The Effect of Delayed Surgical Debridement in the Management of Open Tibial Fractures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Study Identification and Selection
2.4. Data Collection
2.5. Risk of Bias Assessment and Quality of Evidence
2.6. Data Synthesis and Analysis
3. Results
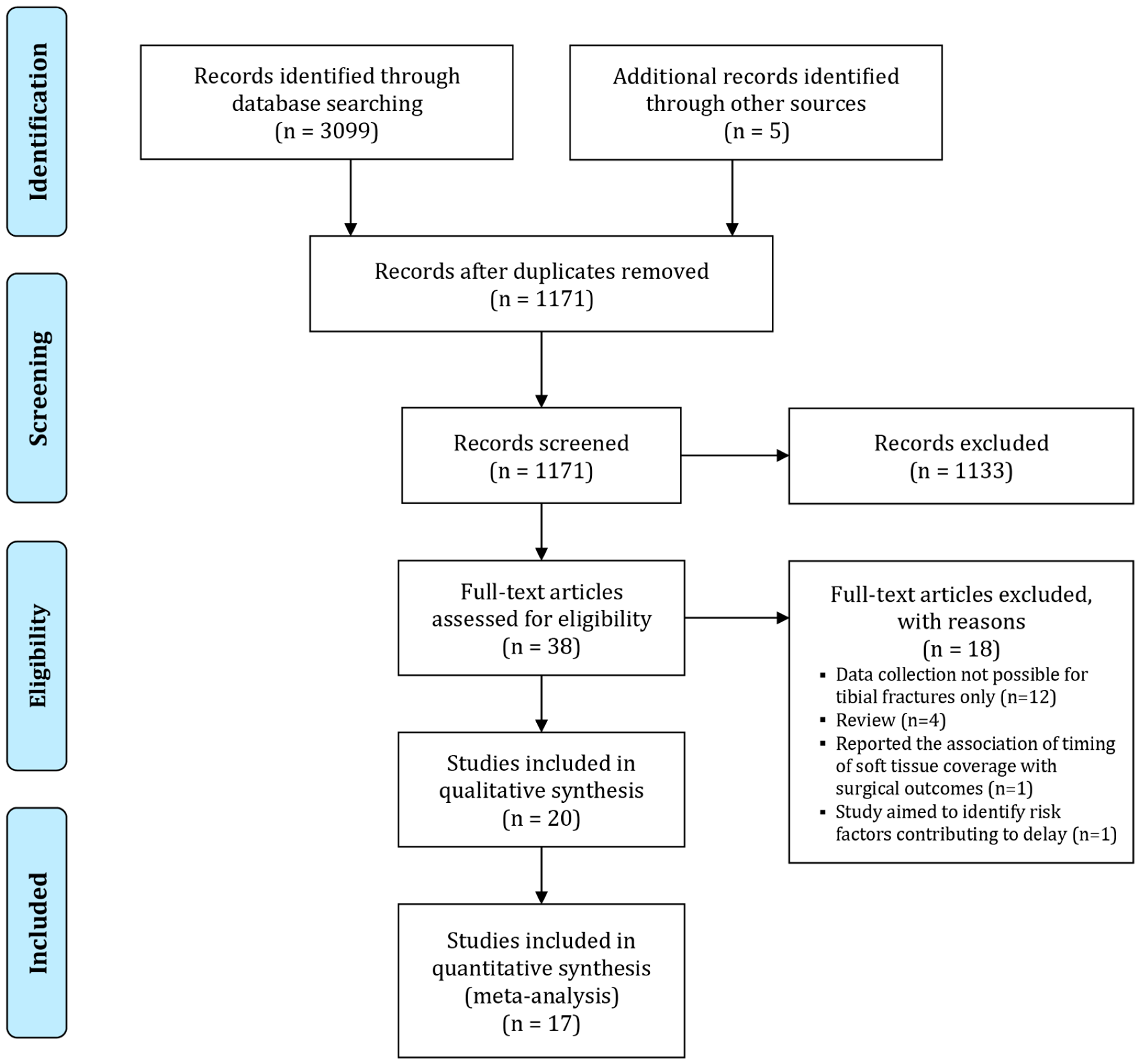
3.1. Search
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment
3.4. Infection
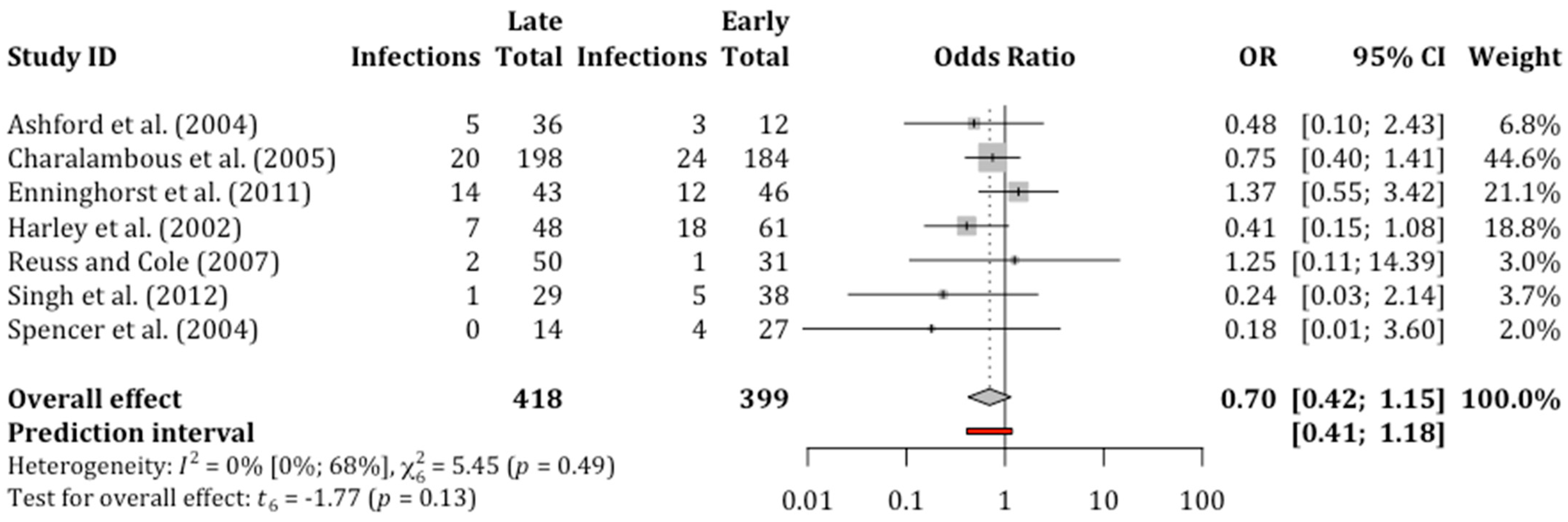
3.5. Non-Union
3.6. Subgroup and Sensitivity Analyses
3.7. Heterogeneity, Outliers and Publication Bias
3.8. Secondary Outcomes
4. Discussion
4.1. Main Findings
4.2. Findings of Excluded Studies
4.3. Comparison with Previous Meta-Analyses
4.4. Strengths and Limitations of This Study
4.5. Interpretation of Results and Current Evidence
4.6. Implications of Our Review
4.7. Impact on National Recommendations
4.8. Future Research and Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustilo, R.B.; Anderson, J.T. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: Retrospective and prospective analyses. J. Bone Jt. Surg. Am. 1976, 58, 453–458. [Google Scholar] [CrossRef]
- Boateng, J. Therapeutic Dressings and Wound Healing Applications; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Schade, A.T.; Hind, J.; Khatri, C.; Metcalfe, A.J.; Harrison, W.J. Systematic review of patient reported outcomes from open tibia fractures in low and middle income countries. Injury 2020, 51, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Santolini, E.; West, R.; Giannoudis, P.V. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015, 46, S8–S19. [Google Scholar] [CrossRef]
- Tay, W.-H.; de Steiger, R.; Richardson, M.; Gruen, R.; Balogh, Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014, 45, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Elniel, A.R.; Giannoudis, P.V. Open fractures of the lower extremity: Current Management and Clinical Outcomes. EFORT Open Rev. 2018, 3, 316–325. [Google Scholar] [CrossRef]
- British Orthopaedic Association and British Association of Plastic, Reconstructive, Aesthetic Surgeons. Open Fractures Audit Standards for Trauma; British Orthopaedic Association: London, UK, 2017. [Google Scholar]
- Lack, W.D.; Karunakar, M.A.; Angerame, M.R.; Seymour, R.B.; Sims, S.; Kellam, J.F.; Bosse, M.J. Type III open tibia fractures: Immediate antibiotic prophylaxis minimizes infection. J. Orthop. Trauma 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Chang, Y.; Kennedy, S.A.; Bhandari, M.; Lopes, L.C.; Bergamaschi, C.d.C.; Carolina de Oliveira e Silva, M.; Bhatnagar, N.; Mousavi, S.M.; Khurshid, S.; Petrisor, B.; et al. Effects of Antibiotic Prophylaxis in Patients with Open Fracture of the Extremities: A Systematic Review of Randomized Controlled Trials. JBJS Rev. 2015, 1–10. [Google Scholar] [CrossRef]
- Friedrich, P. Die aseptische versorgung frischer wundern. Arch. Klin. Chir. 1898, 57, 288–310. [Google Scholar]
