The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment
Abstract
1. Introduction
2. Physiopathological Premises and Current Decisional Algorithm
3. Role of Diagnostic Imaging
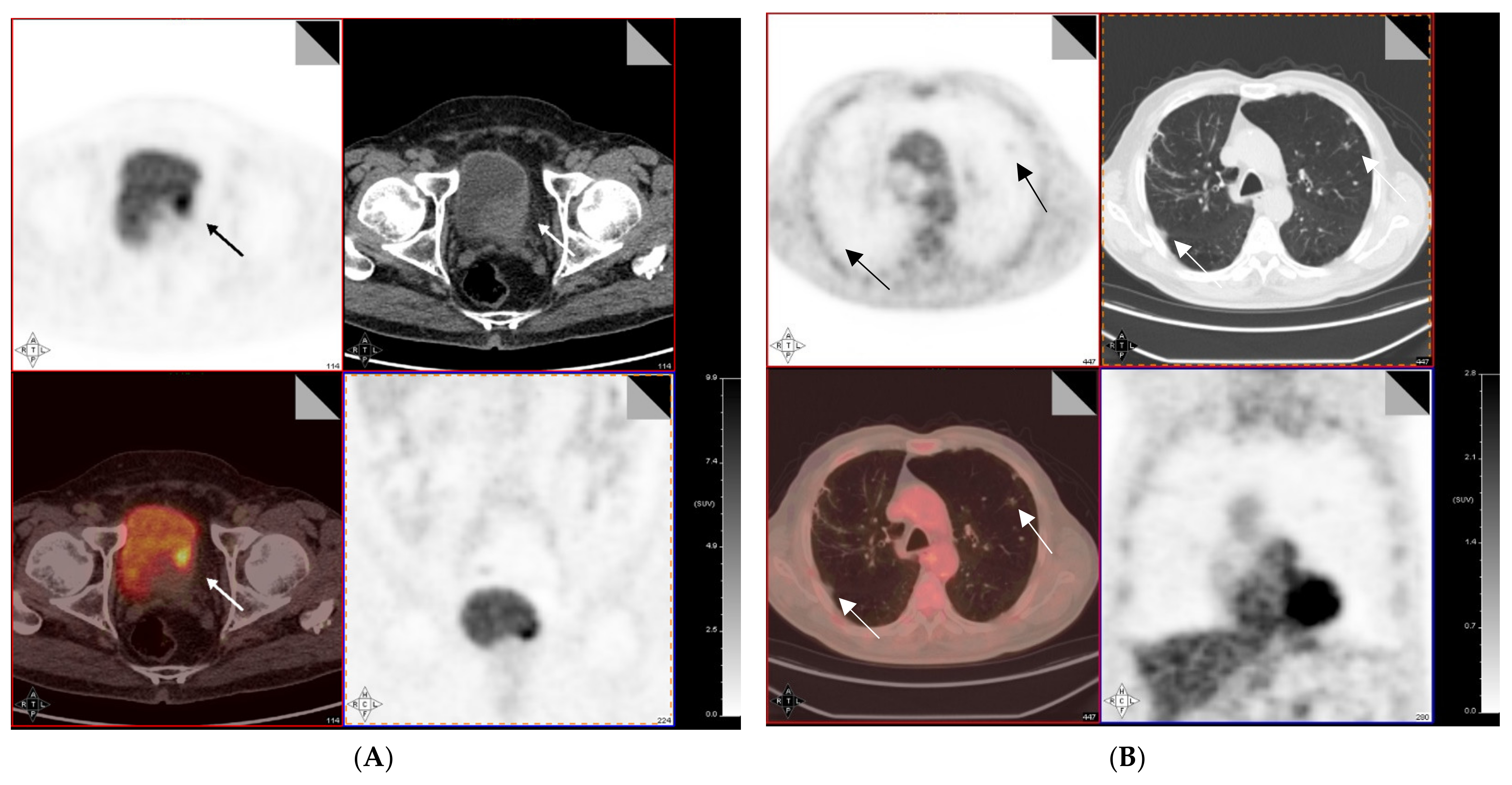
4. 18F-FDG PET/CT for Preoperative Lymph Node Staging of MIBC
5. 18F-FDG PET/CT for Restaging and Response Evaluation of MIBC Following Neoadjuvant Chemotherapy
6. Alternative Tracers for MIBC Functional Imaging
7. Other Diagnostic Imaging Procedures for the Evaluation of Treatment Response
8. Hybrid Imaging with PET-MRI
9. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Erlich, A.; Zlotta, A.R. Molecular Characterization of Bladder Cancer. Curr. Urol. Rep. 2018, 19, 107. [Google Scholar] [CrossRef]
- Alifrangis, C.; McGovern, U.; Freeman, A.; Powles, T.; Linch, M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019, 16, 465–483. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, W.; Yang, X.; Wu, C.; Cheng, F. Traditional Classification and Novel Subtyping Systems for Bladder Cancer. Front. Oncol. 2020, 10, 102. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Phys. 2017, 96, 507–514. [Google Scholar]
- Aragon-Ching, J.B.; Werntz, R.P.; Zietman, A.L.; Steinberg, G.D. Multidisciplinary Management of Muscle-Invasive Bladder Cancer: Current Challenges and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 307–318. [Google Scholar] [CrossRef] [PubMed]
- De Palma, D.; Santos, A.I. Renal radionuclide imaging, an evergreen forty years old. Klin. Pädiatr. 2014, 226, 225–232. [Google Scholar] [CrossRef]
- Mansi, L.; Lopci, E.; Cuccurullo, V.; Chiti, A. (Eds.) Clinical Nuclear Medicine in Pediatrics; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-21370-5. [Google Scholar]
- Dhull, R.S.; Joshi, A.; Saha, A. Nuclear Imaging in Pediatric Kidney Diseases. Indian Pediatr. 2018, 55, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Mansi, L.; Cuccurullo, V.; Prisco, M.R. Peculiar aspects and problems of diagnostic nuclear medicine in pediatrics. In Clinical Nuclear Medicine in Pediatrics; Mansi, L., Lopci, E., Cuccurullo, V., Chiti, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-21370-5. [Google Scholar]
- Cuccurullo, V.; Di Stasio, G.D.; Mansi, L. Physiopathological Premises to Nuclear Medicine Imaging of Pancreatic Neuroendo-crine Tumours. Curr. Radiopharm. 2019, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cistaro, A.; Cuccurullo, V.; Quartuccio, N.; Pagani, M.; Valentini, M.C.; Mansi, L. Role of PET and SPECT in the study of amyo-trophic lateral sclerosis. Biomed. Res. Int. 2014, 2014, 237437. [Google Scholar] [CrossRef]
- Tsao, C.K.; Liaw, B.C.; Oh, W.K.; Galsky, M.D. Muscle invasive bladder cancer: Closing the gap between practice and evidence. Minerva Urol. Nefrol. 2014, 67, 65–73. [Google Scholar]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Cooley, L.F.; McLaughlin, K.A.; Meeks, J.J. Genomic and Therapeutic Landscape of Non-muscle-invasive Bladder Cancer. Urol. Clin. N. Am. 2020, 47, 35–46. [Google Scholar] [CrossRef]
