Relationship between Brain Tissue Changes and Blood Biomarkers of Cyclophilin A, Heme Oxygenase-1, and Inositol-Requiring Enzyme 1 in Patients with Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Correlation Analyses among Subject Characteristics and Blood Biomarkers
2.3. Voxel-Based Multiple Regression Analyses
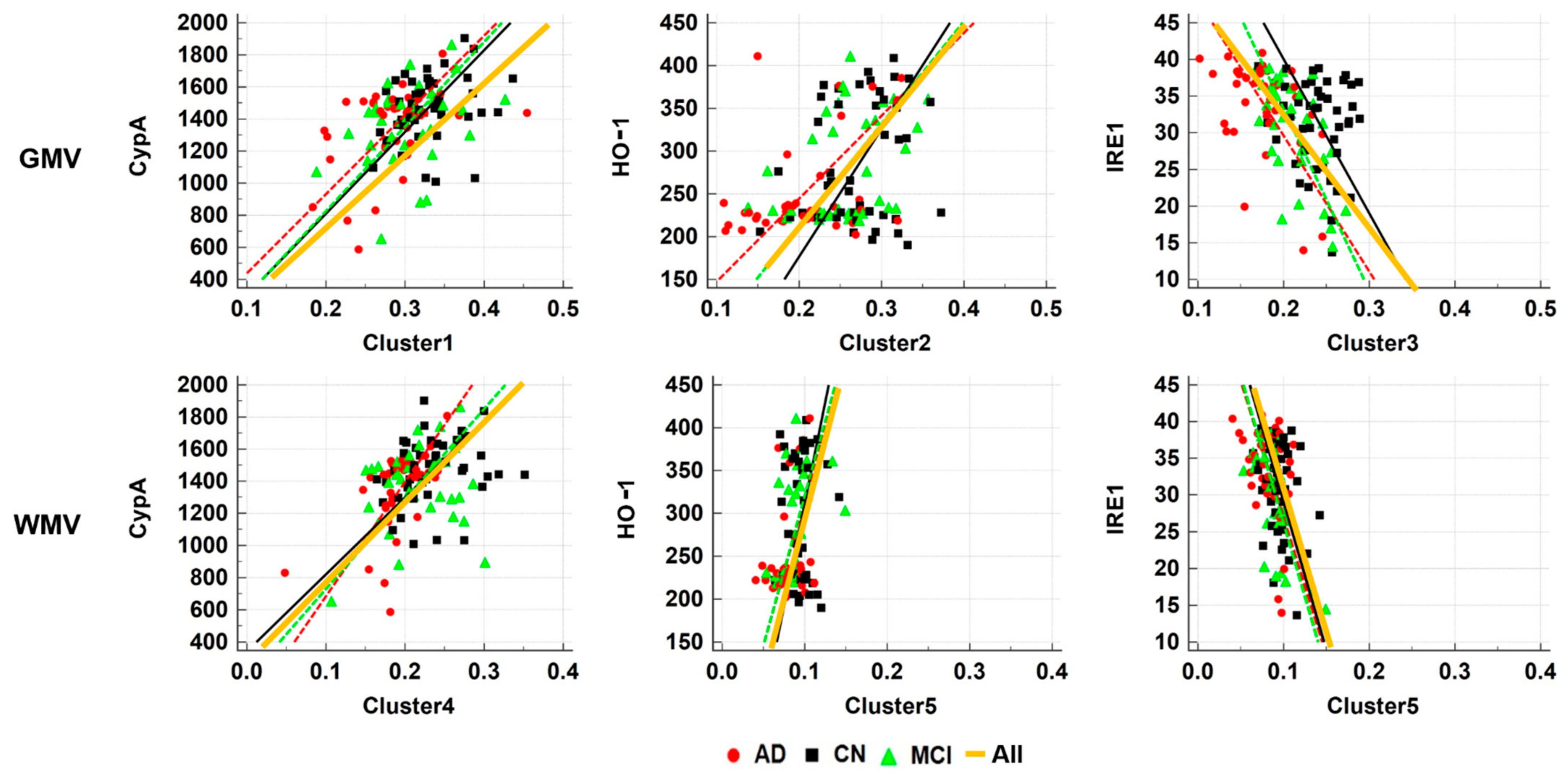
2.4. ROI-Based Correlation Analyses
3. Discussion
3.1. Association of CypA (PPIA) Blood Level
3.2. Association of HO-1 Blood Level
3.3. Association of IRE1 Plasma Level
3.4. Neuroimaging with Plasma Biomarkers
3.5. Limitations
4. Materials and Methods
4.1. Participants
4.2. Plasma Levels of HO-1, CypA, and IRE1
4.3. MRI Acquisition
4.4. Imaging Processing
4.5. Statistical Analyses
4.5.1. Demographic Characteristics, Results of Neuropsychological Tests, and Blood Biomarkers
4.5.2. Voxel-Based Multiple Regression Analyses
4.5.3. ROI-Based Correlation Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, Y.; Tangalos, E.G.; Petersen, R.C. Mild cognitive impairment. When is it a precursor to Alzheimer’s disease? Geriatrics 2000, 55, 65–68. [Google Scholar]
- Apostolova, L.G.; Hwang, K.S.; Avila, D.; Elashoff, D.; Kohannim, O.; Teng, E.; Sokolow, S.; Jack, C.R.; Jagust, W.J.; Shaw, L.; et al. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology 2015, 84, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Youn, Y.C.; Kang, S.; Suh, J.; Park, Y.H.; Kang, M.J.; Pyun, J.M.; Choi, S.H.; Jeong, J.H.; Park, K.W.; Lee, H.W.; et al. Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endres, K.; Reinhardt, S. ER-stress in Alzheimer’s disease: Turning the scale? Am. J. Neurodegener Dis. 2013, 2, 247–265. [Google Scholar]
- Satoh, K.; Shimokawa, H. Cyclophilin A: Novel Biomarker for Oxidative Stress and Cardiovascular Diseases. In General Methods in Biomarker Research and their Applications; Preedy, V.R., Patel, V.B., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 405–438. [Google Scholar]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med Cell Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [Green Version]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Smith, M.A.; Kutty, R.K.; Richey, P.L.; Yan, S.D.; Stern, D.; Chader, G.J.; Wiggert, B.; Petersen, R.B.; Perry, G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am. J. Pathol. 1994, 145, 42–47. [Google Scholar]
- Hettiarachchi, N.; Dallas, M.; Al-Owais, M.; Griffiths, H.; Hooper, N.; Scragg, J.; Boyle, J.; Peers, C. Heme oxygenase-1 protects against Alzheimer’s amyloid-β1-42-induced toxicity via carbon monoxide production. Cell Death Dis. 2014, 5, e1569. [Google Scholar] [CrossRef] [Green Version]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. J. Lab. Clin. Med. 2016, 167, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.; Breker, M.; Bakunts, A.; Pesek, K.; Chas, A.; Argemí, J.; Orsi, A.; Gal, L.; Chuartzman, S.; Wigelman, Y.; et al. Iron affects Ire1 clustering propensity and the amplitude of endoplasmic reticulum stress signaling. J. Cell Sci. 2017, 130, 3222–3233. [Google Scholar] [CrossRef] [Green Version]