- Cross, M.A.; Hahn, M.D.; Marsh, D.; Willett, M.K.; Quaba, M.A.; Small, M.J.; Watson, M.J. A Report by the BOA/BAPS Working Party on the Management of Open Tibial Fractures; British Orthopaedic Association and British Association of Plastic Reconsructive and Aesthetic Surgery: London, UK, 1997. [Google Scholar]
- Association, B.O. BOAST 4: The Management of Severe Open Lower Limb Fractures; British Orthopaedic Association and British Association of Plastic Reconsructive and Aesthetic Surgery: London, UK, 2009. [Google Scholar]
- British Orthopaedic Association. BOAST 4: Open Fractures Audit Standards for Trauma; British Orthopaedic Association: London, UK, 2017. [Google Scholar]
- Trickett, R.; Rahman, S.; Page, P.; Pallister, I. From guidelines to standards of care for open tibial fractures. Ann. R. Coll. Surg. Engl. 2015, 97, 469–475. [Google Scholar] [CrossRef]
- National Institute for Health Care Excellence. Fractures (Complex): Assessment and Management. NICE Guideline [NG37]; NICE: London, UK, 2016. [Google Scholar]
- Schenker, M.L.; Yannascoli, S.; Baldwin, K.D.; Ahn, J.; Mehta, S. Does Timing to Operative Debridement Affect Infectious Complications in Open Long-Bone Fractures? A Systematic Review. J. Bone Jt. Surg. Am 2012, 94, 1057–1064. [Google Scholar] [CrossRef]
- Prodromidis, A.D.; Charalambous, C.P. The 6-hour rule for surgical debridement of open tibial fractures: A systematic review and meta-analysis of infection and nonunion rates. J. Orthop. Trauma 2016, 30, 397–402. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Ashford, R.U.; Mehta, J.A.; Cripps, R. Delayed presentation is no barrier to satisfactory outcome in the management of open tibial fractures. Injury 2004, 35, 411–416. [Google Scholar] [CrossRef]
- Bednar, D.A.; Parikh, J. Effect of time delay from injury to primary management on the incidence of deep infection after open fractures of the lower extremities caused by blunt trauma in adults. J. Orthop. Trauma 1993, 7, 532–535. [Google Scholar] [CrossRef]
- Khatod, M.; Botte, M.J.; Hoyt, D.B.; Meyer, R.S.; Smith, J.M.; Akeson, W.H. Outcomes in Open Tibia Fractures: Relationship between Delay in Treatment and Infection. J. Trauma Acute Care Surg. 2003, 55, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Kindsfater, K.; Jonassen, E.A. Osteomyelitis in grade II and III open tibia fractures with late debridement. J. Orthop. Trauma 1995, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Lu, Y.; Feng, Q.; He, X.; Li Md, Z.; Zhang, K. Relationship between Time to Surgical Debridement and the Incidence of Infection in Patients with Open Tibial Fractures. Orthop. Surg. 2020, 12, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Al-Hourani, K.; Fowler, T.; Whitehouse, M.R.; Khan, U.; Kelly, M. Two-Stage Combined Ortho-Plastic Management of Type IIIB Open Diaphyseal Tibial Fractures Requiring Flap Coverage: Is the Timing of Debridement and Coverage Associated with Outcomes? J. Orthop. Trauma 2019, 33, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, S.A.; Wall, R.A.; Manley, O.; Gibson, W.; Toher, D.; Wallis, K.; Ward, J.; Wallace, D.L.; Lamyman, M.; Giblin, A.V. Time to Initial Debridement and wound Excision (TIDE) in severe open tibial fractures and related clinical outcome: A multi-centre study. Injury 2018, 49, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rambani, R.; Hashim, Z.; Raman, R.; Sharma, H.K. The relationship between time to surgical debridement and incidence of infection in grade III open fractures. Strateg. Trauma Limb Reconstr. 2012, 7, 33–37. [Google Scholar] [CrossRef][Green Version]
- Davis Sears, E.; Davis, M.M.; Chung, K.C. Relationship between timing of emergency procedures and limb amputation in patients with open tibia fracture in the United States, 2003 to 2009. Plast. Reconstr. Surg. 2012, 130, 369–378. [Google Scholar] [CrossRef]
- Enninghorst, N.; McDougall, D.; Hunt, J.J.; Balogh, Z.J. Open tibia fractures: Timely debridement leaves injury severity as the only determinant of poor outcome. J. Trauma Acute Care Surg. 2011, 70, 352–357. [Google Scholar] [CrossRef]
- Mener, A.; Staley, C.A.; Lunati, M.P.; Pflederer, J.; Reisman, W.M.; Schenker, M.L. Is Operative Debridement Greater Than 24 Hours Post-admission Associated With Increased Likelihood of Post-operative Infection? J. Surg. Res. 2020, 247, 461–468. [Google Scholar] [CrossRef]
- Sungaran, J.; Harris, I.; Mourad, M. The effect of time to theatre on infection rate for open tibia fractures. ANZ J. Surg. 2007, 77, 886–888. [Google Scholar] [CrossRef]
- Tripuraneni, K.; Ganga, S.; Quinn, R.; Gehlert, R. The effect of time delay to surgical debridement of open tibia shaft fractures on infection rate. Orthopedics 2008, 31, 1195. [Google Scholar] [CrossRef]
- Spencer, J.; Smith, A.; Woods, D. The effect of time delay on infection in open long-bone fractures: A 5-year prospective audit from a district general hospital. Ann. R. Coll. Surg. Engl. 2004, 86, 108. [Google Scholar] [CrossRef]
- Duyos, O.A.; Beaton-Comulada, D.; Davila-Parrilla, A.; Perez-Lopez, J.C.; Ortiz, K.; Foy-Parrilla, C.; Lopez-Gonzalez, F. Management of Open Tibial Shaft Fractures: Does the Timing of Surgery Affect Outcomes? J. Am. Acad. Orthop. Surg. 2017, 25, 230–238. [Google Scholar] [CrossRef]
- Charalambous, C.P.; Siddique, I.; Zenios, M.; Roberts, S.; Samarji, R.; Paul, A.; Hirst, P. Early versus delayed surgical treatment of open tibial fractures: Effect on the rates of infection and need of secondary surgical procedures to promote bone union. Injury 2005, 36, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.J.; Beaupre, L.A.; Jones, C.A.; Dulai, S.K.; Weber, D.W. The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J. Orthop. Trauma. 2002, 16, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Reuss, B.L.; Cole, J.D. Effect of delayed treatment on open tibial shaft fractures. Am. J. Orthop. 2007, 36, 215–220. [Google Scholar] [PubMed]
