Machine Learning Study in Caries Markers in Oral Microbiota from Monozygotic Twin Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Oral Microbiota Characterization
2.3. Statistical Analysis and Machine Learning Modeling
- Classification accuracy is the proportion of correctly classified examples.
- F-1 is a weighted harmonic mean of precision and recall.
- Precision is the proportion of true positives among instances classified as positive, e.g., the proportion of cavity correctly identified as cavity.
- Recall is the proportion of true positives among all positive instances in the data, e.g., the number of cavity among all diagnosed as cavity.
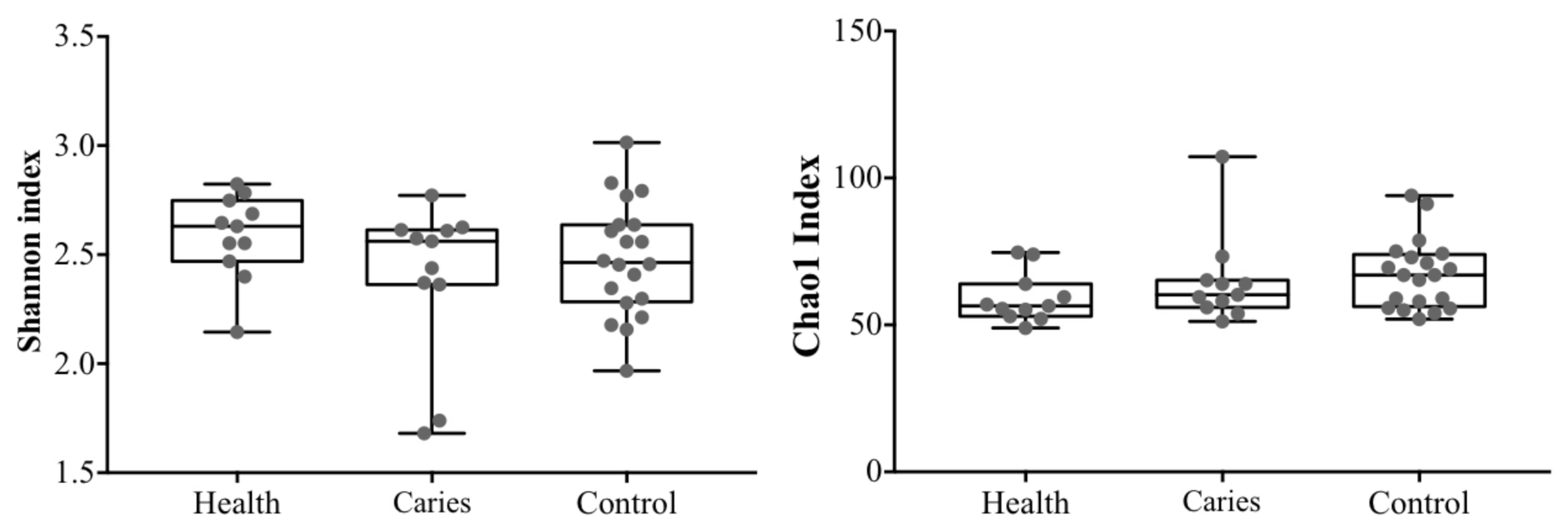
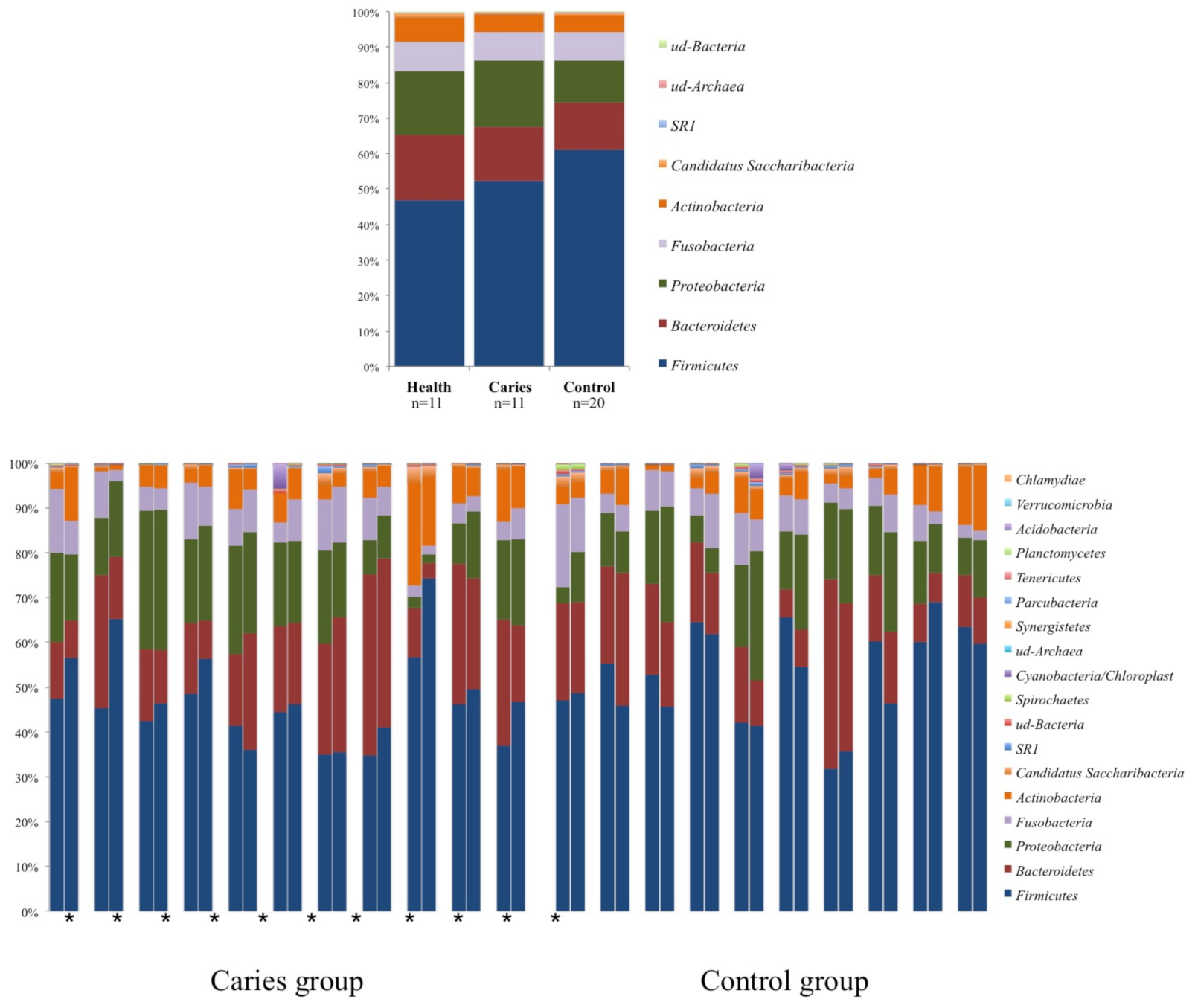
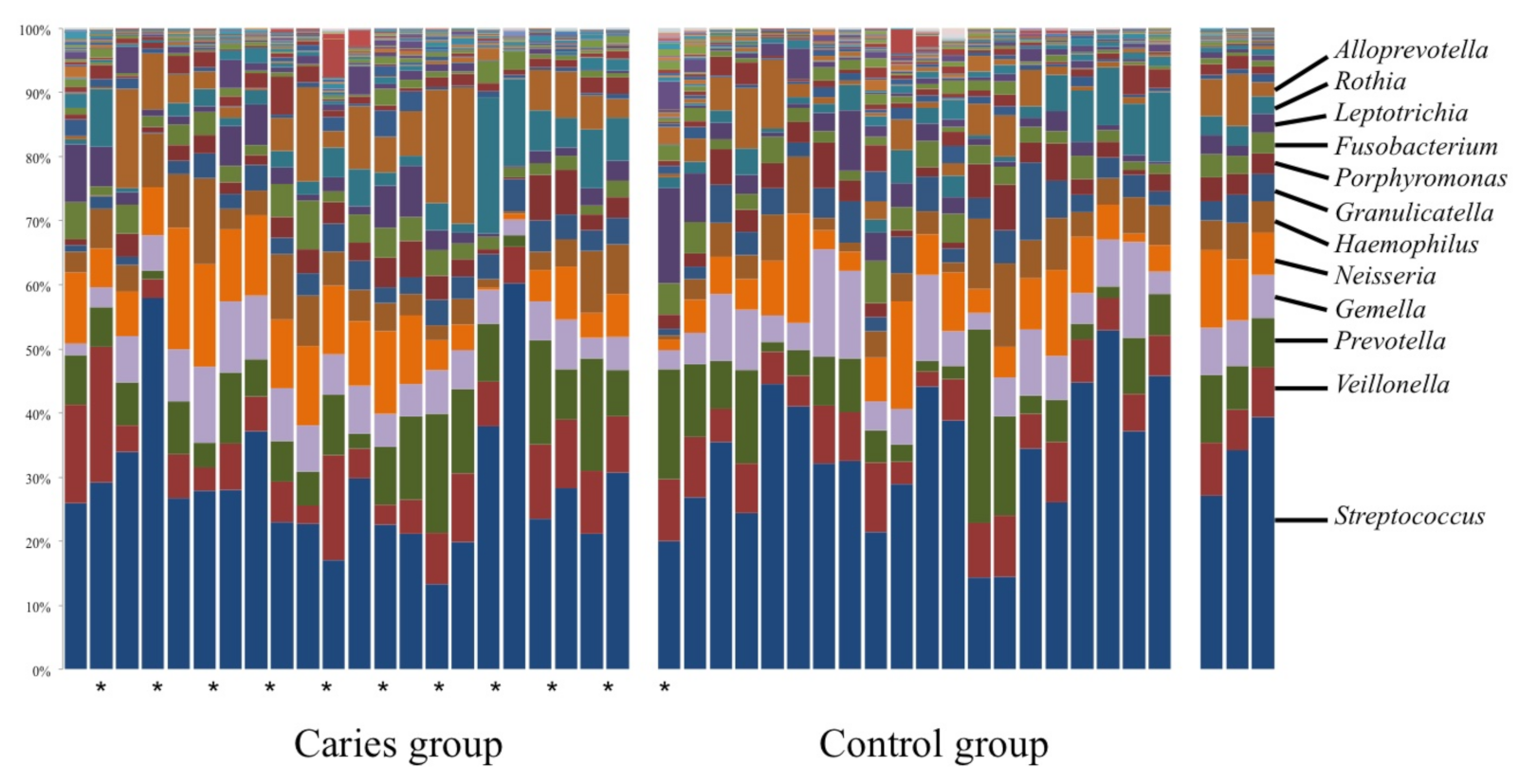
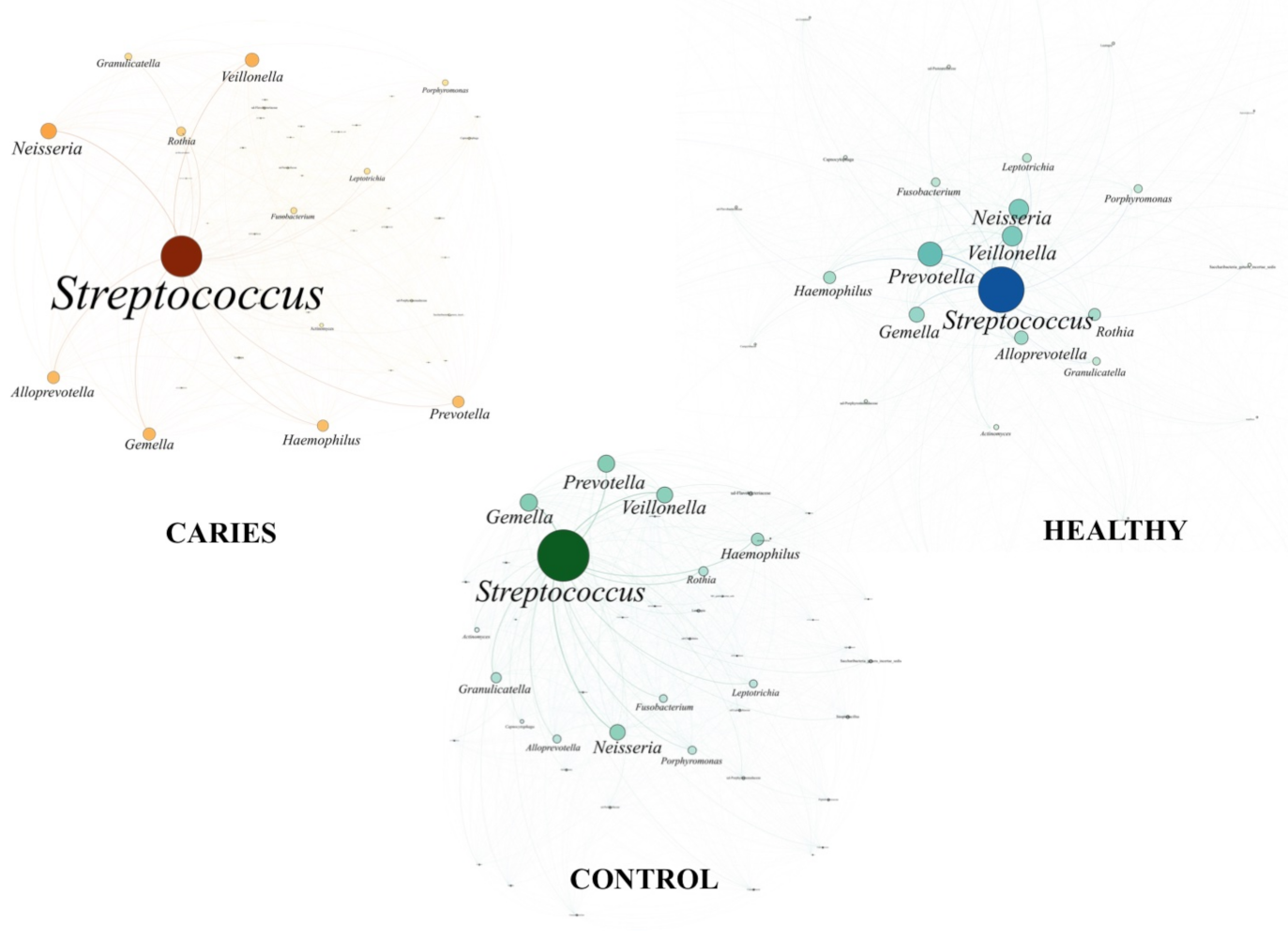
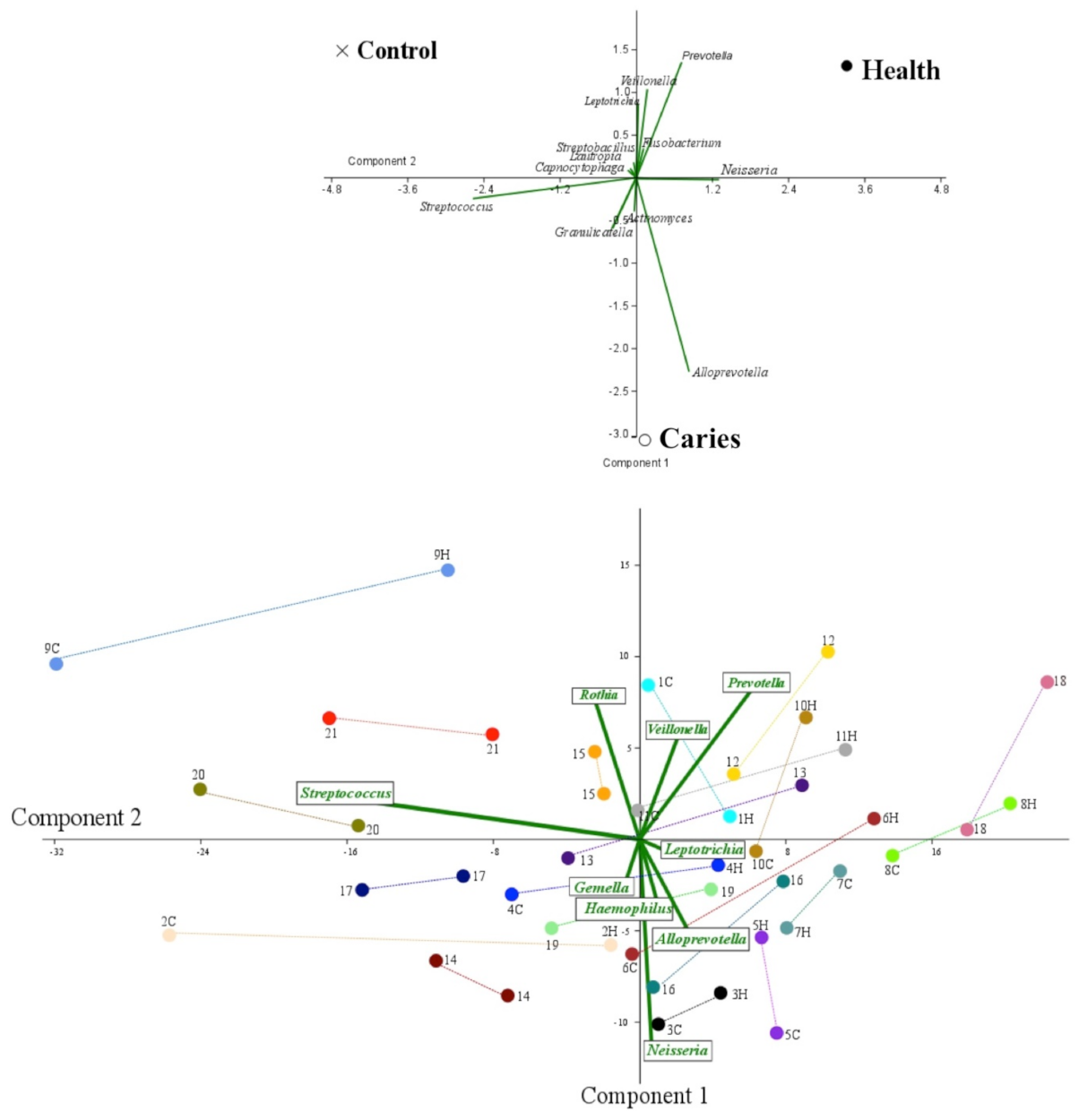
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral microbiome development during childhood: An ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, K.S.; Ngo, H.C.; Samaranayake, L.P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis. 2019, 25, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Nelson, K.E. The oral microbiome of children: Development, disease, and implications beyond oral health. Microb. Ecol. 2017, 73, 492–503. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Chen, T.; Shi, Y.; Wang, X.; Wang, X.; Meng, F.; Yang, S.; Yang, J.; Xin, H. High-throughput sequencing analyses of oral microbial diversity in healthy people and patients with dental caries and periodontal disease. Mol. Med. Rep. 2017, 16, 127–132. [Google Scholar] [CrossRef]
- Fernández-Escapa, I.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New insights into human nostril microbiome from the expanded Human Oral Microbiome Database (eHOMD): A resource for species-level identification of microbiome data from the aerodigestive tract. mSystems 2018, 3, e00187-18. [Google Scholar]
- Solbiati, J.; Frías-Lopez, J. Metatranscriptome of the oral microbiome in health and disease. J. Dent. Res. 2018, 97, 492–500. [Google Scholar] [CrossRef]
- Jiang, W.; Ling, Z.; Lin, X.; Chen, Y.; Zhang, J.; Yu, J.; Xiang, C.; Chen, H. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb. Ecol. 2014, 67, 962–969. [Google Scholar] [CrossRef]
- Xu, H.; Tian, J.; Hao, W.; Zhang, Q.; Zhou, Q.; Shi, W.; Qin, M.; He, X.; Chen, F. Oral Microbiome shifts from caries-free to caries-affected status in 3-year-old chinese children: A longitudinal study. Front. Microbiol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Wang, Y.; Jiang, W.; Wang, S.; Ling, Z.; Chen, H. Dynamic alterations in salivary microbiota related to dental caries and age in preschool children with deciduous dentition: A 2-year follow-up study. Front. Physiol. 2018, 9, 342. [Google Scholar] [CrossRef]