- Sjödahl, G.; Jackson, C.L.; Bartlett, J.M.; Siemens, D.R.; Berman, D.M. Molecular profiling in muscle-invasive bladder cancer: More than the sum of its parts. J. Pathol. 2019, 247, 563–573. [Google Scholar] [CrossRef]
- Da Costa, J.B.; Gibb, E.A.; Nykopp, T.K.; Mannas, M.; Wyatt, A.W.; Black, P.C. Molecular tumor heterogeneity in muscle invasive bladder cancer: Biomarkers, subtypes, and implications for therapy. Urol. Oncol. 2018, in press. [Google Scholar] [CrossRef]
- Faiena, I.; Rosser, C.J.; Chamie, K.; Furuya, H. Diagnostic biomarkers in non-muscle invasive bladder cancer. World J. Urol. 2019, 37, 2009–2016. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Charac-terization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Harshman, L.C.; Preston, M.A.; Bellmunt, J.; Beard, C. Diagnosis of Bladder Carcinoma: A Clinician’s Perspective. Surg. Pathol. Clin. 2015, 8, 677–685. [Google Scholar] [CrossRef]
- Sun, M.; Trinh, Q.-D. Diagnosis and Staging of Bladder Cancer. Hematol. Clin. N. Am. 2015, 29, 205–218. [Google Scholar] [CrossRef]
- Lawrentschuk, N.; Lee, S.T.; Scott, A.M. Current Role of PET, CT, MR for Invasive Bladder Cancer. Curr. Urol. Rep. 2013, 14, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, R.; Singla, N.; Lotan, Y. Treatment Options and Outcomes in Nonmetastatic Muscle Invasive Bladder Cancer. Trends Cancer 2019, 5, 426–439. [Google Scholar] [CrossRef]
- Lemke, E.A.; Shah, A.Y. Management of Advanced Bladder Cancer: An Update. J. Adv. Pr. Oncol. 2018, 9, 410–416. [Google Scholar]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Fransen van de Putte, E.E.; Horenblas, S.; Drabick, J.J. Ne-oadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Cerbone, L.; Sternberg, C.N.; Agerbaek, M.; Van Herpen, C.; Marreaud, S.; Collette, S.; Zhang, J.; Daugaard, G. Results from a Phase I Study of Lapatinib with Gemcitabine and Cisplatin in Advanced or Metastatic Bladder Cancer: EORTC Trial 30061. Oncology 2015, 90, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Bedke, J.; Chamie, K.; Collins, J.W.; Daneshmand, S.; Grivas, P.; Heidenreich, A.; Messing, E.M.; Royce, T.J.; Sankin, A.I.; et al. SIU–ICUD consultation on bladder cancer: Treatment of muscle-invasive bladder cancer. World J. Urol. 2019, 37, 61–83. [Google Scholar] [CrossRef]
- Moschini, M.; Shariat, S.F.; Rouprêt, M.; De Santis, M.; Bellmunt, J.; Sternberg, C.N.; Tombal, B.; Collette, L. Impact of Primary Tumor Location on Survival from the European Organization for the Research and Treatment of Cancer Advanced Urothelial Cancer Studies. J. Urol. 2018, 199, 1149–1157. [Google Scholar] [CrossRef]
