Diagnostic Challenges in Epithelioid Pleural Mesothelioma: Case Series with Support from Electron Microscopy
Abstract
1. Introduction
2. Case Description
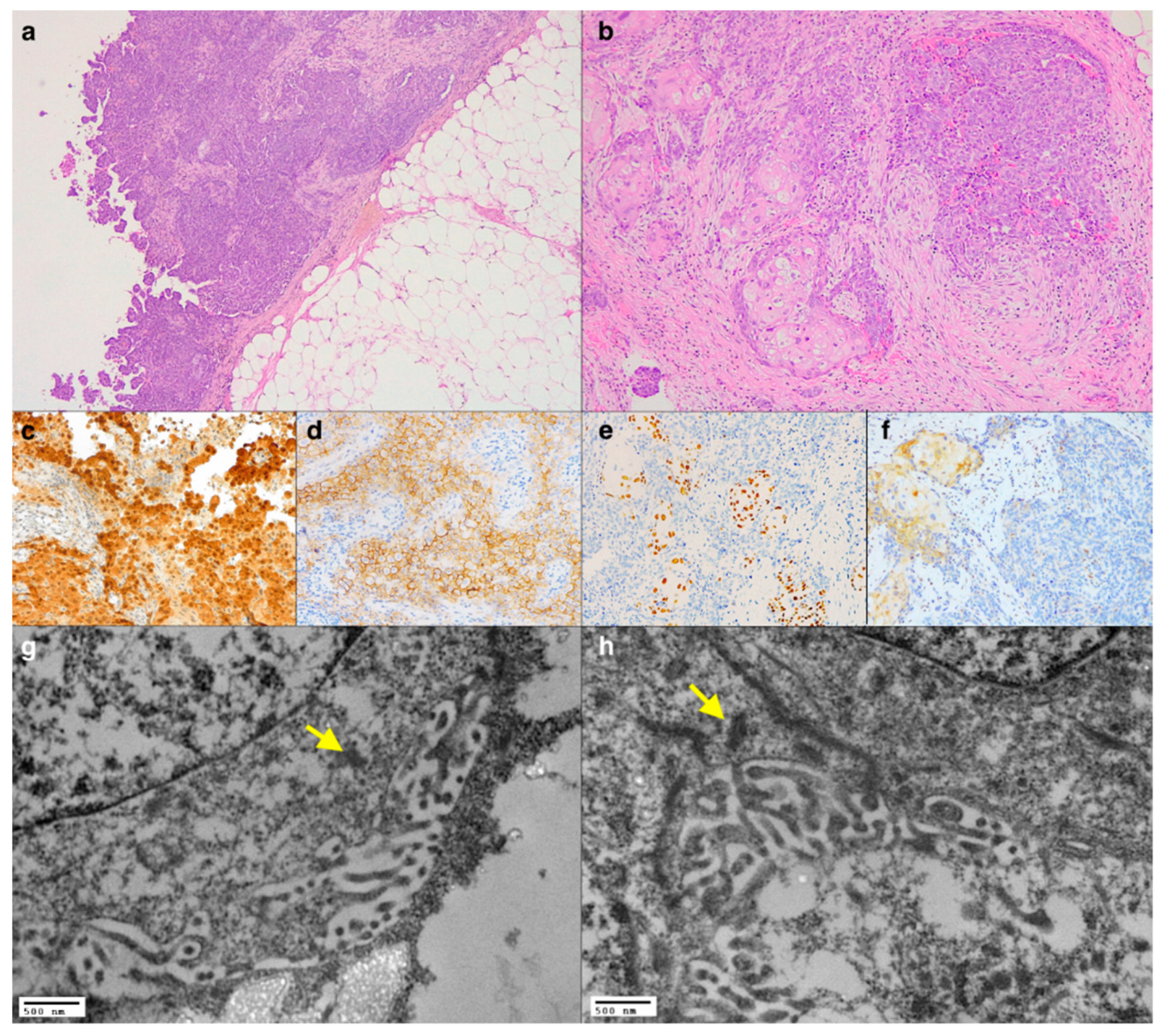
2.1. Case 1: An Unusual Morphological Finding
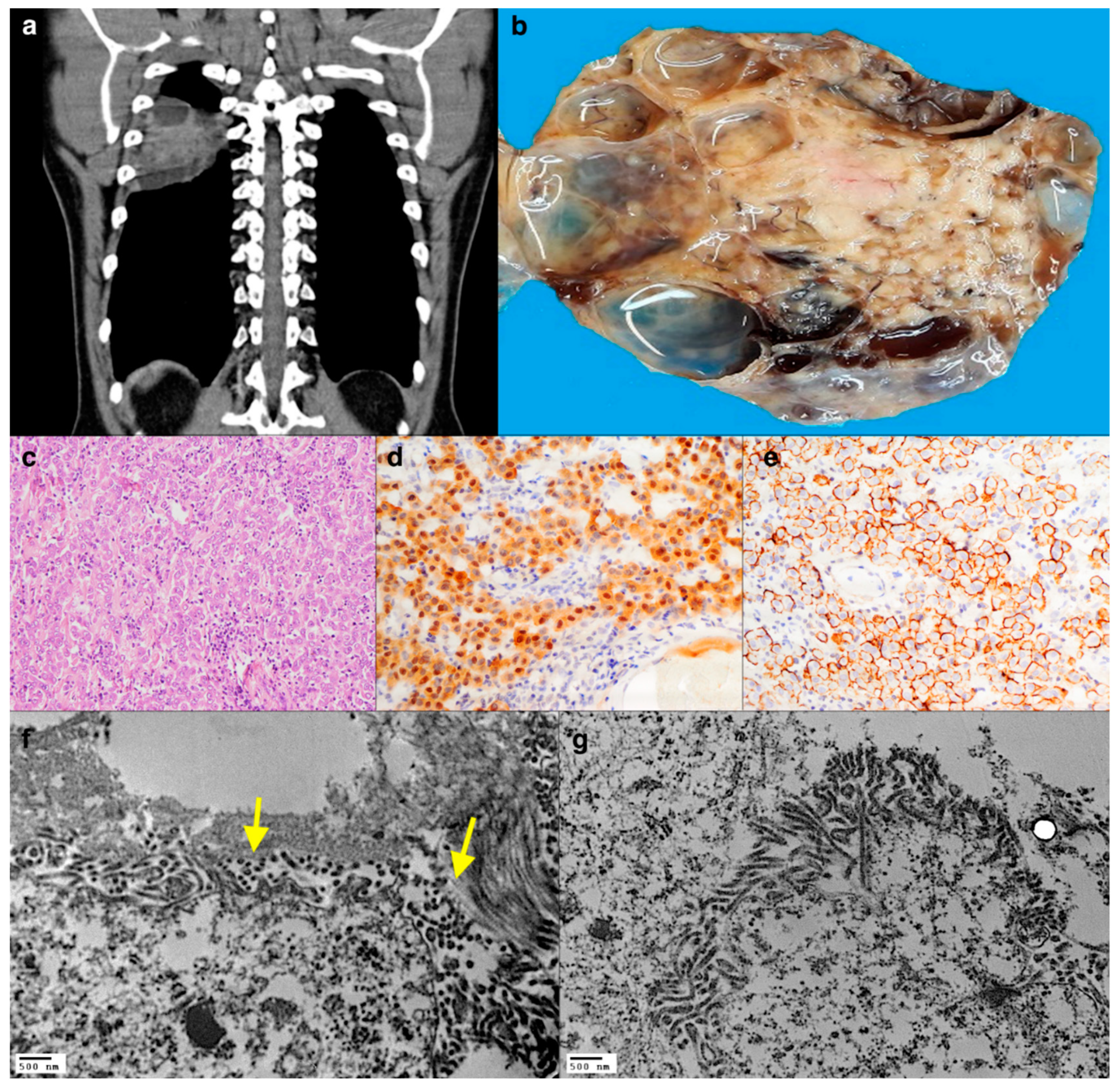
2.2. Case 2: A Single Intrathoracic Nodule
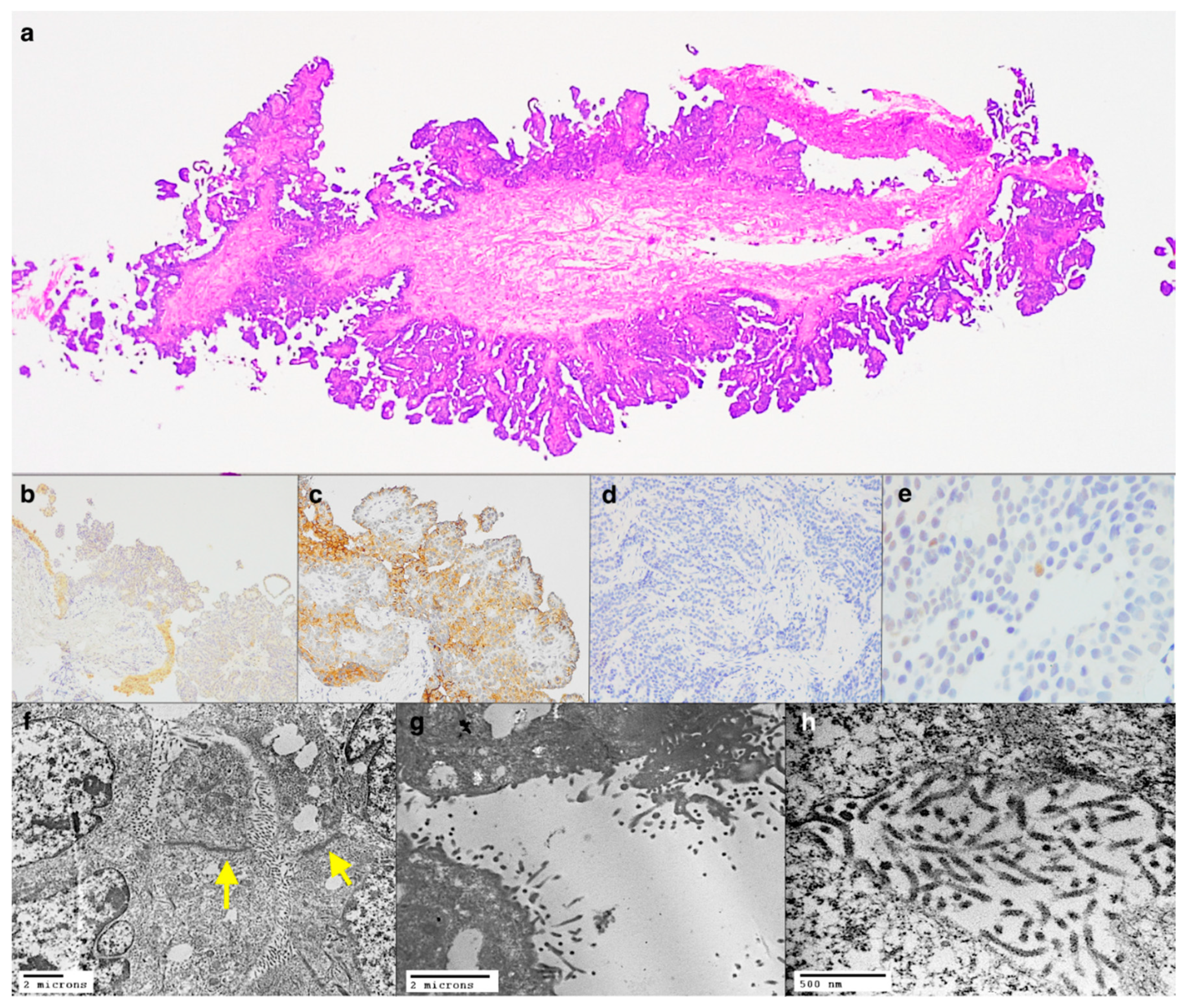
2.3. Case 3: Primitive or Metastasis?
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed]
- Galateau-Salle, F.; Churg, A.; Roggli, V.; Travis, W.D.; World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J. Thorac. Oncol. 2016, 11, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Zatarain-Barron, Z.L.; Carmona, A.; Domínguez-Malagón, H. The use of electron microscopy for diagnosis of malignant pleural mesothelioma. J. Thorac. Dis. 2017, 9, E337–E338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fresco, R. Malignant Mesothelioma Electron Microscopy. In Malignant Mesothelioma; Pass, H.I., Vogelzang, N.J., Carbone, M., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Graham, L.; Orenstein, J.M. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2007, 2, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Oczypok, E.A.; Oury, T.D. Electron microscopy remains the gold standard for the diagnosis of epithelial malignant mesothelioma: A case study. Ultrastruct. Pathol. 