- Young, K.; Aquilina, A.; Chesser, T.; Costa, M.; Hettiaratchy, S.; Kelly, M.; Moran, C.G.; Pallister, I.; Woodford, M. Open tibial fractures in major trauma centres: A national prospective cohort study of current practice. Injury 2019, 50, 497–502. [Google Scholar] [CrossRef]
- Al-Arabi, Y.B.; Nader, M.; Hamidian-Jahromi, A.R.; Woods, D. The effect of the timing of antibiotics and surgical treatment on infection rates in open long-bone fractures: A 9-year prospective study from a district general hospital. Injury 2007, 38, 900–905. [Google Scholar] [CrossRef]
- Campbell, S.; Dhyani, J.; Greenberg, P.; Ahmed, N. Outcomes in patients with late debridement of open long bone fractures of the lower extremities in penetrating trauma: A retrospective review of the National Trauma Data Bank. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.d.C.; Peres, L.R.; Queiroz Neto ACd Lima Neto, J.Q.; Turíbio, F.M.; Matsumoto, M.H. Open fractures and the incidence of infection in the surgical debridement 6 hours after trauma. Acta Ortop. Bras. 2015, 23, 38–42. [Google Scholar] [CrossRef]
- Hull, P.D.; Johnson, S.C.; Stephen, D.J.; Kreder, H.J.; Jenkinson, R.J. Delayed debridement of severe open fractures is associated with a higher rate of deep infection. Bone Jt. J. 2014, 96, 379–384. [Google Scholar] [CrossRef]
- Kasman, R.O.; Albar, H.F. Correlation between early infection and onset of debridement in open diaphysis fracture patient at h. adam malik medan general hospital. Glob. J. Res. Anal. 2019, 8, 242–244. [Google Scholar]
- Leonidou, A.; Kiraly, Z.; Gality, H.; Apperley, S.; Vanstone, S.; Woods, D.A. The effect of the timing of antibiotics and surgical treatment on infection rates in open long-bone fractures: A 6-year prospective study after a change in policy. Strateg. Trauma Limb Reconstr. 2014, 9, 167–171. [Google Scholar] [CrossRef]
- Pollak, A.N.; Jones, A.L.; Castillo, R.C.; Bosse, M.J.; MacKenzie, E.J.; Group, L.S. The relationship between time to surgical debridement and incidence of infection after open high-energy lower extremity trauma. J. Bone Jt. Surg. Am. 2010, 92, 7. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Inaba, K.; Okoye, O.; Chan, C.; Skiada, D.; Schnüriger, B.; Trump, M.; Lam, L.; Demetriades, D. Prospective evaluation of treatment of open fractures: Effect of time to irrigation and debridement. JAMA Surg. 2015, 150, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Dulai, S.K.; Bergman, J.; Buckley, R.; Beaupre, L.A. Time to initial operative treatment following open fracture does not impact development of deep infection: A prospective cohort study of 736 subjects. J. Orthop. Trauma. 2014, 28, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.C.; Eisler, J. Treatment of isolated type I open fractures: Is emergent operative debridement necessary? Clin. Orthop. Relat. Res. (1976–2007) 2003, 410, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.J. Exponential growth of bacteria: Constant multiplication through division. Am. J. Phys. 2010, 78, 1290–1296. [Google Scholar] [CrossRef]