- Van der Pol, C.B.; Sahni, V.A.; Eberhardt, S.C.; Oto, A.; Akin, O.; Alexander, L.F.; Allen, B.C.; Coakley, F.V.; Froemming, A.T.; Fulgham, P.F.; et al. ACR Appropriateness Criteria® Pretreatment Staging of Muscle-Invasive Bladder Cancer. J. Am. Coll. Radiol. 2018, 15, S150–S159. [Google Scholar] [CrossRef]
- Gurram, S.; Muthigi, A.; Egan, J.; Stamatakis, L. Imaging in Localized Bladder Cancer: Can Current Diagnostic Modalities Pro-vide Accurate Local Tumor Staging? Curr. Urol. Rep. 2019, 20, 82. [Google Scholar] [CrossRef]
- Mirmomen, S.M.; Shinagare, A.B.; Williams, K.E.; Silverman, S.G.; Malayeri, A.A. Preoperative imaging for locoregional staging of bladder cancer. Abdom. Radiol. 2019, 44, 3843–3857. [Google Scholar] [CrossRef]
- Green, D.A.; Durand, M.; Gumpeni, N.; Rink, M.; Cha, E.K.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Role of magnetic resonance im-aging in bladder cancer: Current status and emerging techniques. BJU Int. 2012, 110, 1463–1470. [Google Scholar] [CrossRef]
- Yoshida, S.; Koga, F.; Kobayashi, S.; Ishii, C.; Tanaka, H.; Tanaka, H.; Komai, Y.; Saito, K.; Masuda, H.; Fujii, Y.; et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e21–e27. [Google Scholar] [CrossRef]
- Necchi, A.; Bandini, M.; Calareso, G.; Raggi, D.; Pederzoli, F.; Fare, E.; Colecchia, M.; Marandino, L.; Bianchi, M.; Gallina, A.; et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pem-brolizumab in Muscle-Invasive Bladder Cancer: Preliminary Findings from the PURE-01 Study. Eur. Urol. 2020, 77, 636–643. [Google Scholar] [CrossRef]
- Cornelissen, S.W.; Veenboer, P.W.; Wessels, F.J.; Meijer, R.P. Diagnostic Accuracy of Multiparametric MRI for Local Staging of Bladder Cancer: A Systematic Review and Meta-Analysis. Urology 2020, 145, 22–29. [Google Scholar] [CrossRef]
- Kitson, S.L.; Cuccurullo, V.; Ciarmiello, A.; Mansi, L. Targeted Therapy towards Cancer-A Perspective. Anti-Cancer Agents Med. Chem. 2017, 17, 311–317. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Chen, J.-H.; Liang, J.-A.; Wang, H.-Y.; Lin, C.-C.; Lin, W.-Y.; Kao, C.-H. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur. J. Radiol. 2012, 81, 2411–2416. [Google Scholar] [CrossRef]
- Cuccurullo, V.; Di Stasio, G.D.; Schillirò, M.L.; Mansi, L. Small-Animal Molecular Imaging for Preclinical Cancer Research: μPET and μSPECT. Curr. Radiopharm. 2016, 9, 103–113. [Google Scholar] [CrossRef]
- Cascini, G.L.; Cuccurullo, V.; Mansi, L. 18FNa-fluoride has a higher extraction with respect to 99mTc-methylene diphosphonate: Mismatch in a case of meningioma. Rev. Esp. Med. Nucl. Imagen. Mol. 2014, 33, 52–53. [Google Scholar]
- Cuccurullo, V.; Cascini, G.L.; Mansi, L. Structural, pathophysiological and clinical aspects of diagnostic imaging in breast recur-rence: The breast after treatment. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 322–331. [Google Scholar]