- Healy, S.; McMahon, J.; FitzGerald, U. UPR Induction Prevents Iron Accumulation and Oligodendrocyte Loss in ex vivo Cultured Hippocampal Slices. Front. Neurosci. 2018, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Barkhof, F.; Bernstein, M.A.; Cantillon, M.; Cole, P.E.; Decarli, C.; Dubois, B.; Duchesne, S.; Fox, N.C.; Frisoni, G.B.; et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 474–485.e474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leow, A.D.; Yanovsky, I.; Parikshak, N.; Hua, X.; Lee, S.; Toga, A.W.; Jack, C.R., Jr.; Bernstein, M.A.; Britson, P.J.; Gunter, J.L.; et al. Alzheimer’s disease neuroimaging initiative: A one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage 2009, 45, 645–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plácido, A.I.; Pereira, C.M.F.; Duarte, A.I.; Candeias, E.; Correia, S.C.; Santos, R.X.; Carvalho, C.; Cardoso, S.; Oliveira, C.R.; Moreira, P.I. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: Implications for Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1444–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Femminella, G.D.; Dani, M.; Wood, M.; Fan, Z.; Calsolaro, V.; Atkinson, R.; Edginton, T.; Hinz, R.; Brooks, D.J.; Edison, P. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 2019, 92, e1331–e1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain A J. Neurol. 2017, 140, 3286–3300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, B.C.; Stoub, T.R.; Shah, R.C.; Sperling, R.A.; Killiany, R.J.; Albert, M.S.; Hyman, B.T.; Blacker, D.; Detoledo-Morrell, L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011, 76, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Hirose, W.; Ikematsu, K.; Tsuda, R. Age-associated increases in heme oxygenase-1 and ferritin immunoreactivity in the autopsied brain. Leg. Med. 2003, 5, S360–S366. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme oxygenase-1 in Alzheimer disease: A tribute to Moussa Youdim. J. Neural Transm. 2011, 118, 381–387. [Google Scholar] [CrossRef]
- Di Domenico, F.; Barone, E.; Mancuso, C.; Perluigi, M.; Cocciolo, A.; Mecocci, P.; Butterfield, D.A.; Coccia, R. HO-1/BVR-A System Analysis in Plasma from Probable Alzheimer’s Disease and Mild Cognitive Impairment Subjects: A Potential Biochemical Marker for the Prediction of the Disease. J. Alzheimer’s Dis. 2012, 32, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Bürger, K.; Teipel, S.J.; Bokde, A.L.W.; Zetterberg, H.; Blennow, K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease⁎⁎This paper was presented in part by the 1st author at the 10th International Conference of Alzheimer’s Disease and Related Disorders (ICAD), Madrid, Spain, July 2006, as an invited plenary lecture. Alzheimer’s Dement. 2008, 4, 38–48. [Google Scholar] [CrossRef]
