.; Stead, C.M.; Shekerdemian, L.; Raman, L.; Paden, M.L.; Agerstrand, C.; Bartlett, R.H.; Barrett, N.; Combes, A.; et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 485–495. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: A Retrospective Cohort Study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- MacLaren, G.; Combes, A.; Brodie, D. What’s New in ECMO for COVID-19? Intensive Care Med. 2021, 47, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Supady, A.; Taccone, F.S.; Lepper, P.M.; Ziegeler, S.; Staudacher, D.L. Survival after Extracorporeal Membrane Oxygenation in Severe COVID-19 ARDS: Results from an International Multicenter Registry. Crit. Care 2021, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Membrane Oxygenation Support in COVID-19: An International Cohort Study of the Extracorporeal Life Support Organization Registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Biancari, F.; Mariscalco, G.; Dalén, M.; Settembre, N.; Welp, H.; Perrotti, A.; Wiebe, K.; Leo, E.; Loforte, A.; Chocron, S.; et al. Six-Month Survival After Extracorporeal Mem-brane Oxygenation for Severe COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1999–2006. [Google Scholar] [CrossRef]
- Ma, X.; Liang, M.; Ding, M.; Liu, W.; Ma, H.; Zhou, X.; Ren, H. Extracorporeal Membrane Oxygenation (ECMO) in Critically Ill Pa-tients with Coronavirus Disease 2019 (COVID-19) Pneumonia and Acute Respiratory Distress Syndrome (ARDS). Med. Sci. Monit. 2020, 26, p. e925364. Available online: https://www.medscimonit.com/abstract/index/idArt/925364 (accessed on 12 April 2021).
- Lorusso, R.; Combes, A.; Coco, V.L.; De Piero, M.E.; Belohlavek, J. ECMO for COVID-19 Patients in Europe and Israel. Intensive Care Med. 2021, 47, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.H. Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: EOLIA and Beyond. Crit. Care Med. 2019, 47, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Shaefi, S.; Brenner, S.K.; Gupta, S.; O’Gara, B.P.; Krajewski, M.L.; Charytan, D.M.; Chaudhry, S.; Mirza, S.H.; Peev, V. Extracorporeal Membrane Oxygenation in Patients with Severe Respiratory Failure from COVID-19. Intensive Care Med. 2021, 47, 208–221. [Google Scholar] [CrossRef]
- Giraud, R.; Legouis, D.; Assouline, B.; De Charriere, A.; Decosterd, D.; Brunner, M.; Moret-Bochatay, M.; Fumeaux, T.; Bendjelid, K. Timing of VV-ECMO Therapy Implementation Influences Prognosis of COVID-19 Patients. Physiol. Rep. 2021, 9, e14715. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Smits, H.; Knoops, A.J.; Korst, M.B.; Samson, T.; Scholten, E.T.; Schalekamp, S.; Schaefer-Prokop, C.M.; Philipsen, R.H.; Meijers, A.; et al. COVID-19 on Chest Radiographs: A Multi-Reader Evaluation of an Artificial Intelligence System. Radiology 2020, 296, E166–E172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Gerke, A.K.; Tang, F.; Cavanaugh, J.E.; Doerschug, K.C.; Polgreen, P.M. Increased Trend in Extracorporeal Membrane Oxygenation Use by Adults in the United States since 2007. BMC Res. Notes 2015, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and Economic Assessment of Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR): A Multicentre Randomised Controlled Trial. Lancet 2009, 374, 13. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.J.; Martin, I.B.; et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, Q.; Yao, S.; Luo, L.; Zhou, W.; Mao, X.; Li, J.; Duan, J.; Yan, Z.; Yang, M.; et al. Early Prediction of Disease Progression in COVID-19 Pneumonia Patients with Chest CT and Clinical Characteristics. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative Chest CT Analysis in COVID-19 to Predict the Need for Oxygenation Support and Intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.H.; Wani, A.H.; Shah, N.N.; Yaseen, M.; Jehangir, M. Chest CT Features of Coronavirus Disease-19 (COVID-19) Pneumonia: Which Findings on Initial CT can Predict an Adverse Short-Term Outcome? BJR Open 2020, 2, 20200016. [Google Scholar] [CrossRef] [PubMed]