- Cuccurullo, V.; Di Stasio, G.D.; Cascini, G.L. PET/CT in thyroid cancer—The importance of BRAF mutations. Nucl. Med. Rev. 2020, 23, 97–102. [Google Scholar] [CrossRef]
- Apolo, A.B.; Riches, J.; Schöder, H.; Akin, O.; Trout, A.; Milowsky, M.I.; Bajorin, D.F. Clinical Value of Fluorine-18 2-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography in Bladder Cancer. J. Clin. Oncol. 2010, 28, 3973–3978. [Google Scholar] [CrossRef]
- Ali, S.A.; Abdelkawi, M.M.; Hussien, N.M. Delayed post-diuretic 18F-FDG PET/CT: Can it help in determination of the best clinical decision for muscle invasive UB cancer patients? Egypt. J. Radiol. Nucl. Med. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Dason, S.; Wong, N.C.; Donahue, T.F.; Meier, A.; Zheng, J.; Mannelli, L.; Di Paolo, P.L.; Dean, L.W.; McPherson, V.A.; Rosenberg, J.E.; et al. Utility of Routine Preoperative 18 F-Fluorodeoxyglucose Positron Emission Tomography-Computerized Tomography in Identifying Pathological Lymph Node Metastases at Radical Cystectomy. J. Urol. 2020, 204. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, A.; Komori, T.; Juri, H.; Inada, Y.; Azuma, H.; Narumi, Y. Detectability of residual invasive bladder cancer in de-layed 18F-FDG PET imaging with oral hydration using 500 mL of water and voiding-refilling. Ann. Nucl. Med. 2018, 32, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Uttam, M.; Pravin, N.; Anish, B.; Nandita, K.; Arup, M. Is [F-18]-fluorodeoxyglucose FDG-PET/CT better than ct alone for the preoperative lymph node staging of muscle invasive bladder cancer? Int. Braz. J. Urol. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Goodfellow, H.; Viney, Z.; Hughes, P.; Rankin, S.; Rottenberg, G.; Hughes, S.; Evison, F.; Dasgupta, P.; O’Brien, T.; Khan, M.S. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2013, 114, 389–395. [Google Scholar] [CrossRef]
- Pichler, R.; De Zordo, T.; Fritz, J.; Kroiss, A.; Aigner, F.; Heidegger, I.; Virgolini, I.; Horninger, W.; Uprimny, C. Pelvic Lymph Node Staging by Combined 18 F-FDG-PET/CT Imaging in Bladder Cancer Prior to Radical Cystectomy. Clin. Genitourin. Cancer 2017, 15, e387–e395. [Google Scholar] [CrossRef]
- Crozier, J.; Papa, N.; Perera, M.; Ngo, B.; Bolton, D.; Sengupta, S.; Lawrentschuk, N. Comparative sensitivity and specificity of im-aging modalities in staging bladder cancer prior to radical cystectomy: A systematic review and meta-analysis. World J. Urol. 2019, 37, 667–690. [Google Scholar] [CrossRef]
- Soubra, A.; Hayward, D.; Dahm, P.; Goldfarb, R.; Froehlich, J.; Jha, G.; Konety, B.R. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: A single-institution study and a systematic review with meta-analysis. World J. Urol. 2016, 34, 1229–1237. [Google Scholar] [CrossRef]