- Williams, T.; Meynell, G. Time-dependence and count-dependence in microbial infection. Nature 1967, 214, 473–475. [Google Scholar] [CrossRef]
- Merritt, K. Factors increasing the risk of infection in patients with open fractures. J. Trauma Acute Care Surg. 1988, 28, 823–827. [Google Scholar] [CrossRef]
- Kale, A.R.; Sonawane, C.S.; Waghmare, V.U.; Kalambe, H. Open Fractures and Incidence of Infection in Tertiary Care Government Hospital. Int. J. Sci. Study 2017, 5, 24–28. [Google Scholar]
- Townley, W.; Nguyen, D.; Rooker, J.; Dickson, J.; Goroszeniuk, D.; Khan, M.; Camp, D. Management of open tibial fractures–a regional experience. Ann. R. Coll. Surg. Engl. 2010, 92, 693–696. [Google Scholar] [CrossRef][Green Version]
- Gopal, S.; Majumder, S.; Batchelor, A.; Knight, S.; De Boer, P.; Smith, R. Fix and flap: The radical orthopaedic and plastic treatment of severe open fractures of the tibia. J. Bone Jt. Surg. 2000, 82, 959–966. [Google Scholar] [CrossRef]
- Skaggs, D.; Kautz, S.; Kay, R.; Tolo, V. Effect of delay of surgical treatment on rate of infection in open fractures in children. J. Pediatric Orthop. 2000, 20, 19. [Google Scholar] [CrossRef]
- Azoury, S.C.; Stranix, J.T.; Kovach, S.J.; Levin, L.S. Principles of orthoplastic surgery for lower extremity reconstruction: Why is this important? J. Reconstr. Microsurg. 2019, 37, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Boriani, F.; Haq, A.U.; Baldini, T.; Urso, R.; Granchi, D.; Baldini, N.; Tigani, D.; Tarar, M.; Khan, U. Orthoplastic surgical collaboration is required to optimise the treatment of severe limb injuries: A multi-centre, prospective cohort study. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Gans, I.; Baldwin, K.D.; Levin, L.S.; Nance, M.L.; Chang, B.; Kovach, S.J., III; Serletti, J.M.; Flynn, J.M. A lower extremity musculoskeletal and vascular trauma protocol in a children’s hospital may improve treatment response times and appropriate microvascular coverage. J. Orthop. Trauma 2015, 29, 239–244. [Google Scholar] [CrossRef]
- Stammers, J.; Williams, D.; Hunter, J.; Vesely, M.; Nielsen, D. The impact of trauma centre designation on open tibial fracture management. Ann. R. Coll. Surg. Engl. 2013, 95, 184–187. [Google Scholar] [CrossRef]
- Sommar, P.; Granberg, Y.; Halle, M.; Skogh, A.-C.D.; Lundgren, K.T.; Jansson, K.-Å. Effects of a formalized collaboration between plastic and orthopedic surgeons in severe extremity trauma patients; a retrospective study. J. Trauma Manag. Outcomes 2015, 9, 3. [Google Scholar] [CrossRef]