- Maes, O.C.; Kravitz, S.; Mawal, Y.; Su, H.; Liberman, A.; Mehindate, K.; Berlin, D.; Sahlas, D.J.; Chertkow, H.M.; Bergman, H.; et al. Characterization of alpha1-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiol. Dis. 2006, 24, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kimpara, T.; Takeda, A.; Watanabe, K.; Itoyama, Y.; Ikawa, S.; Watanabe, M.; Arai, H.; Sasaki, H.; Higuchi, S.; Okita, N.; et al. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 1997, 100, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Ohnuma, T.; Baba, H.; Arai, H. No genetic association between polymorphisms of heme oxygenase 1 and 2 and Alzheimer’s disease in a Japanese population. Dement Geriatr. Cogn. Disord. 2009, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Hwang, K.S.; Lazaris, A.; Chow, N.; Ramirez, L.; Babakchanian, S.; Woo, E.; Thompson, P.M.; Apostolova, L.G. Cortical thickness and semantic fluency in Alzheimer’s disease and mild cognitive impairment. Am. J. Alzheimers Dis. 2013, 1, 81–92. [Google Scholar] [CrossRef]
- de Jong, L.W.; van der Hiele, K.; Veer, I.M.; Houwing, J.J.; Westendorp, R.G.; Bollen, E.L.; de Bruin, P.W.; Middelkoop, H.A.; van Buchem, M.A.; van der Grond, J. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: An MRI study. Brain A J. Neurol. 2008, 131, 3277–3285. [Google Scholar] [CrossRef] [Green Version]
- Haroutunian, V.; Katsel, P.; Schmeidler, J. Transcriptional vulnerability of brain regions in Alzheimer’s disease and dementia. Neurobiol. Aging 2009, 30, 561–573. [Google Scholar] [CrossRef]
- Cornejo, V.H.; Hetz, C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013, 35, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Duran-Aniotz, C.; Cornejo, V.H.; Espinoza, S.; Ardiles, Á.O.; Medinas, D.B.; Salazar, C.; Foley, A.; Gajardo, I.; Thielen, P.; Iwawaki, T.; et al. IRE1 signaling exacerbates Alzheimer’s disease pathogenesis. Acta Neuropathol. 2017, 134, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, P.; Huang, R.; Shang, H. A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2012, 36, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Z.; Li, K.; Li, Z.; Qi, Z.; Jin, Z.; Yao, L.; Chen, K. Voxel-based assessment of gray and white matter volumes in Alzheimer’s disease. Neurosci. Lett. 2010, 468, 146–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Seiger, R.; Ganger, S.; Kranz, G.S.; Hahn, A.; Lanzenberger, R. Cortical Thickness Estimations of FreeSurfer and the CAT12 Toolbox in Patients with Alzheimer’s Disease and Healthy Controls. J. Neuroimaging 2018, 28, 515–523. [Google Scholar] [CrossRef] [PubMed]