- Ha, H.K.; Koo, P.J.; Kim, S.J. Diagnostic Accuracy of F-18 FDG PET/CT for Preoperative Lymph Node Staging in Newly Diag-nosed Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Oncology 2018, 95, 31–38. [Google Scholar] [CrossRef]
- Van de Putte, E.E.F.; Vegt, E.; Mertens, L.S.; Bruining, A.; Hendricksen, K.; van der Heijden, M.S.; Horenblas, S.; van Rhijn, B.W.G. FDG-PET/CT for response evaluation of invasive bladder cancer following neoadjuvant chemotherapy. Int. Urol. Nephrol. 2017, 49, 1585–1591. [Google Scholar] [CrossRef]
- Kollberg, P.; Almquist, H.; Bläckberg, M.; Cwikiel, M.; Gudjonsson, S.; Lyttkens, K.; Patschan, O.; Liedberg, F. [18F] Fluorodeoxyglucose-positron emission tomography/computed tomography response evaluation can predict histological response at surgery after induction chemotherapy for oligometastatic bladder cancer. Scand. J. Urol. 2017, 51, 308–313. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, X.; Wang, X.; Li, R.; Shi, Y.; Xia, Q.; Wan, L.; Huang, G.; Liu, J. Delayed 18F FDG PET/CT Imaging in the Assessment of Residual Tumors after Transurethral Resection of Bladder Cancer. Radiology 2019, 293, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Harkirat, S.; Anand, S.; Jacob, M. Forced diuresis and dual-phase18F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J. Radiol. Imaging 2010, 20, 13–19. [Google Scholar] [CrossRef]
- Alongi, P.; Caobelli, F.; Gentile, R.; Stefano, A.; Russo, G.; Albano, D.; Baldari, S.; Gilardi, M.C.; Midiri, M. Recurrent bladder carcinoma: Clinical and prognostic role of 18 F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 44, 224–233. [Google Scholar] [CrossRef]
- Cuccurullo, V.; Di Stasio, G.D.; Evangelista, L.; Castoria, G.; Mansi, L. Biochemical and Pathophysiological Premises to Positron Emission Tomography With Choline Radiotracers. J. Cell. Physiol. 2016, 232, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.; Chiaravalloti, A.; Cicciò, C.; Gangemi, V.; Gullà, D.; Rocca, F.; Gallo, G.; Cascini, G.L.; Schillaci, O. PET/CT with (18)F-choline: Physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients: (18)F-choline PET/CT bio-distribution and pitfalls. A southern Italian experience. Nucl. Med. Biol. 2017, 51, 40–54. [Google Scholar] [CrossRef]
- Calabria, F.; Gallo, G.; Schillaci, O.; Cascini, G.L. Bio-Distribution, Imaging Protocols and Diagnostic Accuracy of PET with Trac-ers of Lipogenesis in Imaging Prostate Cancer: A Comparison between 11C-Choline, 18FFluoroethylcholine and 18F-Methylcholine. Curr. Pharm. Des. 2015, 21, 4738–4747. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Guttilla, A.; Zattoni, F.; Cuccurullo, V.; Mansi, L. 18F-fluoromethylcholine or 18F-fluoroethylcholine pet for prostate cancer imaging: Which is better? A literature revision. Nucl. Med. Biol. 2015, 42, 340–348. [Google Scholar] [CrossRef]