- Fernandez, M.; Wallis, K.; Venus, M.; Skillman, J.; Young, J.; Costa, M. The impact of a dedicated orthoplastic operating list on time to soft tissue coverage of open lower limb fractures. Ann. R. Coll. Surg. Engl. 2015, 97, 456–459. [Google Scholar] [CrossRef]
- Yang, N.; Elmatite, W.M.; Elgallad, A.; Gajdos, C.; Pourafkari, L.; Nader, N.D. Patient outcomes related to the daytime versus after-hours surgery: A meta-analysis. J. Clin. Anesth. 2019, 54, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ricci, W.M.; Gallagher, B.; Brandt, A.; Schwappach, J.; Tucker, M.; Leighton, R. Is after-hours orthopaedic surgery associated with adverse outcomes?: A prospective comparative study. JBJS 2009, 91, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Wixted, J.J.; Reed, M.; Eskander, M.S.; Millar, B.; Anderson, R.C.; Bagchi, K.; Kaur, S.; Franklin, P.; Leclair, W. The effect of an orthopedic trauma room on after-hours surgery at a level one trauma center. J. Orthop. Trauma 2008, 22, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Ippolito, M.; Misseri, G.; Helviz, Y.; Ingoglia, G.; Bonanno, G.; Giarratano, A.; Rochwerg, B.; Einav, S. Association between night/after-hours surgery and mortality: A systematic review and meta-analysis. Br. J. Anaesth. 2020, 124, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.L.; Ahn, J.; Donegan, D.; Mehta, S.; Baldwin, K.D. The cost of after-hours operative debridement of open tibia fractures. J. Orthop. Trauma 2014, 28, 626–631. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Author | Year | Country | Study Design | Fractures | Gustilo Anderson | Time Threshold | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIB | IIIC | |||||||
| Al-Hourani et al. [29] | 2019 | UK | Retrospective | 45 | 0 | 0 | 0 | 45 | 0 | 6 | Deep infection Non-union Flap failure |
| Ashford et al. [24] | 2004 | Australia | Retrospective | 48 | 3 | 10 | 14 | 21 | 0 | 6 | Infection Non-union/delayed union Length of stay |
| Bednar and Parikh [25] | 1993 | Canada | Retrospective | 52 | NR | 6 | Deep infection | ||||
| Charalambous et al. [37] | 2005 | UK | Retrospective | 383 | 33 | 38 | 64 | 0 | 0 | 6 | Infection Deep infection Secondary procedure to promote bone union |
| David Sears et al. [32] | 2012 | US | Retrospective | 7560 | NR | 24, 48, 96 and 120 | Amputation | ||||
| Duyos et al. [38] | 2017 | Puerto Rico | Retrospective | 227 | NR | 48, 72 and 96 | Deep infection | ||||
| Enninghorst et al. [33] | 2011 | Australia | Prospective | 89 | 21 | 27 | 18 | 21 | 1 | 6 | Deep infection Non-union |
| Harley et al. [39] | 2002 | Canada | Retrospective | 109 | 19 | 53 | 37 | 8 | Deep infection Non union | ||
| Hendrickson et al. [30] | 2018 | UK | Retrospective | 116 | 0 | 0 | 0 | 116 | 0 | 12 | Deep Infection |
| Kamat et al. [40] | 2011 | New Zealand | Retrospective | 103 | 49 | 32 | 22 | 6 | Infection | ||
| Khatod et al. [26] | 2003 | US | Retrospective | 101 | 17 | 46 | 23 | 8 | 7 | 6 | Infection |