- Kressirer, C.A.; Smith, D.J.; King, W.F.; Dobeck, J.M.; Starr, J.R.; Tanner, A.C.R. Scardovia wiggsiae and its potential role as a caries pathogen. J. Oral Biosci. 2017, 59, 135–141. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Murabiato, P.; Indelicato, F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch. Oral Biol. 2021. [Google Scholar] [CrossRef]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Ribeiro, A.A.; Azcarate-Peril, M.A.; Cadenas, M.B.; Butz, N.; Paster, B.J.; Chen, T.; Bair, E.; Arnold, R.R. The oral bacterial microbiome of occlusal surfaces in children and its association with diet and caries. PLoS ONE 2017, 12, e0180621. [Google Scholar] [CrossRef]
- Ledder, R.G.; Kampoo, K.; Teanpaisan, R.; McBain, A.J. Oral microbiota in severe early childhood caries in Thai children and their families: A pilot study. Front. Microbiol. 2018, 9, 2420. [Google Scholar] [CrossRef]
- Silva, M.J.; Kilpatrick, N.M.; Craig, J.M.; Manton, D.J.; Leong, P.; Burgner, D.P.; Scurrah, K.J. Genetic and early-life environmental influences on dental caries risk: A twin study. Pediatrics 2019, 2019. 143, e20183499. [Google Scholar] [CrossRef]