- Kim, S.J.; Koo, P.J.; Pak, K.; Kim, I.J.; Kim, K. Diagnostic accuracy of C-11 choline and C-11 acetate for lymph node staging in pa-tients with bladder cancer: A systematic review and meta-analysis. World J. Urol. 2018, 36, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Sopov, V.; Baniel, J.; Groshar, D. Comparison of 11C-choline with 18F-FDG in positron emission tomogra-phy/computerized tomography for staging urothelial carcinoma: A prospective study. J. Urol. 2011, 186, 436–441. [Google Scholar] [CrossRef]
- Vargas, H.A.; Akin, O.; Schöder, H.; Olgac, S.; Dalbagni, G.; Hricak, H.; Bochner, B.H. Prospective evaluation of MRI, 11C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur. J. Radiol. 2012, 81, 4131–4137. [Google Scholar] [CrossRef]
- Picchio, M.; Treiber, U.; Beer, A.J.; Metz, S.; Bössner, P.; Van Randenborgh, H.; Paul, R.; Weirich, G.; Souvatzoglou, M.; Hartung, R.; et al. Value of 11C-choline PET and contrast-enhanced CT for staging of bladder cancer: Correlation with histopathologic findings. J. Nucl. Med. 2006, 47, 938–944. [Google Scholar]
- Schöder, H.; Ong, S.C.; Reuter, V.E.; Cai, S.; Burnazi, E.; Dalbagni, G.; Larson, S.M.; Bochner, B.H. Initial results with (11)C-acetate pos-itron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol. Imaging Biol. 2012, 14, 245–251. [Google Scholar] [CrossRef]
- Escorcia, F.E.; Steckler, J.M.; Abdel-Atti, D.; Price, E.W.; Carlin, S.D.; Scholz, W.W.; Lewis, J.S.; Houghton, J.L. Tumor-Specific Zr-89 Immuno-PET Imaging in a Human Bladder Cancer Model. Mol. Imaging Biol. 2018, 20, 808–815. [Google Scholar] [CrossRef]
- Yoshida, S.; Koga, F.; Kawakami, S.; Ishii, C.; Tanaka, H.; Numao, N.; Sakai, Y.; Saito, K.; Masuda, H.; Fujii, Y.; et al. Initial experi-ence of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology 2010, 75, 387–391. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Taher, M.G.A.; Ali, W.A.; Ebrahem, M.A.E.S. Diagnostic performance of contrast-enhanced dynamic and diffusion-weighted MR imaging in the assessment of tumor response to neoadjuvant therapy in muscle-invasive bladder cancer. Abdom. Radiol. 2021, 1–10. [Google Scholar] [CrossRef]
- Deserno, W.M.L.L.G.; Harisinghani, M.G.; Taupitz, M.; Jager, G.J.; Witjes, J.A.; Mulders, P.F.; Van De Kaa, C.A.H.; Kaufmann, D.; Barentsz, J.O. Urinary Bladder Cancer: Preoperative Nodal Staging with Ferumoxtran-10–enhanced MR Imaging. Radiology 2004, 233, 449–456. [Google Scholar] [CrossRef]
- Fortuin, A.S.; Meijer, H.; Thompson, L.C.; Witjes, J.A.; Barentsz, J.O. Ferumoxtran-10 ultrasmall superparamagnetic iron ox-ide-enhanced diffusion-weighted imaging magnetic resonance imaging for detection of metastases in normal-sized lymph nodes in patients with bladder and prostate cancer: Do we enter the era after extended pelvic lymph node dissection? Eur. Urol. 2013, 64, 961–963. [Google Scholar] [PubMed]