| Group | CN (1) | aMCI (2) | AD (3) | Statistics (Post Hoc) |
|---|---|---|---|---|
| Demographic Data and Neuropsychologic Tests | ||||
| No. of subjects | 45 | 34 | 39 | 118 |
| * Age (years) | 63.64 ± 9.18 | 70.35 ± 6.88 | 75.03 ± 7.92 | F = 20.697/p < 0.001 (1,2)(1,3) |
| Sex (male/female) | 12/33 | 6/28 | 4/35 | & 0.1541, χ2 = 3.741 |
| * K-MMSE(/30) | 27.73 ± 2.32 | 24.12 ± 4.00 | 18.49 ± 4.60 | F = 65.904/p < 0.001 (1,2,3) |
| CDR (range) | 0.000 (0.0–0.5) | 0.500 (0.5–0.5) | 1.000 (0.50–2.0) | N/A |
| * Education (years) | 9.53 ± 5.98 | 8.48 ± 5.11 | 6.26 ± 4.65 | F = 4.051/p = 0.020 (1,3) |
| * Global Brain Tissue Segmented Volume | ||||
| * Global GMV (mm3) | 586.36 ± 48.07 | 564.48 ± 42.50 | 525.41 ± 52.26 | F = 17.053/p < 0.001 (1,3) (2,3) |
| * Global WMV (mm3) | 471.59 ± 50.50 | 456.32 ± 39.88 | 436.34 ± 46.22 | F = 6.080/p = 0.003 (1,3) |
| * Global CSF volume (mm3) | 365.61 ± 54.77 | 419.54 ± 62.10 | 486.51 ± 69.61 | F = 39.614/p < 0.001 (1,2,3) |
| * TIV (mm3) | 1423.56 ± 101.07 | 1440.34 ± 108.99 | 1448.25 ± 134.34 | F = 0.505/p = 0.605 |
| * Plasma Levels of Three Blood Biomarkers | ||||
| * CypA (PPIA)ng/mL) | 1470.44 ± 201.27 | 1372.13 ± 250.96 | 1352.80 ± 250.35 | F = 3.089/p = 0.049 (none) |
| * HO-1 (ng/mL) | 285.05 ± 70.71 | 280.87 ± 61.25 | 250.91 ± 56.61 | F = 3.415/p = 0.036 (none) |
| * IRE1(pg/mL) | 31.30 ± 6.04 | 30.62 ± 7.01 | 34.01 ± 6.19 | F = 2.995/p = 0.054 |
| Regressor | Subjects | CypA | HO-1 | IRE1 |
|---|---|---|---|---|
| Age | All | −0.096/0.303 | −0.226/0.014 | 0.222/0.016 |
| CN | −0.118/0.439 | −0.126/0.409 | 0.011/0.944 | |
| aMCI | −0.215/0.222 | −0.403/0.018 | 0.482/0.004 | |
| AD | 0.349/0.029 | 0.042/0.800 | 0.118/0.474 | |
| MMSE | All | 0.215/0.019 | 0.250/0.006 | −0.241/0.009 |
| CN | 0.098/0.523 | 0.009/0.952 | 0.049/0.7515 | |
| aMCI | 0.126/0.478 | −0.194/0.272 | −0.091/0.607 | |
| AD | 0.088/0.594 | 0.493/0.001 | −0.344/0.032 | |
| * adjMMSE | All | 0.195/0.036 | 0.160/0.085 | −0.153/0.101 |
| CN | 0.054/0.730 | −0.048/0.756 | 0.0584/0.707 | |
| aMCI | 0.072/0.689 | −0.343/0.051 | 0.0456/0.801 | |
| AD | 0.152/0.363 | 0.506/0.001 | −0.332/0.042 | |
| Education | All | 0.012/0.901 | 0.092/0.325 | −0.038/0.684 |
| TIV | All | 0.048/0.609 | −0.063/0.498 | −0.006/0.947 |
| Global GMV | All | 0.220/0.017 | 0.193/0.037 | −0.213/0.021 |
| CN | 0.156/0.305 | 0.043/0.777 | −0.080/0.604 | |
| aMCI | 0.182/0.304 | 0.091/0.607 | −0.165/0.353 | |
| AD | 0.114/0.489 | 0.174/0.288 | −0.192/0.241 | |
| Global WMV | All | 0.154/0.097 | 0.059/0.530 | −0.100/0.282 |
| CypA | All | - | 0.131/0.156 | −0.106/0.252 |
| HO-1 | All | 0.131/0.156 | - | −0.664/<0.001 |