- Stahringer, S.S.; Clemente, J.C.; Corley, R.P.; Hewitt, J.; Knights, D.; Walters, W.A.; Knight, R.; Krauter, K.S. Nurture trumps nature in a longitudinal survey of salivary bacterial communities in twins from early adolescence to early adulthood. Genome Res. 2012, 22, 2146–2152. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, B.; Li, B.; Ren, B.; Zhao, J.; Li, M.; Peng, X.; Feng, M.; Li, J.; Wei, H.; et al. Research on oral microbiota of monozygotic twins with discordant caries experience—In vitro and in vivo study. Sci. Rep. 2018, 8, 7267. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, N.; Wang, S.; Hu, X.; Jiao, K.; He, X.; Li, Z.; Wang, J. Exploration of human salivary microbiomes–insights into the novel characteristics of microbial community structure in caries and caries-free subjects. PLoS ONE 2016, 11, e0147039. [Google Scholar] [CrossRef]
- Lovelina, F.D.; Shastri, S.M.; Kumar, P.D. Assessment of the oral health status of monozygotic and dizygotic twins—A comparative study. Oral Health Prev. Dent. 2012, 10, 135–139. [Google Scholar]
- Zheng, Y.; Zhang, M.; Li, J.; Li, Y.; Teng, F.; Jiang, H.; Du, M. Comparative analysis of the microbial profiles in supragingival plaque samples obtained from twins with discordant caries phenotypes and their mothers. Front Cell Infect Microbiol. 2018, 8, 361. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, X.; Jin, L.; Lo, E.C. Salivary microbiome diversity in caries-free and caries-affected children. Int. J. Mol. Sci. 2016, 17, 1978. [Google Scholar] [CrossRef] [PubMed]