- Gresser, E.; Rueckel, J.; Puhr-Westerheide, D.; Schwarze, V.; Fink, N.; Kunz, W.G.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; Ingrisch, M.; et al. Prognostic Value of Admission Chest CT Findings for Invasive Ventilation Therapy in COVID-19 Pneumonia. Diagnostics 2020, 10, 1108. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-End Lung Cancer Screening with Three-Dimensional Deep Learning on Low-Dose Chest Computed Tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef]
- Huang, P.; Lin, C.T.; Li, Y.; Tammemagi, M.C.; Brock, M.V.; Atkar-Khattra, S.; Xu, Y.; Hu, P.; Mayo, J.R.; Schmidt, H.; et al. Prediction of Lung Cancer Risk at Follow-Up Screening with Low-Dose CT: A Training and Validation Study of a Deep Learning Method. Lancet Digit. Health 2019, 1, e353–e362. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kawata, N.; Yokota, H.; Sugiura, T.; Matsumura, Y.; Higashide, T.; Horikoshi, T.; Oda, S.; Tatsumi, K.; Uno, T. A Predictive Factor for Patients with Acute Respiratory Distress Syndrome: CT Lung Volumetry of the Well-Aerated Region as an Automated Method. Eur. J. Radiol. 2020, 122, 108748. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-Acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N.; Fang, C.; Shan, F.; Mei, X.; Shi, J.; et al. CT Quantification of Pneumonia Lesions in Early Days Predicts Progression to Severe Illness in a Cohort of COVID-19 Patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Supady, A.; DellaVolpe, J.; Taccone, F.; Scharpf, D.; Ulmer, M.; Lepper, P.; Halbe, M.; Ziegeler, S.; Vogt, A.; Ramanan, R.; et al. Outcome Prediction in Patients with Severe COVID-19 Requiring Extracorporeal Membrane Oxygenation—A Retrospective International Multicenter Study. Membranes 2021, 11, 170. [Google Scholar] [CrossRef]
| COVID-19 ICU-patients (n = 95) | ||
|---|---|---|
| Patient Data | ||
| Age | 66 | (55–74) |
| Male Sex | 74 | (77.9%) |
| Body Mass Index | 27 | (25–33) |
| SOFA Score on Admission * | 8 | (5–11) |
| Lactate on Admission | 1.3 | (1.0–1.8) |
| Oxygenation Index on Admission ** | 168 | (110–229) |
| Comorbidities | ||
| Diabetes | 33 | (34.7%) |
| Hypertension | 59 | (62.1%) |
| Heart Disease | 32 | (33.7%) |
| Pulmonary Disease | 19 | (20.0%) |
| Chronic Kidney Disease | 9 | (9.5%) |
| Active Malignancy | 10 | (10.5%) |
| Immunosuppression | 7 | (7.4%) |
| ARDS Type on Admission ** | ||
| Mild | 25 | (30.1%) |
| Moderate | 40 | (48.2%) |
| Severe | 15 | (18.1%) |
| No ARDS on Admission | 3 | (3.6%) |
| CT Features on Admission ** | ||
| CT-Severity Score | 15 | (10–20) |
| CT-Percentage of Lung Involvement | 36 | (19–56) |
| Standard ICU Therapy (n = 81) | ECMO Therapy (n = 14) | p Value | |||
|---|---|---|---|---|---|
| Comparison of Patient Characteristics | |||||
| Age | 68 | (55–75) | 62 | (55–68) | p = 0.164 |
| Male Sex | 64 | (79.0%) | 10 | (71.4%) | p = 0.528 |
| BMI | 27 | (25–30) | 31 | (27–37) | p = 0.031 |
| ARDS Type | |||||
| Mild | 7 | (9.3%) | 0 | (0.0%) | n/a |
| Moderate | 34 | (45.3%) | 2 | (14.3%) | p = 0.006 |
| Severe | 34 | (45.3%) | 12 | (85.7%) | p = 0.029 |
| Patient Data during ICU Stay | |||||
| Days on ICU (including external ICUs) | 12.5 | (5.5–23.9) | 22.3 | (8.4–29.1) | p = 0.120 |
| Days on ICU (Survivors) | 12.1 | (5.5–20.6) | 51.9 | (39.6–64.2) | p = 0.014 |
| Number of Patients on Mechanical Ventilation | 59 | (72.8%) | 14 | (100%) | p = 0.026 |
| Hours on Ventilator | 157.6 | (0.0–401.1) | 516.0 | (192.9–698.5) | p = 0.003 |