- Birkhäuser, F.D.; Studer, U.E.; Froehlich, J.M.; Triantafyllou, M.; Bains, L.J.; Petralia, G.; Vermathen, P.; Fleischmann, A.; Thoeny, H.C. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur. Urol. 2013, 64, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Mansi, L.; Ciarmiello, A.; Cuccurullo, V. PET/MRI and the revolution of the third eye. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1519–1524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catalano, O.A.; Rosen, B.R.; Sahani, D.V.; Hahn, P.F.; Guimaraes, A.R.; Vangel, M.G.; Nicolai, E.; Soricelli, A.; Salvatore, M. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: Initial experience in 134 patients—A hypothesis-generating exploratory study. Radiology 2013, 269, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Friedman, K.P.; Ponzo, F.; Raad, R.A.; Jackson, K.; Huang, W.C.; Balar, A.V. Prospective Pilot Study to Evaluate the Incremental Value of PET Information in Patients with Bladder Cancer Undergoing 18F-FDG Simultaneous PET/MRI. Clin. Nucl. Med. 2017, 42, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Eulitt, P.J.; Altun, E.; Sheikh, A.; Wong, T.Z.; Woods, M.E.; Rose, T.L.; Wallen, E.M.; Pruthi, R.S.; Smith, A.B.; Nielsen, M.E.; et al. Pilot Study of [18F] Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)/Magnetic Resonance Imaging (MRI) for Staging of Muscle-invasive Bladder Cancer (MIBC). Clin. Genitourin. Cancer 2020, 18, 378–386.e1. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Jambor, I.; Merisaari, H.; Ettala, O.; Virtanen, J.; Koskinen, I.; Veskimae, E.; Sairanen, J.; Taimen, P.; Kemppainen, J.; et al. 11C-acetate PET/MRI in bladder cancer staging and treatment response evaluation to neoadjuvant chemo-therapy: A prospective multicenter study (ACEBIB trial). Cancer Imaging 2018, 18, 25. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuccurullo, V.; Di Stasio, G.D.; Manti, F.; Arcuri, P.; Damiano, R.; Cascini, G.L. The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment. Diagnostics 2021, 11, 863. https://doi.org/10.3390/diagnostics11050863
Cuccurullo V, Di Stasio GD, Manti F, Arcuri P, Damiano R, Cascini GL. The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment. Diagnostics. 2021; 11(5):863. https://doi.org/10.3390/diagnostics11050863
Chicago/Turabian StyleCuccurullo, Vincenzo, Giuseppe Danilo Di Stasio, Francesco Manti, Pierpaolo Arcuri, Rocco Damiano, and Giuseppe Lucio Cascini. 2021. "The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment" Diagnostics 11, no. 5: 863. https://doi.org/10.3390/diagnostics11050863
APA StyleCuccurullo, V., Di Stasio, G. D., Manti, F., Arcuri, P., Damiano, R., & Cascini, G. L. (2021). The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment. Diagnostics, 11(5), 863. https://doi.org/10.3390/diagnostics11050863