| CN | 0.145/0.343 | - | −0.627/<0.001 | |
| aMCI | 0.060/0.737 | - | −0.756/<0.001 | |
| AD | 0.082/0.620 | - | −0.583/0.001 |
| ROI | Tissue | CypA r/p-Value | HO-1 r/p-Value | IRE1 r/p-Value | |||
|---|---|---|---|---|---|---|---|
| Cluster 1 | GMV | 0.387 | <0.0001 | −0.043 | 0.642 | −0.025 | 0.789 |
| WMV | 0.054 | 0.564 | 0.079 | 0.394 | −0.083 | 0.372 | |
| Cluster 2 | GMV | −0.023 | 0.805 | 0.307 | 0.0008 | −0.239 | 0.010 |
| WMV | 0.012 | 0.898 | 0.013 | 0.886 | −0.065 | 0.489 | |
| Cluster 3 | GMV | 0.080 | 0.391 | 0.287 | 0.002 | −0.335 | 0.0002 |
| WMV | 0.054 | 0.567 | −0.045 | 0.629 | 0.027 | 0.772 | |
| Cluster 4 | GMV | 0.223 | 0.016 | −0.024 | 0.801 | 0.017 | 0.856 |
| WMV | 0.369 | <0.0001 | 0.058 | 0.537 | −0.092 | 0.323 | |
| Cluster 5 | GMV | 0.129 | 0.165 | −0.058 | 0.532 | −0.137 | 0.140 |
| WMV | 0.655 | 0.483 | 0.225 | 0.015 | −0.405 | <0.0001 | |
| Hippocampus | GMV | 0.156 | 0.093 | 0.197 | 0.034 | −0.175 | 0.059 |
| WMV | 0.106 | 0.256 | −0.042 | 0.650 | 0.015 | 0.869 | |
| Posterior Cingulate | GMV | 0.196 | 0.034 | −0.007 | 0.937 | −0.072 | 0.442 |
| WMV | 0.158 | 0.089 | 0.014 | 0.880 | −0.061 | 0.513 | |
| Precuneus | GMV | 0.148 | 0.112 | −0.070 | 0.451 | 0.013 | 0.887 |
| WMV | 0.138 | 0.137 | 0.037 | 0.696 | −0.063 | 0.495 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-I.; Kim, K.; Lee, J.; Chang, Y.; Rhee, H.Y.; Park, S.; Lee, W.-I.; Choe, W.; Ryu, C.-W.; Jahng, G.-H. Relationship between Brain Tissue Changes and Blood Biomarkers of Cyclophilin A, Heme Oxygenase-1, and Inositol-Requiring Enzyme 1 in Patients with Alzheimer’s Disease. Diagnostics 2021, 11, 740. https://doi.org/10.3390/diagnostics11050740
Choi H-I, Kim K, Lee J, Chang Y, Rhee HY, Park S, Lee W-I, Choe W, Ryu C-W, Jahng G-H. Relationship between Brain Tissue Changes and Blood Biomarkers of Cyclophilin A, Heme Oxygenase-1, and Inositol-Requiring Enzyme 1 in Patients with Alzheimer’s Disease. Diagnostics. 2021; 11(5):740. https://doi.org/10.3390/diagnostics11050740
Chicago/Turabian StyleChoi, Hyon-Il, Kiyoon Kim, Jiyoon Lee, Yunjung Chang, Hak Young Rhee, Soonchan Park, Woo-In Lee, Wonchae Choe, Chang-Woo Ryu, and Geon-Ho Jahng. 2021. "Relationship between Brain Tissue Changes and Blood Biomarkers of Cyclophilin A, Heme Oxygenase-1, and Inositol-Requiring Enzyme 1 in Patients with Alzheimer’s Disease" Diagnostics 11, no. 5: 740. https://doi.org/10.3390/diagnostics11050740
APA StyleChoi, H.-I., Kim, K., Lee, J., Chang, Y., Rhee, H. Y., Park, S., Lee, W.-I., Choe, W., Ryu, C.-W., & Jahng, G.-H. (2021). Relationship between Brain Tissue Changes and Blood Biomarkers of Cyclophilin A, Heme Oxygenase-1, and Inositol-Requiring Enzyme 1 in Patients with Alzheimer’s Disease. Diagnostics, 11(5), 740. https://doi.org/10.3390/diagnostics11050740