| Kindsfater and Jonassen [27] | 1995 | US | Retrospective | 47 | 0 | 25 | 13 | 7 | 2 | 5 | Deep infection (osteomyelitis) |
| Konbaz et al. [41] | 2019 | Saudi Arabia | Retrospective | 113 | 13 | 45 | 20 | 28 | 7 | 6 | Infection |
| Li et al. [28] | 2020 | China | Retrospective | 215 | 62 | 98 | 26 | 25 | 4 | 6, 12 and 24 | Infection |
| Mener et al. [34] | 2020 | Georgia | Retrospective | 259 | NR | 24 | Infection | ||||
| Reuss and Cole [42] | 2007 | US | Retrospective | 81 | 14 | 19 | 9 | 34 | 5 | 8 | Deep infection Non-union |
| Singh et al. [31] | 2012 | UK | Retrospective | 67 | 0 | 0 | 26 | 39 | 2 | 6 | Deep infection Non-union |
| Spencer et al. [43] | 2004 | UK | Prospective | 41 | 0 | 5 | 14 | 11 | 0 | 6 | Deep infection Non-union |
| Sungaran et al. [35] | 2007 | Australia | Retrospective | 161 | 28 | 35 | 95 | 6 and 12 | Infection | ||
| Tripuraneni et al. [36] | 2008 | US | Retrospective | 215 | 62 | 98 | 26 | 25 | 4 | 6, 12 and 24 | Infection |
| Total | 10,032 | 321 | 531 | 819 | |||||||
| Outcome | No of Participants (Studies) | Relative Effect (95% CI) | p-Value | Anticipated Absolute Effects (95% CI) | Certainty (GRADE) | Interpretation | ||
|---|---|---|---|---|---|---|---|---|
| Early Debridement | Late Debridement | Difference | ||||||
| Infection | 2193 (17) | OR 0.87 (0.68 to 1.11) | 0.23 | 14.0% | 12.4% (10 to 15.3) | 1.6% fewer (4 fewer to 1.3 more) | ⨁◯◯◯ VERY LOW a,b,c | The evidence suggests that late debridement results in little to no difference in infection. |
| Non-union | 817 (7) | OR 0.70 (0.42 to 1.15) | 0.13 | 16.8% | 12.4% (7.8 to 18.8) | 4.4% fewer (9 fewer to 2 more) | ⨁◯◯◯ VERY LOW a,c,d | The evidence suggests that late debridement results in little to no difference in non-union. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaides, M.; Vris, A.; Heidari, N.; Bates, P.; Pafitanis, G. The Effect of Delayed Surgical Debridement in the Management of Open Tibial Fractures: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1017. https://doi.org/10.3390/diagnostics11061017
Nicolaides M, Vris A, Heidari N, Bates P, Pafitanis G. The Effect of Delayed Surgical Debridement in the Management of Open Tibial Fractures: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11(6):1017. https://doi.org/10.3390/diagnostics11061017
Chicago/Turabian StyleNicolaides, Marios, Alexandros Vris, Nima Heidari, Peter Bates, and Georgios Pafitanis. 2021. "The Effect of Delayed Surgical Debridement in the Management of Open Tibial Fractures: A Systematic Review and Meta-Analysis" Diagnostics 11, no. 6: 1017. https://doi.org/10.3390/diagnostics11061017
APA StyleNicolaides, M., Vris, A., Heidari, N., Bates, P., & Pafitanis, G. (2021). The Effect of Delayed Surgical Debridement in the Management of Open Tibial Fractures: A Systematic Review and Meta-Analysis. Diagnostics, 11(6), 1017. https://doi.org/10.3390/diagnostics11061017