2015, 39, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.T.; Smith, M.L.; Roden, A.C.; Sukov, W.R.; Hornychová, H.; Thirumala, S.; Colby, T.V.; Tazelaar, H.D. Molecular and Ultrastructural Features of Diffuse Intrapulmonary Malignant Mesothelioma. Am. J. Surg. Pathol. 2019, 43, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Felner, K.J.; Wieczorek, R.; Kline, M.; Smith, R.L.; Sidhu, G.S. Malignant mesothelioma masquerading as a multinodular bronchioloalveolar cell adenocarcinoma with widespread pulmonary nodules. Int. J. Surg. Pathol. 2006, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Sheffield, B.S.; Ionescu, D.; Churg, A. Loss of BRCA1-associated protein 1 (BAP1) expression is rare in non-small cell lung cancer. Hum. Pathol. 2017, 60, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Akiyama, Y.; Kitamura, A.; Matsumoto, N.; Tomita, M.; Kataoka, H. Malignant mesothelioma with squamous differentiation. Histopathology 2018, 72, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.; Fraia, A.S.; Bellini, A.; Fortarezza, F.; Rea, F.; Calabrese, F. Unexpected pleural finding after a fall. Lancet Oncol. 2019, 20, e606. [Google Scholar] [CrossRef]
- Hung, Y.P.; Dong, F.; Dubuc, A.M.; Dal Cin, P.; Bueno, R.; Chirieac, L.R. Molecular characterization of localized pleural mesothelioma. Mod. Pathol. 2020, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Khoor, A.; Walts, A.E.; Nicholson, A.G.; Zhang, Y.Z.; Roggli, V.; Carney, J.; Roden, A.C.; Tazelaar, H.D.; Larsen, B.T.; et al. Localized malignant mesothelioma, an unusual and poorly characterized neoplasm of serosal origin: Best current evidence from the literature and the International Mesothelioma Panel. Mod. Pathol. 2020, 33, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Warhol, M.J.; Corson, J.M. An ultrastructural comparison of mesotheliomas with adenocarcinomas of the lung and breast. Hum. Pathol. 1985, 16, 50–55. [Google Scholar] [CrossRef]
- Chirieac, L.R.; Barletta, J.A.; Yeap, B.Y.; Richards, W.G.; Tilleman, T.; Bueno, R.; Baldini, E.H.; Godleski, J.; Sugarbaker, D.J. Clinicopathologic characteristics of malignant mesotheliomas arising in patients with a history of radiation for Hodgkin and non-Hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 4544–4549. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortarezza, F.; Della Barbera, M.; Pezzuto, F.; Lunardi, F.; Faccioli, E.; Pasello, G.; Rea, F.; Rizzo, S.; Calabrese, F. Diagnostic Challenges in Epithelioid Pleural Mesothelioma: Case Series with Support from Electron Microscopy. Diagnostics 2021, 11, 841. https://doi.org/10.3390/diagnostics11050841
Fortarezza F, Della Barbera M, Pezzuto F, Lunardi F, Faccioli E, Pasello G, Rea F, Rizzo S, Calabrese F. Diagnostic Challenges in Epithelioid Pleural Mesothelioma: Case Series with Support from Electron Microscopy. Diagnostics. 2021; 11(5):841. https://doi.org/10.3390/diagnostics11050841
Chicago/Turabian StyleFortarezza, Francesco, Mila Della Barbera, Federica Pezzuto, Francesca Lunardi, Eleonora Faccioli, Giulia Pasello, Federico Rea, Stefania Rizzo, and Fiorella Calabrese. 2021. "Diagnostic Challenges in Epithelioid Pleural Mesothelioma: Case Series with Support from Electron Microscopy" Diagnostics 11, no. 5: 841. https://doi.org/10.3390/diagnostics11050841
APA StyleFortarezza, F., Della Barbera, M., Pezzuto, F., Lunardi, F., Faccioli, E., Pasello, G., Rea, F., Rizzo, S., & Calabrese, F. (2021). Diagnostic Challenges in Epithelioid Pleural Mesothelioma: Case Series with Support from Electron Microscopy. Diagnostics, 11(5), 841. https://doi.org/10.3390/diagnostics11050841