- Belstrom, D.; Constancias, F.; Liu, Y.; Yang, L.; Drautz-Moses, D.I.; Schuster, S.C.; Kohli, G.S.; Jakobsen, T.H.; Holmstrup, P.; Givskov, M. Metagenomic and metatranscriptomic analysis of saliva reveals disease-associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms Microbiomes 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Richards, V.P.; Alvarez, A.J.; Luce, A.R.; Bedenbaugh, M.; Mitchell, M.L.; Burne, R.A.; Nascimento, M.M. Microbiomes of site-specific dental plaques from children with different caries status. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Yasunaga, H.; Takeshita, T.; Shibata, Y.; Furuta, M.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; Takahashi, I.; Yamashita, Y. Exploration of bacterial species associated with the salivary microbiome of individuals with a low susceptibility to dental caries. Clin. Oral Investig. 2017, 21, 2399–2406. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, X.; Jiang, W.; Wang, S.; Xu, L.; Tu, Y.; Zheng, P.; Wang, Y.; Lin, X.; et al. Profiling of oral microbiota in early childhood caries using single-molecule real-time sequencing. Front. Microbiol. 2017, 8, 2244. [Google Scholar] [CrossRef]
- López-López, A.; Camelo-Castillo, A.; Ferrer, M.D.; Simon-Soro, A.; Mira, A. Health-associated niche inhabitants as oral probiotics: The case of Streptococcus dentisani. Front. Microbiol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Kim, B.S.; Han, D.H.; Lee, H.; Oh, B. Association of salivary microbiota with dental caries incidence with dentine involvement after 4 years. J. Microbiol. Biotechnol. 2018, 28, 454–464. [Google Scholar] [CrossRef]
| Caries Group 22 Infants | Sex | Age |
|---|---|---|
| 1C/1H | females | 12 |
| 2C/1H | females | 7 |
| 3C/1H | females | 8 |
| 4C/1H | females | 6 |
| 5C/1H | females | 11 |
| 6C/1H | males | 9 |
| 7C/1H | females | 8 |
| 8C/1H | males | 10 |
| 9C/1H | females | 12 |
| 10C/1H | females | 9 |
| 11C/1H | males | 9 |
| Control Group 20 Infants | Sex | Age |
| 12 | females | 12 |
| 13 | females | 7 |
| 14 | males | 4 |
| 15 | females | 8 |
| 16 | females | 9 |
| 17 | females | 5 |
| 18 | females | 9 |
| 19 | males | 4 |
| 20 | females | 4 |
| 21 | males | 5 |
| Model | CA | F1 | Precision | Recall |
|---|---|---|---|---|
| kNN | 0.666 | 0.655 | 0.646 | 0.665 |
| SVM | 0.738 | 0.627 | 0.545 | 0.738 |
| Random Forest | 0.881 | 0.874 | 0.880 | 0.881 |
| Neural Network | 0.810 | 0.814 | 0.823 | 0.810 |