| Hours on Ventilator (Survivors) | 113.9 | (0.0–327.2) | 1012.4 | (946.7–1078.1) | p = 0.014 |
| Hours on NIV | 3.3 | (0.0–12.3) | 0.8 | (0.0–25.3) | p = 0.006 |
| Number of Patients with HDF | 25 | (30.9%) | 13 | (92.9%) | p < 0.001 |
| Hours on Hemodiafiltration | 90.5 | (34.0–280.2) | 143.5 | (41.7–346.5) | p = 0.361 |
| Prone Position | 25 | (30.9%) | 8 | (57.1%) | p = 0.057 |
| Hours of Prone Position | 23.5 | (15.5–36.0) | 22.5 | (18–56.4) | p = 0.636 |
| SOFA mean* | 7.5 | (5.1–10.6) | 14.5 | (12.5–18.8) | p < 0.001 |
| SOFA max** | 12 | (8–15) | 18 | (15–22) | p < 0.001 |
| SOFA on Admission* | 8 | (4–11) | 12 | (10–14) | p < 0.001 |
| Oxygenation Index on Admission*** | 178 | (121–232) | 110 | (90–161) | p = 0.009 |
| CT Severity Score on Admission*** | 14 | (10–19) | 21 | (19–22) | p < 0.001 |
| CT Percentage of Lung Involvement on Admission*** | 30 | (17–53) | 66 | (49–72) | p < 0.001 |
| Disease Progression | |||||
| Time from Admission to SOFA max | 2 | (1–8) | 13 | (2–5) | p = 0.012 |
| Time from Admission to Death | 17 | (5–28) | 19 | (7–23) | p = 0.932 |
| Time from Admission to ECMO Placement (days) | n/a | 1.4 | (0.2–4.0) | n/a | |
| Delta SOFA from admission to max per day | 0.8 | (0.0–4.0) | 0.5 | (0.3–2.0) | p = 0.836 |
| Time from CT to ICU Admission (days) | 1 | (1–3) | 1 | (0–1) | p = 0.078 |
| Time from CT to ECMO Placement (days) | n/a | 2.5 | (1–5) | n/a | |
| Time from Hospital Admission to ICU Admission | 1 | (1–4) | 1 | (1–1) | p = 0.119 |
| Outcome | |||||
| In-hospital mortality | 24 | (29.6%) | 12 | (85.7%) | p < 0.001 |
| ECMO Therapy | |||
|---|---|---|---|
| Independent Variables | Odds Ratio | CI | p Value |
| Age | 1.003 | 0.924–1.089 | 0.936 |
| Sex | 0.509 | 0.064–4.073 | 0.525 |
| BMI | 1.065 | 0.934–1.214 | 0.350 |
| SOFA on Admission | 1.320 | 1.077–1.617 | 0.008 * |
| Lactate on Admission | 0.991 | 0.528–1.859 | 0.977 |
| CT Lung Involvement (%) on Admission | 1.059 | 1.013–1.106 | 0.011 * |
| Standard ICU Therapy (n = 68) vs. ECMO Therapy (n = 14) | AUC (95% CI) | Y-Index | Discriminative Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| SOFA Score on Admission | 0.82 | 0.72–0.91 | 0.50 | 8.5 | 0.93 | 0.57 |
| Standard ICU Therapy (n = 76) vs. ECMO Therapy (n = 14) | ||||||
| Lung Involvement on CT (%) | 0.83 | 0.73–0.93 | 0.54 | 55.7 | 0.71 | 0.82 |
| Standard ICU Therapy (n = 64) vs. ECMO Therapy (n = 14) | ||||||
| SOFA Score on Admission and Lung Involvement on CT (%) combined | 0.91 | 0.84–0.97 | 0.77 | 435 | 0.93 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gresser, E.; Reich, J.; Sabel, B.O.; Kunz, W.G.; Fabritius, M.P.; Rübenthaler, J.; Ingrisch, M.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; et al. Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission. Diagnostics 2021, 11, 1029. https://doi.org/10.3390/diagnostics11061029
Gresser E, Reich J, Sabel BO, Kunz WG, Fabritius MP, Rübenthaler J, Ingrisch M, Wassilowsky D, Irlbeck M, Ricke J, et al. Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission. Diagnostics. 2021; 11(6):1029. https://doi.org/10.3390/diagnostics11061029
Chicago/Turabian StyleGresser, Eva, Jakob Reich, Bastian O. Sabel, Wolfgang G. Kunz, Matthias P. Fabritius, Johannes Rübenthaler, Michael Ingrisch, Dietmar Wassilowsky, Michael Irlbeck, Jens Ricke, and et al. 2021. "Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission" Diagnostics 11, no. 6: 1029. https://doi.org/10.3390/diagnostics11061029
APA StyleGresser, E., Reich, J., Sabel, B. O., Kunz, W. G., Fabritius, M. P., Rübenthaler, J., Ingrisch, M., Wassilowsky, D., Irlbeck, M., Ricke, J., & Puhr-Westerheide, D. (2021). Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission. Diagnostics, 11(6), 1029. https://doi.org/10.3390/diagnostics11061029