| Logistic Regression | 0.595 | 0.601 | 0.607 | 0.595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alia-García, E.; Ponce-Alonso, M.; Saralegui, C.; Halperin, A.; Cortés, M.P.; Baquero, M.R.; Parra-Pecharromán, D.; Galeano, J.; del Campo, R. Machine Learning Study in Caries Markers in Oral Microbiota from Monozygotic Twin Children. Diagnostics 2021, 11, 835. https://doi.org/10.3390/diagnostics11050835
Alia-García E, Ponce-Alonso M, Saralegui C, Halperin A, Cortés MP, Baquero MR, Parra-Pecharromán D, Galeano J, del Campo R. Machine Learning Study in Caries Markers in Oral Microbiota from Monozygotic Twin Children. Diagnostics. 2021; 11(5):835. https://doi.org/10.3390/diagnostics11050835
Chicago/Turabian StyleAlia-García, Esther, Manuel Ponce-Alonso, Claudia Saralegui, Ana Halperin, Marta Paz Cortés, María Rosario Baquero, David Parra-Pecharromán, Javier Galeano, and Rosa del Campo. 2021. "Machine Learning Study in Caries Markers in Oral Microbiota from Monozygotic Twin Children" Diagnostics 11, no. 5: 835. https://doi.org/10.3390/diagnostics11050835
APA StyleAlia-García, E., Ponce-Alonso, M., Saralegui, C., Halperin, A., Cortés, M. P., Baquero, M. R., Parra-Pecharromán, D., Galeano, J., & del Campo, R. (2021). Machine Learning Study in Caries Markers in Oral Microbiota from Monozygotic Twin Children. Diagnostics, 11(5), 835. https://doi.org/10.3390/diagnostics11050835








